Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Research on which this chapter is based was supported by National Institutes of Health grant HD-008783-39 and the George L. MacGregor Professorship in Pediatrics. I thank the many co-investigators and students who, while in my laboratories, facilitated many of our studies.
Pregnancy is associated with numerous alterations to the maternal cardiovascular system; however, it is the development of the low-resistance, high-flow placental vascular bed that characterizes this physiologic state. Moreover, this vascular bed is unique in that it is composed of maternal and fetal components separated by several cell layers that differ in structure and function among the mammalian species studied. Although these two vascular beds are intimately associated with each other, they function independently. An understanding of the mechanisms that control their vascular tone and the magnitude of blood flow is important in that alterations in either the maternal or the fetal aspect of placental blood flow may modify the delivery of oxygen and/or nutrients to the fetus, as well as affect the removal of carbon dioxide and other fetal metabolic wastes. Thus, the integrity of the maternal uteroplacental and fetal umbilicoplacental vascular beds is essential to fetal growth and development, minute-to-minute fetal well-being, and possibly alterations in fetal programming that contribute to the subsequent occurrence of adult-onset cardiovascular and/or metabolic disease. ,
In view of the importance of the placental vascular beds to fetal well-being and survival of the species, research in this area has been intense since the time of Barcroft’s research. Because detailed studies in humans remain problematic and are generally descriptive, current understanding reflects in large part experimental observations in various animal models and, in particular, the pregnant ewe. Thus, conclusions about the relative importance of the mechanisms responsible for the control of blood flow to these vascular beds in the human continue to require careful interpretation. Nonetheless, recent knowledge obtained from animal models—and in particular, sheep—has been replicated in studies of human uterine arteries. Thus, sheep studies provide an excellent basis for characterizing this aspect of pregnancy and improving our understanding of clinical data obtained in Doppler flow velocity studies in women.
Although the placenta is of fetal origin and composed solely of fetal cells, its blood supply is derived from the maternal uterine and fetal umbilical arteries, the former serving as the source of placental and fetal oxygen and nutrients. Both vascular beds undergo substantial independent modification throughout pregnancy. For example, the maternal uterine vasculature undergoes remodeling, and the fetal vasculature exhibits ongoing vasculogenesis, angiogenesis, and branching morphogenesis. These changes occur in parallel and are essential in providing the increasing requirements for oxygen and nutrient delivery necessary for the logarithmic fetal growth in the last third of pregnancy, and the increasing endocrine and metabolic functions of the placenta. Understanding these independent, yet parallel, modifications will facilitate our knowledge of fetal growth, maintenance of fetal well-being, and possibly the origins of some pregnancy-related diseases (e.g., preeclampsia).
In women, the uteroplacental vascular bed is composed of cotyledons, lobes, or placentomes similar to those seen in many species. In contrast with their counterparts in the sheep or cow, these structures adhere to each other, rather than existing as separate entities. Nonetheless, they can be identified as individual structures when viewed from the basal or maternal side of the placenta after its delivery. Each placentome receives its maternal arterial blood by way of a single spiral artery, and alterations in the tone of these and/or more proximal branches of the uterine artery are responsible for modifying the magnitude and distribution of maternal uteroplacental blood flow. , As in pregnant sheep, a fixed number of placentomes are ultimately available in normal human pregnancy. Therefore, the rise in maternal uterine blood flow during pregnancy is initially due to the development of the placenta (i.e., implantation and growth) and vasodilation of the spiral arteries, allowing for increases in perfusion of the intervillous space.
