Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Access video and video lecture content for this chapter online at Elsevier eBooks+ ![]()
![]()
Plastic surgeons are frequently called upon to reconstruct soft-tissue and bony defects of the chest wall. Chest wall defects are exciting and challenging as thewy have multiple etiologies, can have a significant impact on cardiopulmonary dynamics, often occur in patients with multiple medical comorbidities, and require multidisciplinary care. While algorithms for sternal and lateral chest wall have been numerously published, this field continues to evolve and improve. There are more robust options for sternal fixation, better synthetics to solve bony defects, and technical advances to limit donor site morbidity and improve viability of soft-tissue flaps. This chapter will provide a comprehensive overview of how to evaluate chest wall defects, an evidence-based guide of wound bed preparation, and will review the pros and cons of the multitude of options for bony and soft-tissue reconstruction, outline technical pearls, and focus on newer techniques and technologies that will allow continued growth towards improved outcomes in this field.
There are both congenital and acquired causes of chest wall defects. The most common congenital defects include those seen in Poland syndrome, anterior thoracic hypoplasia, pectus excavatum, and pectus carinatum. Management of these conditions will be addressed briefly at the end of the chapter. Acquired defects can occur in the setting of infection, oncologic resection, and trauma.
The incidence of deep sternal wounds following a sternotomy ranges from 1% to 4%. Patient risk factors include increased age, obesity, diabetes, smoking, poor nutrition, and MRSA colonization. There is a stepwise increase in risk of deep infection after a coronary artery bypass graft (CABG) with increasing BMI class. For each unit increase in hemoglobin A1c, there is an increase in deep infection after a CABG, and an A1c greater than 8.6% is associated with a fourfold increased risk in mortality. Post-sternotomy infections increase in patients with an unexpected weight loss of 10% within 6 months, BMI below 20, and pre-albumin below 20 mg/dl. Technical risk factors include harvest of both internal mammary arteries (IMAs) and duration of surgery. Prompt and aggressive treatment of infected sternal wounds is critical as deep sternal wounds increase hospital stay and medical costs up to fourfold, and mortality rates can be as high as 50%.
The pathogenesis of deep sternal wounds is varied and unlike that of other surgical site infections. Gram-positive and Gram-negative bacteria and fungi can be the causative organism, though S. aureus and coagulase-negative Staphylococcus species are isolated in cultures in 60%–80% of cases of mediastinitis. Mediastinitis caused by skin flora pathogens requires both an infection or dehiscence of the superficial skin and sternal instability. Similar to lower extremity fractures, there is the school of thought that micromotion and lack of rigid stability at the fracture site may cause infection rather than result from it. Multiple studies have demonstrated that primary rigid sternal fixation with plates is associated with decreased rates of mediastinitis and mortality in comparison to cerclage wiring. Infections caused by organisms such as Klebsiella , E. coli , Enterobacter , etc. likely result from translocation of those bacteria from other sites of infection: pneumonia, urinary tract infections, intra-abdominal infections, bacteremia, etc., so it is important to treat the source. Finally, infections caused by more virulent, drug-resistant organisms such as methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus (VRE), Pseudomonas , etc. may result from contamination in the operating room and are particularly challenging to treat.
Pairolero published the most commonly used classification system, which divides infections into three types. A type I infection occurs within the first week after surgery and is characterized by an incisional dehiscence with non-purulent, culture-negative drainage without osteomyelitis. A type II infection occurs from the first week to several weeks after surgery and is characterized by purulent, culture-positive drainage often extending into the mediastinum and frequently associated with osteomyelitis. A type III infection occurs months to years after surgery and is characterized by a chronic draining sinus typically secondary to culture-positive osteomyelitis.
It is important to note any prosthetics used in the sentinel surgery. The wires, plates, and screws used to initially fixate the sternum are frequently infected or chronically colonized in type II and III infections. If the infection extends deeper into the mediastinum, one should evaluate the integrity of any grafts or patches alongside the cardiothoracic team.
Ventricular assist device (VAD) infections should be considered separately from infections following cardiac bypasses, valve repairs/replacements, and other cardiac surgeries. A VAD is a prosthetic electromechanical device that assists the left, right, or both ventricles. VADs can be used as a bridge to heart transplantation in eligible patients or as destination therapy in those who are not transplant candidates. Both cases present unique challenges when deciding how to best treat VAD infections.
