Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
According to conventional clinical and surgical standards of the early 1960s, removal of a diseased liver and replacing it with a working, undamaged one was a concept that, although visionary and sound, had a low probability of being successfully executed. Only because of the technical brilliance and stubborn perseverance of Thomas E. Starzl did the experiment continue and slowly develop into the refined surgical procedure that we know today. To perform liver replacement in an environment of unsophisticated hemodynamic monitoring, slow turnaround of biochemical and coagulation monitoring profiles, inadequate blood component replacement therapy, and crude surgical instruments that did not even include atraumatic needles is almost beyond comprehension. During the last 1½ years of Starzl’s liver transplant program at Colorado General Hospital (1979 to 1980) in Denver, the fastest procedure took 14½ hours, and only cases lasting longer than 20 hours were considered poor from a technical point of view. The preoperative blood order for type and cross-match for 15 units of packed cells was designated for opening the abdomen, during which time the blood bank would have the opportunity to cross-match and supply more blood for the remainder of the case.
Since these pioneering times, innumerable improvements have been made, each one making a small contribution that, as a whole, has transformed today’s liver transplant procedure into an evolutionary descendant that barely resembles its ancestor. Of the factors that have done the most to develop liver transplantation from an experimental undertaking to a therapeutic procedure, we first list the development of anesthesia, including central hemodynamic monitoring and coagulation control. We believe that anesthesiologists are the unsung heroes of this operation, and they deserve much credit. Second, the introduction of electrocautery, the argon beam coagulator, and new hemostatic agents created not only a safe technique of controlled coagulation of bleeding areas but also a more sophisticated technique of dissection. Today the entire dissection is performed with electrocautery in a coagulation mode, most commonly directly by the surgeon or by the assistant cauterizing on the surgeon’s dissecting tonsil clamp. Whereas suture ligation was used for all vessels during the recipient hepatectomy, it is now reserved for only major vascular and tissue structures. Third, the introduction of venovenous bypass and its early implementation in the operation have provided a certain equanimity to the conduct of the operation, thereby providing a more suitable atmosphere for the education of liver transplant trainees. A fourth advancement is an adaptable technique of using either the classic orthotopic procedure or the caval-sparing transplant (piggyback). Fifth, the use of modern mechanical retractors permits an unrestricted, nontiring visualization of the field, which allows the procedure to be performed with only two assistants. In Denver, fellows and residents hanging for dear life onto the retractors were rotated every 2 to 4 hours or until they dropped to give the best possible exposure. Nonetheless, most adult patients required a thoracotomy to allow access to the suprahepatic vena cava, a procedure that is now virtually never used.
Liver transplantation today is performed principally with two different techniques: the classic technique with vena cava interposition and a piggyback technique that leaves the native cava behind. Venovenous bypass is not mandatory with either one. Until 1983, liver transplantation was performed by what we now call the classic technique , which is when Starzl and coworkers developed the bypass. The bypass maintains cardiovascular stability and normal hemodynamics to a degree that caval-side occlusion is unable to do; it requires fewer intraoperative fluid transfusions and results in a diminished frequency of reperfusion syndrome and a less edematous posttransplant patient. Furthermore, it is most helpful in the high Model for End-Stage Liver Disease (MELD) patient who is receiving vasopressors and/or in need of hemodialysis at the time of transplantation. The most important indication for the piggyback technique may be cases in which a small donor liver with a narrow cava is to be transplanted into a large recipient in whom the narrow donor cava causes clinically significant stenosis. Another important advantage of the piggyback technique is in difficult retransplantation, when the vena cava is embedded in an inflamed retroperitoneum.
For a technically adroit surgeon, surgical time and blood loss with the piggyback technique may approach that of the classic technique with the use of venovenous bypass. In a regular transplant procedure using the classic technique, we expect the patient to be anhepatic on bypass for 40 to 50 minutes with a warm ischemia time of the graft of approximately 25 to 30 minutes. Of note, both authors of this chapter routinely use the classic technique in the vast majority of transplants.
After the patient has been carefully positioned on the table, prepared, and draped, the abdomen is opened via a bilateral subcostal incision with midline extension ( Fig. 45-1 ), so appropriately named the Mercedes incision by Sir Roy Calne. Except for the superficial skin incision, the skin, subcutaneous tissue, and muscle layers are opened with the electrocautery unit. The right-sided incision is extended far enough laterally to allow a horizontal view into the vena cava. The left-sided incision is shorter and extends beyond the lateral border of the rectus. If splenomegaly is present, care should be taken to avoid making this incision very long because the spleen is then easily injured, which could possibly cause serious bleeding and necessitate an otherwise unnecessary splenectomy. The midline incision is extended to the point at which the surgeon has a direct perpendicular view of the suprahepatic vena cava; at times, complete or partial xiphoidectomy may be necessary. With this incision, exposure is excellent, and of more than 6000 patients, only 2 required extension into the chest via a median thoracotomy.
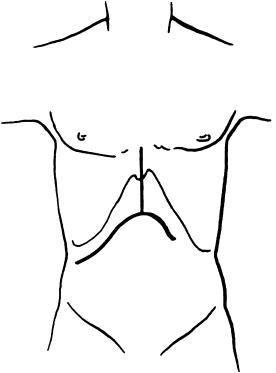
A mechanical retractor is placed with the blades under both costal margins to pull the rib cage laterally and anteriorly to spread the aperture ( Fig. 45-2 ). The falciform ligament is divided, and the tissue with all the collaterals along the falciform ligament and the midline fascia are removed up to the xiphoid, thereby totally preventing further collateral bleeding and obstruction of the field from the tissue mass itself. The fatty tissue below the upper midline extension often contains large collaterals from the umbilical vein that should be controlled with ligatures and entirely removed, which is done without blood loss if the correct tissue plane is found. On opening the rectus sheath, a ligature placed on the round ligament, when left long and “draped” over the retractor ring, can serve as excellent exposure to the porta hepatis.