When maternal uteroplacental blood flow is examined throughout ovine gestation, a complex scenario is observed. Total uterine blood flow ( Fig. 9.1B ) increases nearly 40-fold during the course of a normal singleton pregnancy, and levels are even higher in multiple gestations. This increase is coincident with a doubling of maternal cardiac output and an increase in the proportion of cardiac output directed to the uterus, increasing from less than 1% of cardiac output in nonpregnant sheep to nearly 25% at term. The pattern of this increase in total ovine uterine blood flow (mL/min) occurs in three phases (see Fig. 9.1B ). The first is associated with relatively low absolute blood flows, which on the basis of uterine plus conceptus wet weight is actually high, achieving values of 0.8 mL/min•g, compared with 0.3 mL/min•g for the nonpregnant uterus (see Fig. 9.1C ). This increase in uterine blood flow is believed to reflect vasodilation secondary to the increased production of maternal ovarian and possibly fetal hormones (e.g., estrogen, progesterone), which may be required for survival of the conceptus before implantation and the initial phase of implantation and placentation. , In primates and women, this occurs in the first days or weeks of pregnancy. The second phase is associated with the development of the fetal placentomes and in the primate, with the development of the intervillous spaces or the maternal placental vascular bed. At this time, the anatomic maternal placental vascular bed achieves its maximum size. If this does not occur, placentation will be restricted, resulting in a small placenta and a fetus characterized by proportionate growth restriction. In sheep, total uterine blood flow plateaus at this time and averages approximately 500 mL/min; however, blood flow expressed on a weight basis at the same point in gestation (which takes into account metabolically active tissues) has fallen by 50% to 0.3 to 0.4 mL/min•g, reflecting the ever-increasing weight of the fetus and placenta (see Fig. 9.1C ). The final phase in the “growth” of uterine blood flow is exponential and associated with a threefold increase in fetal weight in the last third of pregnancy, or after 110 days in sheep (75% of gestation) and beyond 30 weeks in humans (75%). Because neither the total number of spiral arteries nor that of placentomes changes, this increase in uterine blood flow must be due to vasodilation.
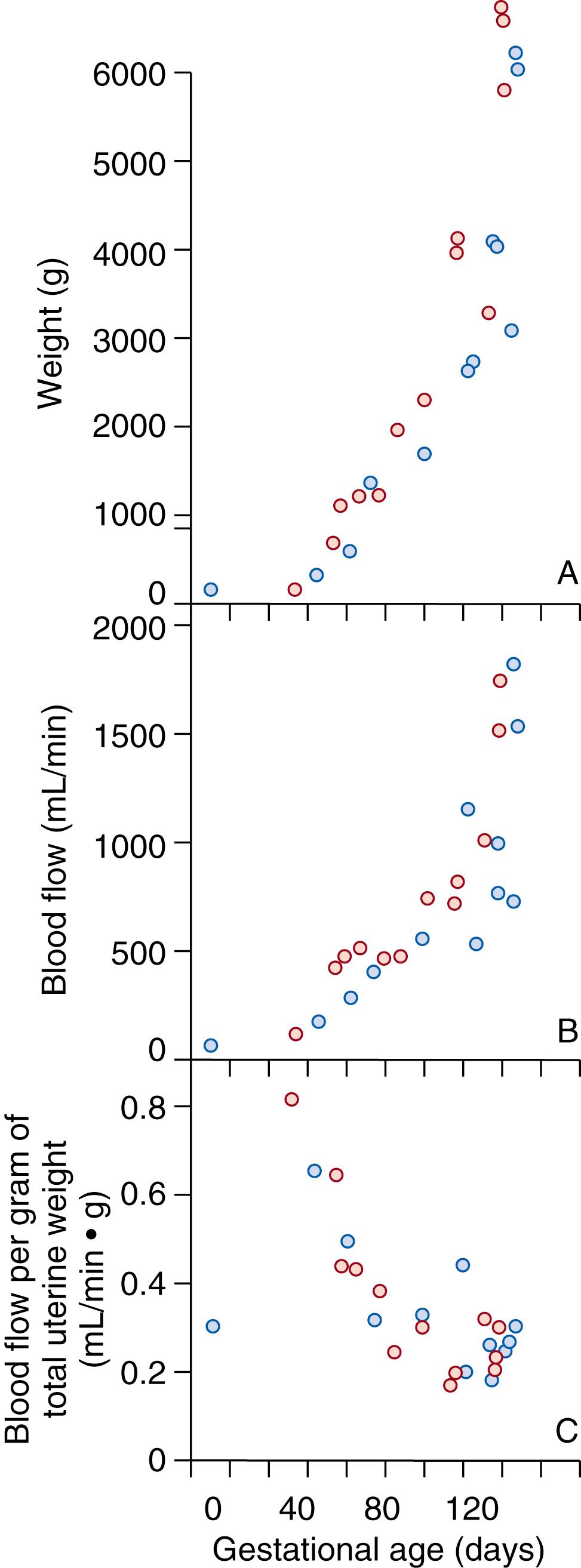