In addition to the external battery pack and controller, the main components of a VAD include the driveline, pump, and flow tract. The driveline is percutaneous and connects the external battery pack and controller to the internal pump. The pump, often placed in a pocket within the abdominal wall or peritoneal cavity, helps propel blood from the ventricles through the flow tract to the aorta or pulmonary artery.
The driveline, pump, and flow tract all can be infected, with overall incidence of infection ranging from 30% to 50%. One review identified the driveline as the most likely component to be infected at 26%, followed by pocket infections at 8%, and flow tract infections at 4%. Management of these infections remains incredibly challenging, and it is important to discuss with the cardiac surgery team whether the VAD is a bridge or definitive treatment.
The VAD can be exchanged, though one review observed that 88% of infections are managed without device exchange. Regardless of exchange or device salvage, one-year mortality rates remain high at 15%–25%, and infection recurrence ranges from 25% to 40%.
Cardiac implantable electronic devices (CIEDs) include implantable cardioverter defibrillators (ICDs) and permanent pacemakers (PPMs), both of which detect and help prevent complications from potentially fatal arrhythmias. Infection rates are relatively low between 2% and 5%. Proper management includes obtaining blood cultures and an echocardiogram as bacteremia and/or endocarditis can cause or result from CIED infections. While superficial infections can be managed with antibiotics alone, deeper infections require implant removal, a bridge period of medical management only for the patient’s underlying arrhythmia, followed by delayed reinsertion of the CIED by at least 72 hours, but preferably 7 days from time of explant.
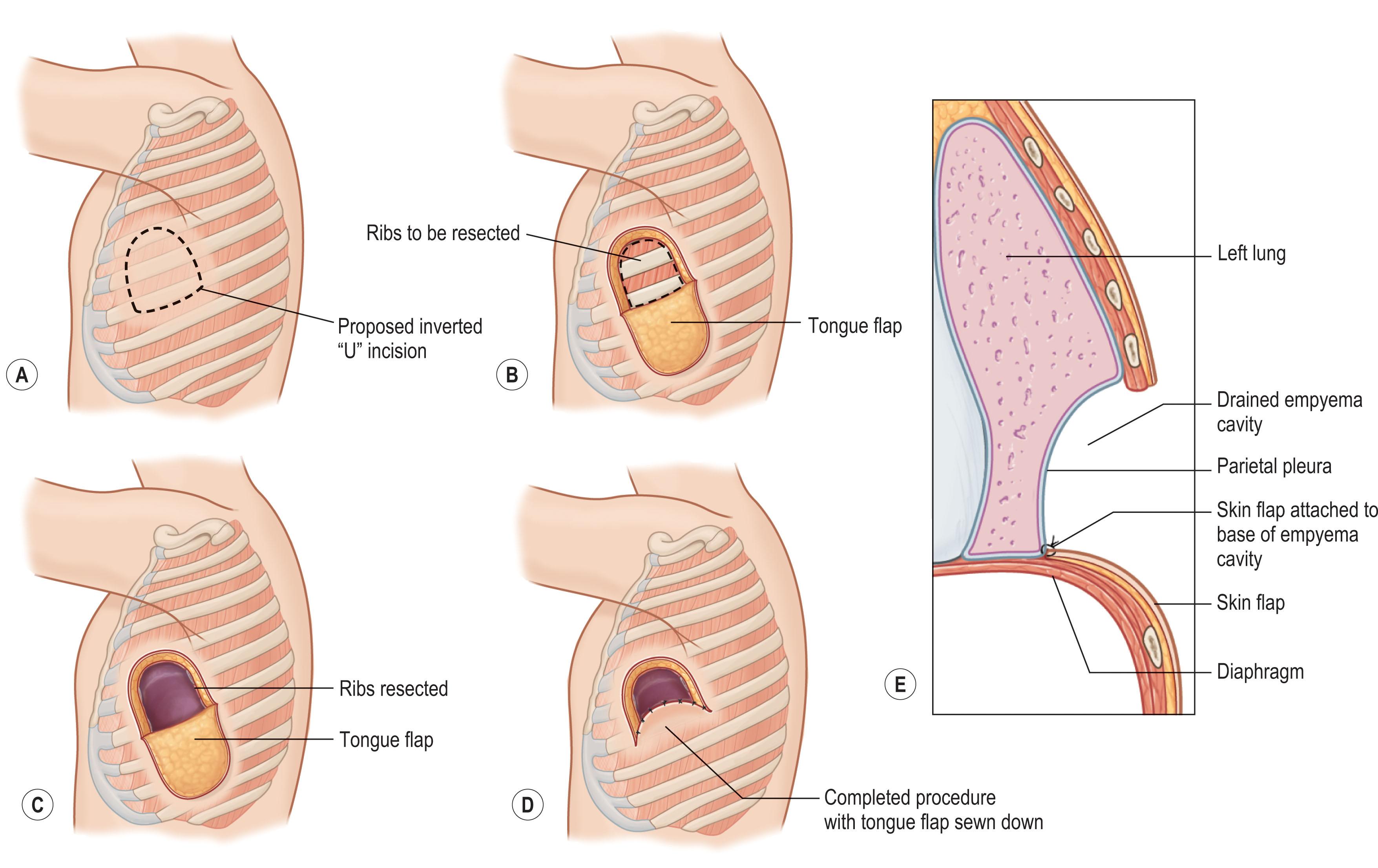
An empyema is an infection of the pleural space that can be caused by pneumonia, penetrating chest wall injuries, and as a complication following thoracic procedures. In the acute phase, the infected pleural fluid must be drained via either minimally invasive techniques such as a thoracentesis or thoracostomy – which also allows for injection of fibrinolytics – or surgical open thoracotomy or video-assisted thoracoscopic surgery (VATS). There is no significant difference in mortality between minimally invasive and surgical approaches. Chronic empyema may be treated with surgical decortication with or without lung resection or obliteration of the pleural space with a flap or rib resection via a