In recent studies, this final stage of vasodilation has been shown to involve activation of large-conductance, calcium-sensitive, potassium channels (BK Ca ) in the uterine vascular smooth muscle and membrane hyperpolarization. Although the initiating events are unclear, the signaling cascade appears to involve nitric oxide synthase (NOS), nitric oxide (NO), cyclic guanosine monophosphate (cGMP), and cGMP-dependent protein kinase ( Table 9.1 ). Evolving evidence suggests that voltage-regulated K + channels (K V ) might also play a role in maintaining basal uterine vascular tone by attenuating the vasoconstrictor effects of angiotensin II (ANG II) and increased sympathetic outflow in response to the fall in maternal systemic vascular resistance. Similar mechanisms appear to exist in women. Of importance, the rise in blood flow in milliliters per minute is proportionate to and parallels the rise in wet weight of the uterus and its metabolically active tissues, which includes the growing fetus, membranes, and placenta. Thus, blood flow per gram of wet weight remains low (0.2 to 0.3 mL/min•g) during the remainder of pregnancy. In the presence of late-onset fetal growth restriction (i.e., after 70% of gestation), blood flow per gram of fetoplacental mass is lower, and nutrient and oxygen delivery is decreased. Fetal growth is attenuated until adaptation has occurred and this ratio is reestablished, at which time a new growth curve is established that lies below the curve for normally growing fetuses.
| Uteroplacental Circulation | Umbilicoplacental Circulation | |
|---|---|---|
| Angiotensin II (ANG II) | Type 2 ANG II receptor accounts for >80% of binding in UA VSM and decreases ANG II sensitivity, as does reciprocal synthesis of endothelium-derived nitric oxide (NO) and prostacyclin (PGI 2 ), all contributing to the refractoriness to ANG II in pregnancy. | Type 1 ANG II receptor accounts for >90% of binding, and with advanced functional maturation of umbilical artery, VSM accounts for the increased sensitivity to vasoconstricting effects of ANG II compared to fetal systemic arteries and vascular beds. |
| Catecholamines | Potent vasoconstrictor effects mediated via VSM α-adrenergic receptors, but in pregnancy is attenuated by substantial increases in endothelium-derived NO. | Refractoriness to the vasoconstrictor effects are poorly studied but may reflect low receptor expression or function and/or high endothelial NO synthesis. |
| Arginine vasopressin (AVP) | VSM refractoriness to the vasoconstrictor effects, but the mechanisms are unclear. Could reflect alterations in receptor expression and/or function. | VSM is refractory to the vasoconstrictor effects; mechanisms are unclear. |
| Endothelin-1 (ET-1) | Potent vasoconstrictor mediated via ET-A and ET-B 2 receptors in VSM. | Vasoconstrictor effects mediated via ET-A and ET-B 2 receptors in VSM. |
| Estrogens | Potent UA vasodilators (E2β>> estrone >> estriol >> E2α) mediated via endothelial estrogen receptor-α (ERα), activation of endothelial and VSM NOS-NO and VSM NO-cGMP-PKG pathway, resulting in increased opening of VSM large conductance calcium-activated potassium channels (BK Ca ), which decrease cytosolic Ca 2+ . Estrogens also increase β1-regulatory subunit expression, which regulates Ca 2+ entry. Progesterone antagonizes the UA vasodilator effects. | Unclear and poorly studied. |
| Hydrogen sulfide (H 2 S) | Estrogens and pregnancy increase endothelial and VSM cystathionine β-synthase (CBS) expression and activity, thereby increasing H 2 S synthesis and UA vasodilation by activating ATP-dependent potassium channels (K ATP ) and VSM BK Ca channels. | Unclear and not studied. |
| Potassium channels | VSM BK Ca channels are activated by NO and estrogens via VSM NO-cGMP-PKG pathway to decrease cytosolic Ca 2+ (entry and translocation) via effects of β1-regulatory subunits, which increase channel opening and VSM depolarization, resulting in UA vasodilation. In late pregnancy, VSM β1-regulatory subunit expression is increased. K v channels are more complex and their role in placental circulation is unclear, but their expression may also be estrogen sensitive. | Unclear and not studied. |
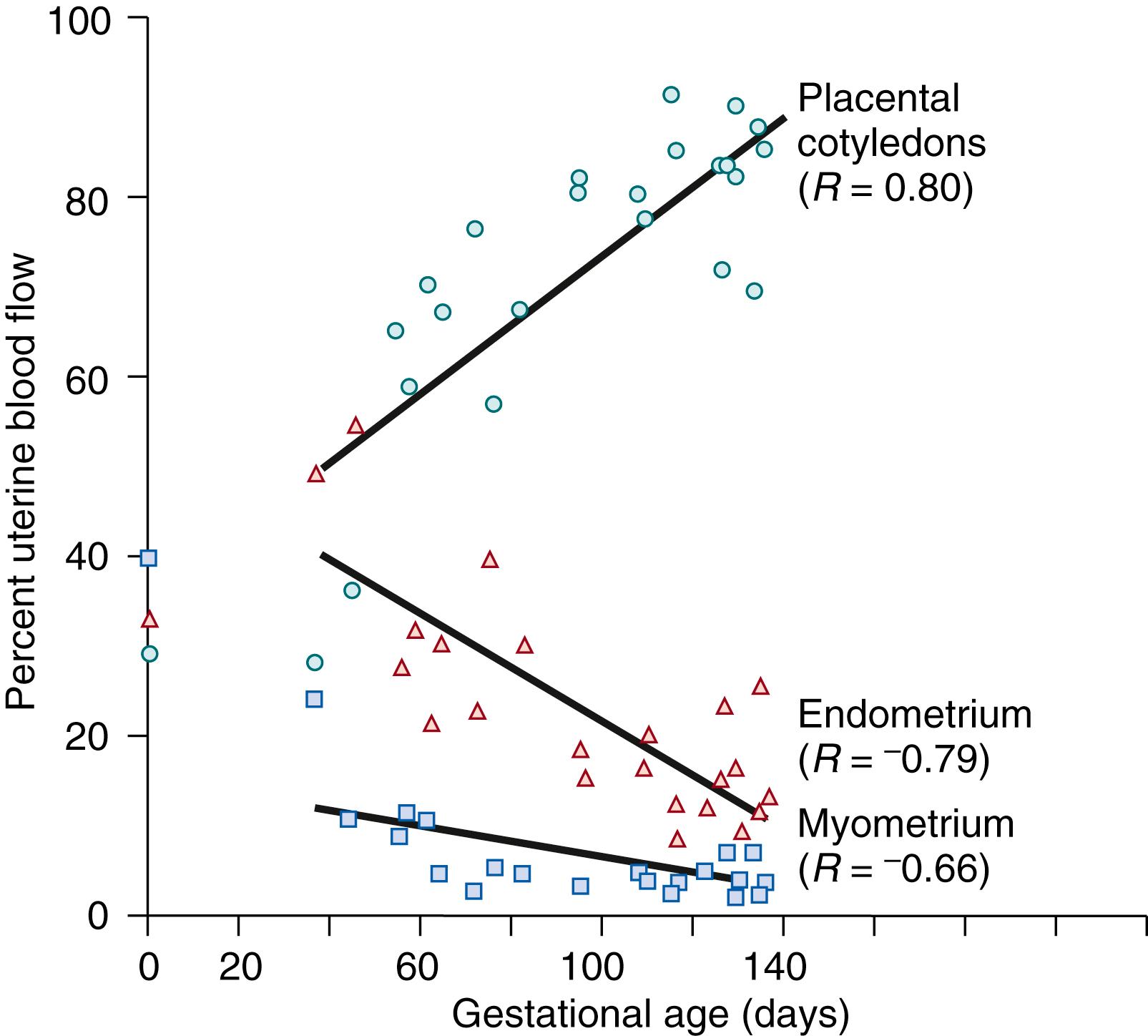
The dramatic rise in total uterine blood flow during pregnancy actually reflects changes in three separate vascular beds within the gravid uterus: the placentomes, endometrium, and myometrium. The change in distribution of blood flow to these tissues cannot be determined in women but has been studied in sheep and other species. , This limitation is an important consideration because measurement of changes in total uterine blood flow or blood flow in a single, large uterine artery in women by Doppler technology does not necessarily reflect the issue of primary importance—that is, placental perfusion. As illustrated in Fig. 9.2 , blood flow to these three tissues is evenly distributed in the nonpregnant uterus, each receiving approximately one third. As pregnancy progresses, a gradual redistribution of uterine blood flow occurs, such that at term the placentomes (cotyledons) receive nearly 90% of total uterine blood flow. Thus in the last third of pregnancy, perfusion of the placentomes accounts for most of the observed increase in uterine blood flow, and it is more likely that measures of Doppler flow in the main uterine artery parallel placental blood flow.