thoracoplasty. Patients who have failed all reconstructive options or who are acutely ill and need a bridging treatment prior to more radical treatment, creation of a thoracostomy window to allow for controlled drainage from the pleural space can be performed. The most common procedure is an Eloesser flap, which involves a U-shaped skin incision, resection of a segment of two to four adjacent ribs, and suturing of the skin flap to the base of the empyema cavity ( Fig. 11.1 ).
A bronchopleural fistula is an abnormal connection between the bronchi and pleural space. This condition is potentially fatal due to development of tension pneumothorax or asphyxiation from pulmonary flooding. A bronchopleural fistula occurs in 5%–20% of patients following a pneumonectomy and has mortality rates up to 70%.
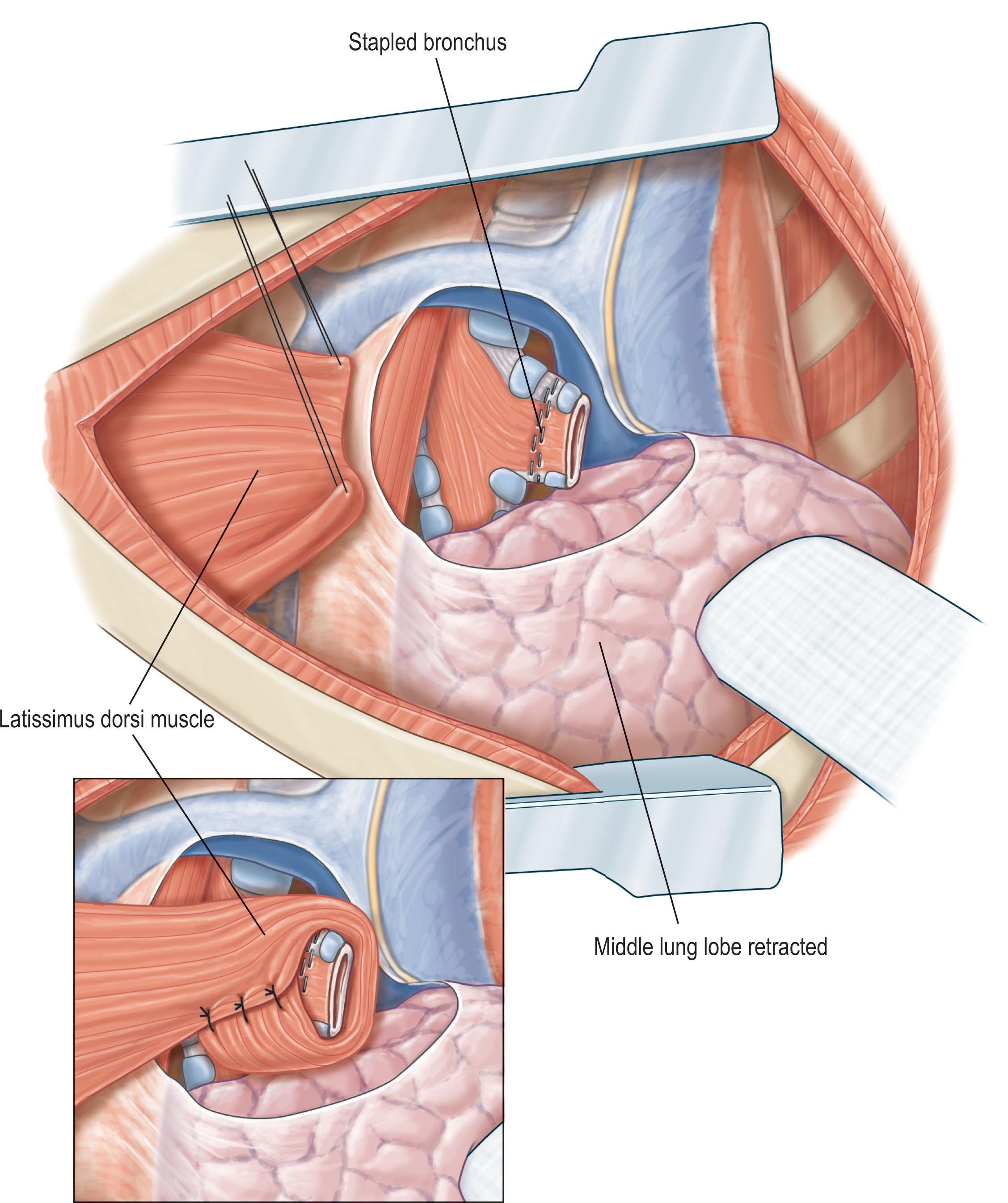
Plastic surgeons may be called upon to assist with soft-tissue reconstruction in the setting of open wounds following empyema treatment or to help protect a newly sewn bronchial stump to prevent or treat a bronchopleural fistula ( Fig. 11.2 ).
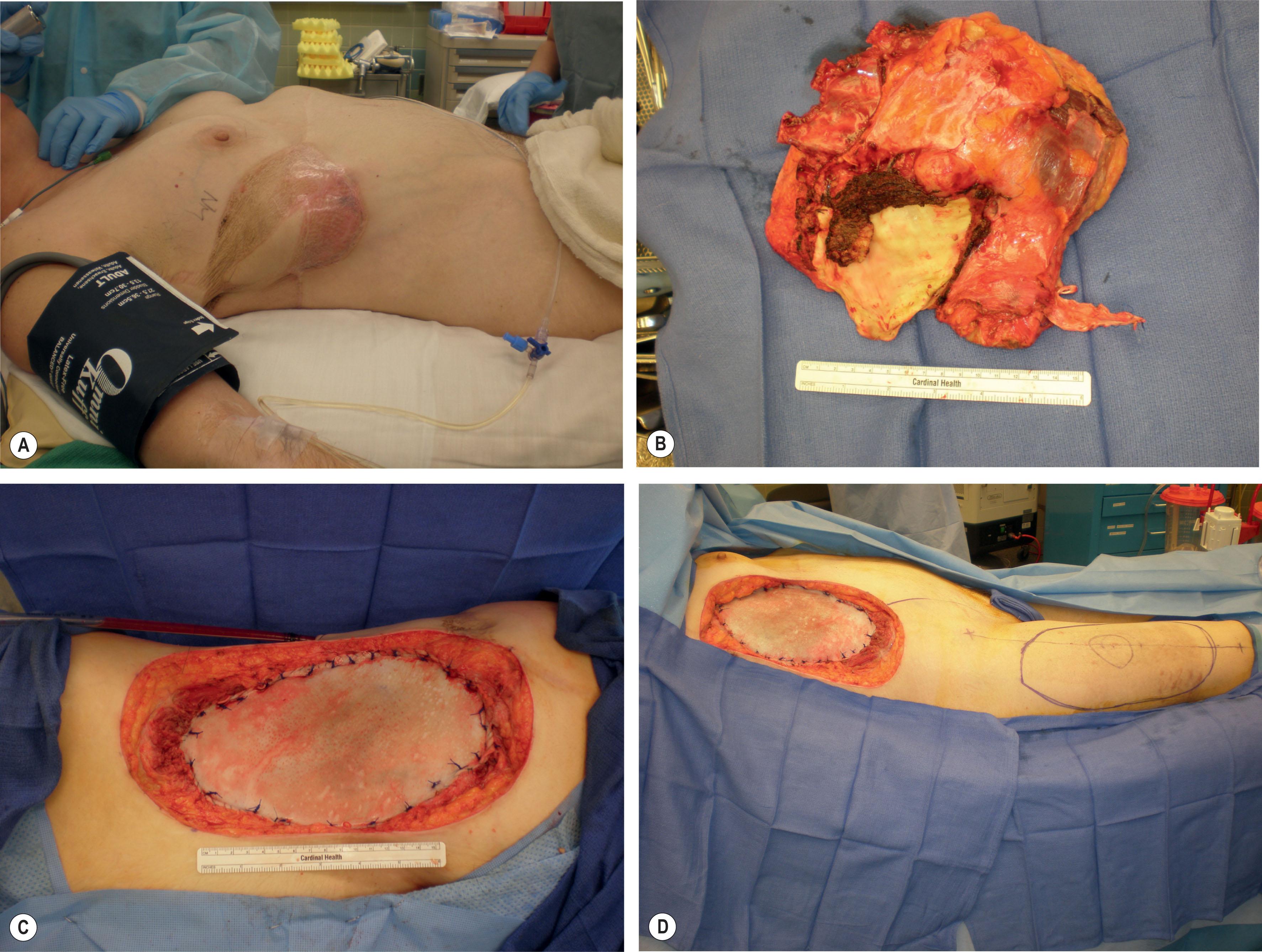
Chest wall tumors are relatively rare but can result in significant soft-tissue and bony defects following proper surgical resection. Primary chest wall tumors occur in less than 2% of the population and represent only 5% of all thoracic neoplasms. Between 50% and 80% of primary chest wall tumors are malignant, and they arise relatively equally from soft tissue and bone. Chondrosarcomas are the most common primary malignant chest wall tumor, with osteosarcomas, rhabdomyosarcomas, plastocytomas, histiocytomas, and other histologic variations of sarcomas as other common lesions. Chondromas are the most common primary benign chest wall tumor; others include fibrous dysplasia, osteochondromas, aneurysmal bone cysts, desmoid tumors, chondroblastomas, and osteoblastomas. The remaining chest wall tumors are metastases, most commonly from primary breast or lung cancer.
Management of these tumors should be conducted in a multidisciplinary fashion, and any patients initially seen with a chest wall mass should be appropriately referred to cardiothoracic surgery and/or surgical oncology. If operative intervention is an option for a given tumor, plastic surgeons are often called upon for reconstruction following resection. Proper resection of these tumors, especially malignant ones, requires large soft-tissue margins – up to 4 cm depending on the type of tumor – and resection of multiple ribs and/or part or all of the sternum depending on the location ( Fig. 11.3 ).