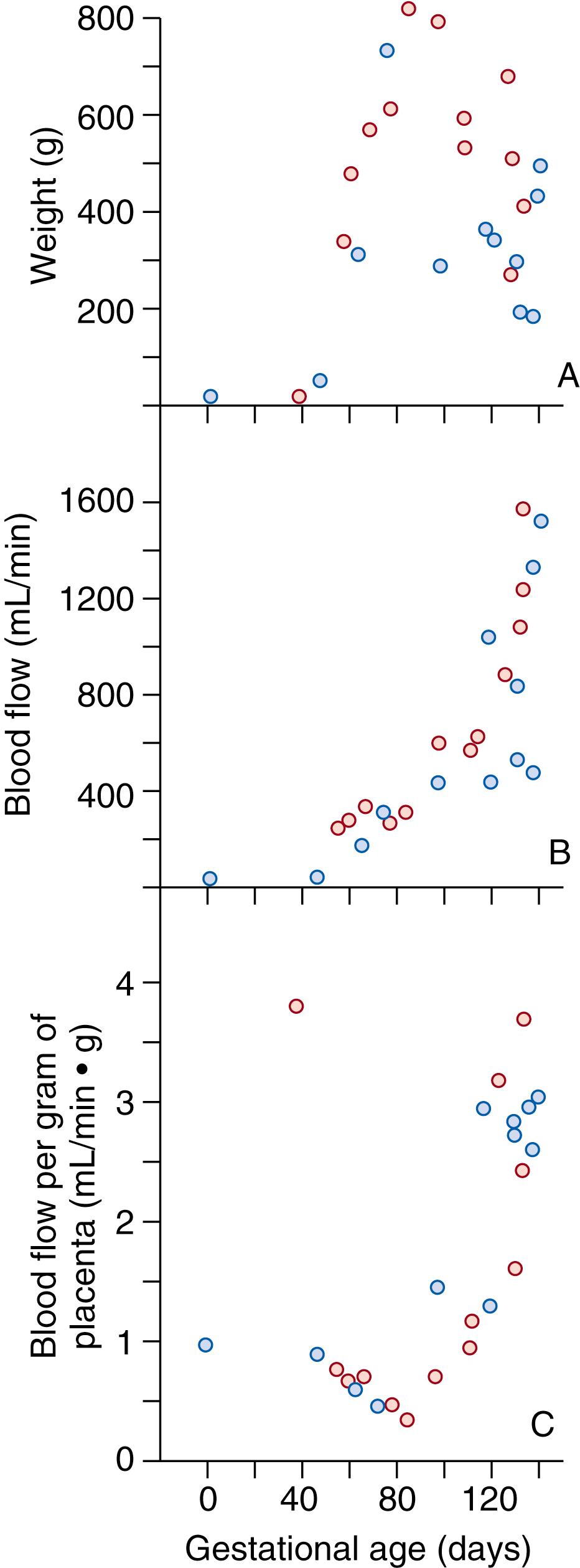
The placentome is the site of nutrient and gas exchange; thus fetal well-being is determined by changes in placental development, growth, and perfusion. Placental weight is relatively unchanged after the middle third of ovine gestation, yet maternal placental blood flow continues to increase exponentially ( Fig. 9.3A and B ). This discordance in placental growth and blood flow occurs earlier in pregnancy in women and other primates compared with sheep. The pattern of change in absolute blood flow (see Fig. 9.3B ) resembles that seen for total uterine blood flow (see Fig. 9.1B ). Because placental blood flow accounts for more than 60% of total uterine blood flow in the last two thirds of pregnancy (see Fig. 9.2 ), it is not surprising that the “patterns” are similar. The principal difference between the two patterns is obvious when blood flow per gram of metabolically active tissue is examined (see Fig. 9.3C ). In contrast to the high value for total uterine blood flow early in pregnancy, followed by a fall and leveling off, placental blood flow falls in mid-pregnancy and then progressively increases from about 0.5 mL/min•g to nearly 4 mL/min•g at term—a value 5 times greater than that observed for the total uterus. This increase in placental blood flow is due to progressive vasodilation, because maternal arterial blood pressure (i.e., perfusion pressure) is minimally changed and the number of maternal spiral arteries is unchanged . , The rise in maternal placental blood flow parallels a more general process of functional maturation of the placenta, which involves the simultaneous increase in fetoplacental perfusion and permeability ; these physiologic changes are necessary to provide adequate nutrients and oxygen for the rapidly growing fetus. Of note, the volume of placental blood flow greatly exceeds that required for normal fetal growth. , Thus, there is a substantial “margin” of safety (i.e., an excess of blood flow) that protects the fetus from episodic decreases in uteroplacental blood flow associated with transient increases in maternal plasma levels of vasoconstrictors (e.g., ANG II) or increases in myometrial tone during parturition, which may alter placental perfusion.
Although much has been learned about the functional and anatomic aspects of the maternal uteroplacental circulation, few investigators have tried to describe in detail the pattern of change in umbilicoplacental blood flow over the entire course of pregnancy. This reflects the difficulty in performing such studies, even in an animal model. Nonetheless, many similarities exist between species. For example, a villous stem originating from the fetal side of the placenta contains a distal branch of the umbilical artery and vein. As pregnancy progresses, a villous tree (in the human) or vascular bed originating from this villous stem undergoes angiogenesis and branching morphogenesis throughout pregnancy, providing an ever-enlarging fetal placental vascular bed and surface area for nutrient and gas exchange by term pregnancy. Although the sheep has no villous tree, continued growth of the fetal placental vascular bed occurs as in the human. , In both species, therefore, the size of the fetal placental vascular bed and the magnitude of umbilicoplacental blood flow increase, the latter increasing exponentially in the last third of normal pregnancy. In contrast with the rise in uteroplacental blood flow, which reflects vasodilation, the rise in umbilicoplacental blood flow is due primarily to vascular growth and, to a lesser extent, vasodilation. Thus, umbilicoplacental blood flow remains approximately 100 to 200 mL/min/kg of fetal weight throughout gestation in most species. Conditions that attenuate the growth of the fetal placental vascular bed in the last third of gestation (e.g., pregnancy-induced hypertension) or increased fetal placental vascular resistance may result in insufficient nutrient delivery and fetal growth restriction. Although this correlation is not well studied, Doppler technology has shown increases in umbilical artery resistance associated with decreased fetal growth.
With the advent of Doppler ultrasound, this has become a routine approach for determining uterine blood flow during pregnancy and has been widely applied to assess blood flow through the maternal uteroplacental circulation noninvasively, at low cost and with high temporal resolution. Doppler ultrasound assesses placental perfusion by measuring impedance to flow in the uterine arteries, which has been reported to increase in pregnancies with preeclampsia and fetal growth restriction. However, Doppler ultrasound measurements of placental perfusion lack sufficient reproducibility and sensitivity, and quantification remains challenging. In contrast, the introduction of magnetic resonance imaging (MRI) produces spatially resolved perfusion images and quantitative assessment. Arterial spin labeling (ASL) is a noninvasive imaging method for measuring tissue perfusion and has extensively been applied in the brain for measurement of cerebral blood flow. While conventional perfusion MRI techniques require infusion of contrast agents, which are contraindicated during pregnancy, ASL utilizes water molecules in arterial blood as an endogenous contrast agent, making it optimal for examining placental perfusion in pregnancy. However, ASL measures the movement of all blood within the placenta and thus reflects both maternal and fetal blood flows. As a result, ASL overestimates maternal placental perfusion and complicates the understanding of changes in maternal blood supply and the contributions from the maternal and fetal circulation. Various noninvasive techniques are being examined that might selectively label maternal placental blood flow, including pseudo-continuous ASL and velocity-selective ASL.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here