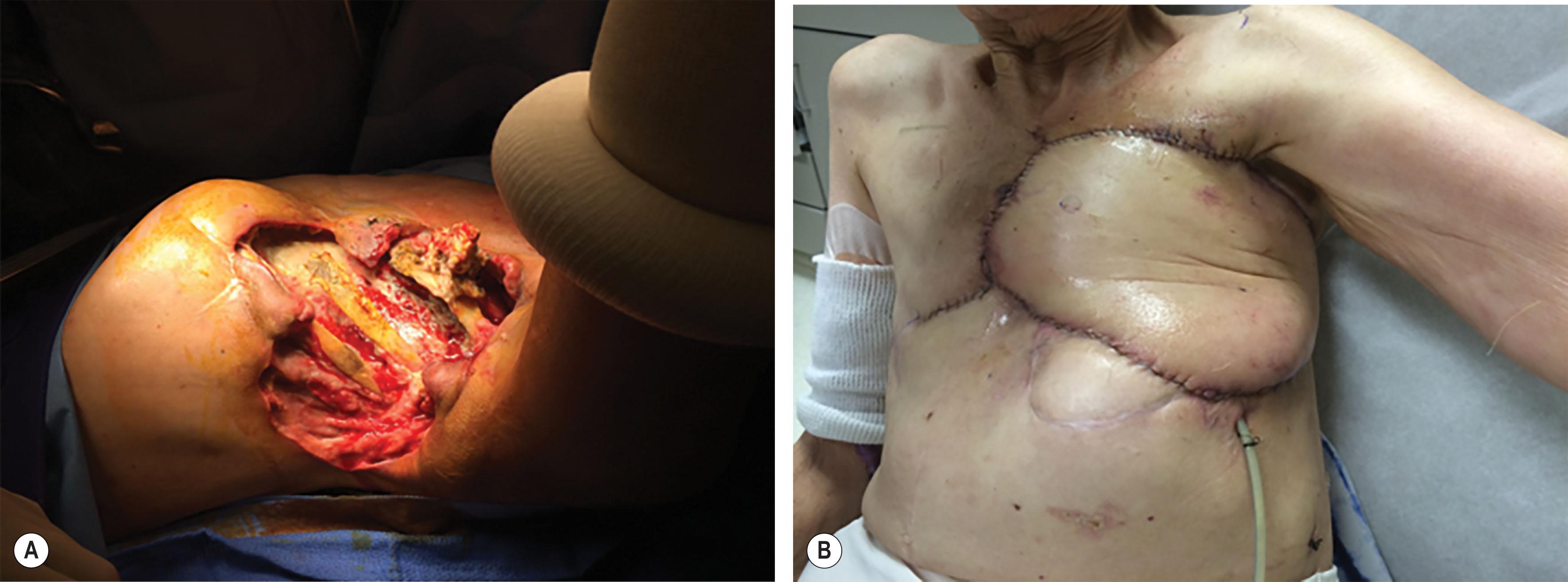
Osteoradionecrosis is a complication of radiation therapy whereby irradiated bone becomes necrotic and exposed through a defect in the soft tissue. Radiation is often used as an adjunctive treatment for breast cancer, lung cancer, and primary chest wall tumors. Generation of reactive oxygen species, increased inflammatory cytokines, and relative hypoxia cause chronic inflammation and damage to soft-tissue cells, vascular endothelial cells, and progenitor cells. The severity of the radiation-induced injury may be dependent on total dosage of radiation administered, the frequency and interval timing of administration, and host factors.
The incidence of osteoradionecrosis of the chest wall following radiation for breast cancer or other chest wall tumors has not been reported extensively, but it should be suspected when a patient with a history of chest wall radiation presents with a chronic draining wound and exposed bone. It is again important to manage these patients in a multidisciplinary fashion and obtain a tissue biopsy to rule out recurrent cancer and radiation-induced cancers. Hyperbaric oxygen (HBO) therapy has been trialed in managing osteoradionecrosis in other areas of the body with limited success. These wounds must be treated with radical resection of the involved tissue, often creating a large composite defect that must be reconstructed ( Fig. 11.4 ).
Penetrating and blunt trauma can create chest wall deformities and defects. As with any trauma patient, Advanced Trauma Life Support (ATLS) protocols should be followed, delaying any non-urgent reconstruction until the patient is stabilized. Good communication and coordination with the trauma surgery team and any other involved teams is critical.
The chest wall serves multiple critical functions. It protects the intrathoracic and upper abdominal contents, facilitates the function of the diaphragm and accessory muscles of respiration, provides stability to the shoulder and upper extremity, and supports the normal aesthetic contour of the breasts and female and male chest.
Respiration requires a complex interplay between the muscles of respiration and the skeletal thorax to which these muscles attach. The primary muscles involved with inspiration are the diaphragm and, less so, the intercostal muscles. Accessory muscles of inspiration include the scalenes, sternocleidomastoid, and pectoralis muscles. Expiration, on the other hand, is largely due to the elastic recoil of the semi-rigid skeletal structures of the thoracic cavity – specifically, the ribs, sternum, and vertebral column. Expiration via passive recoil is often aided by accessory muscles including the rectus abdominis, transversus abdominis, and oblique muscles.
In terms of skeletal contributions to respiration, the ribs move in relation to thoracic deformation through motion generated by contraction of the intercostal muscles. The axis of movement is determined by its attachments anteriorly at the costochondral junction and posteriorly at the costovertebral joint. The flexibility and dynamic nature of the skeletal thorax can be viewed on a spectrum. For example, the anterior chest exhibits a greater degree of compliance as compared to the posterior chest. This is due to both the greater flexibility afforded by the costocartilaginous junctions as well as the more restricted movement of the posterior thorax due to its anchoring to the scapula, vertebral column, and shoulder girdle. As a result, the anterior chest plays a more dynamic role in respiration, and may tolerate more skeletal loss. Similarly, defects of the posterior chest are less predisposed to paradoxical respiration in the event of a flail segment, making reconstruction of these regions less compulsory.
There are also differences in respiratory biomechanics in the craniocaudal axis. The upper ribs (1–7, considered true ribs), have more limited mobility due to their attachments to the thoracic vertebrae and sternum. This is in comparison to the false ribs (8–10), which attach anteriorly to costal cartilage, and the floating ribs (11, 12) that do not have any anterior attachments. As a result, the upper ribs are more restricted in their movement, whereas the lower ribs exhibit more mobility and may tolerate more skeletal loss. The directionality of movement also differs in the craniocaudal axis. Specifically, the upper ribs rotate in a lateral axis during the inspiration–expiration cycle, whereas the lower ribs move in a more dorsal–ventral axis.
The thoracic cage is very different from the bony elements of the extremities. When managing fractures of the extremities, the extremity can be immobilized and prevent motion at the fracture site. It is impossible to immobilize the thoracic cage during respiration, which is why rib and sternal fractures are so painful. Furthermore, because of the articulation of the shoulder girdle, any movement of the upper extremities will similarly stress any rib or sternum fracture or osteotomy sites. For these reasons, it is challenging to achieve radiographic union at 6 months. Sternal separation of just 2 mm can result in significantly decreased healing, and this degree of separation has been demonstrated in a cadaver model to occur under daily loads of physiologic force. Multiplanar motion to this degree can be achieved even at 3 months postoperatively when performing daily activities such as inspiration, coughing, and use of upper extremities to sit and stand.
Sternal non-union is a well-known complication after cardiac surgery or traumatic injuries, occurring in 0.5% to 5% of cardiac procedures, with rates up to 15% in high-risk patients. It is defined as clinical instability without signs of healing for more than 3 months, which often manifests as pain and clicking, without any signs of underlying infection. A stable sternum is important for normal cardiopulmonary function. Patients who do not have sternal stability develop long-term pulmonary dysfunction, which is exacerbated by painful chest-wall motion. If the sternum is not united, the chronic motion at the sternal edges creates inflammatory fluid collections, which also may impair pulmonary function. Respiration is further limited by pain that accompanies this condition. When the sternum is left in discontinuity, there can be asymmetric movement of the left and right hemithoraces, which hinders expansion and contraction of the thoraces, impairing thoracic respiration. Nagasao et al . examined the effect of different sternal defects on efficiency of thoracic respiration using a dynamic computer model. They found that defects that left the sternum in discontinuity along the entire vertical length caused an 80% reduction in respiratory function. Partial defects of the superior half of the sternum caused a 50% reduction in respiratory function, while partial defects of the inferior half were well tolerated. Compromised blood flow either leading to or as a result of sternal non-union can lead to sternal osteomyelitis and mediastinitis. The manubrium is thought to be the most critical structural component of the sternum, and loss of the manubrium and its adjoining ribs causes the greatest decrease in respiratory function.
In addition to providing biomechanical advantages, maintaining sternal integrity makes repeat operations easier by preserving normal anatomic delineations between the skin and subcutaneous tissues, the sternum, and the mediastinal structures. Maintaining the plane between the sternum and heart is particularly important in cardiac patients, who frequently need additional surgery for their underlying condition.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here