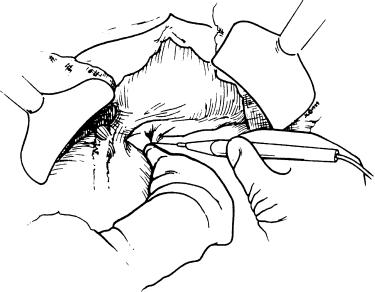
The falciform ligament is divided all the way down to the suprahepatic vena cava, and the left triangular ligament is then opened with the cautery. Frequently, collateral veins are present at the very tip of the left lateral segment that require ligature for hemostasis. The left lateral segment is then retracted up out of the wound and to the right. The gastrohepatic ligament is now visualized and requires either cautery division or suture ligation, depending on the extent of the collateral vessels. Obviously, if an accessory left hepatic artery is present, it must be ligated and divided.
The anteroinferior edge of the liver or the ligated round ligament is now lifted up by an assistant to allow an unobstructed view of the porta hepatis ( Fig. 45-3 ). The dissection is carried down to the hepatic artery, which is then divided above its bifurcation. Before division we find it helpful to isolate the common hepatic and gastroduodenal arteries and place an atraumatic vascular bulldog clamp on the common hepatic artery. This maneuver prevents dissection of the hepatic artery, which can occur if the artery is simply ligated distally. This maneuver virtually eliminates initial dissection of the hepatic artery. We then proceed to the right side of the porta hepatis and divide the common duct. Normally it is unnecessary to divide the cystic duct separately; however, division above the cystic duct entrance can easily be accomplished when necessary to preserve sufficient length of the common duct. In more than 95% of patients with an accessory right hepatic artery, it traverses posterior to the common bile duct and is divided when present. We then complete the dissection around the portal vein. In many cases the dorsal pancreatic vein drains into the anterior portion of the portal vein and must be divided and ligated.
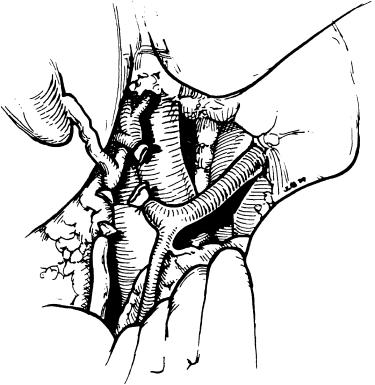
If adhesions to the liver are present after previous operations or from spontaneous bacterial peritonitis, they are taken down with electrocautery. The tip of the electrocautery is run parallel to the liver surface at all times, never venturing more than 1 mm from the liver surface but never violating the liver capsule. The liver is to be “shaved.” In cases in which the liver is encased in adhesions, it is often easier to commence with dissection of the lateral aspects of the right lobe followed by dissection of the stomach and duodenum from segments II and III before venturing into the porta hepatis.
In an uncomplicated case we now proceed to dissect the right triangular ligament ( Fig. 45-4 ). This dissection is performed completely with cautery, beginning at the lateral inferior aspect and dividing the ligament carefully all the way into the vena cava. If there are technical problems with an unusual amount of collaterals, scarring, and inflammation, this part of the procedure is deferred until after the patient is placed on total venovenous bypass. With the portal vein transected, the anterior aspect of the infrahepatic inferior vena cava (IVC) is exposed to allow easy circumferential mobilization for placement of a vascular clamp. If the portal vein is not transected at this point, the vena cava is dissected in an inferior-to-superior direction with cautery. The right adrenal vein is ligated and divided. At this time we allow the right lobe to fall back into the hepatic fossa and expose the left side of the vena cava by retracting the left lateral segment and the caudate lobe to the right. The peritoneal reflection is opened longitudinally along the vena cava with cautery. In most cases, the retrohepatic caval tissue can be taken down with finger dissection. Any resistance to such dissection usually signifies retrohepatic collaterals entering the vena cava, which require ligation. With this dual-sided approach, the posterior aspect of the retrohepatic cava can be quickly and safely liberated from the retroperitoneum.
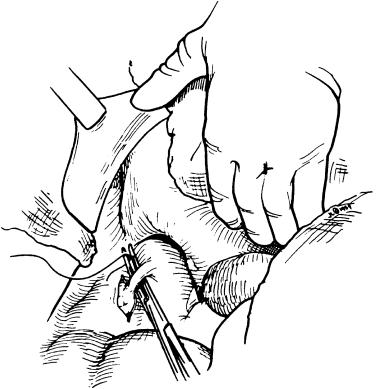
At this juncture the patient is prepared for venovenous bypass by cannulation of the femoral vein. Cannulation is performed via the Seldinger technique in the groin by using a premade kit (Medtronic, catalog No. 96530-015). We use wire-reinforced cannulas, 15F for the femoral vein. Forced cannulation is contraindicated. The position of the wire is easily checked by palpation of the infrahepatic vena cava. The return cannula is inserted by the anesthesia team into the right intrajugular vein by using a 12F wire-reinforced cannula (Medtronic, catalog No. 96830-012) with a pediatric arterial kit (Argon Medical, catalog No. 401135B). This cannula is always inserted when the patient has been intubated before preparing the abdomen. An alternative option is to cut down directly on the axillary vein (usually the left) and the left saphenofemoral junction and then insert 20F and 18F cannulas, respectively.
Once the cannulas have been introduced and secured, we proceed to final dissection of the portal vein. The portal vein is secured with the left hand and cleaned with scissors by simply pushing the tissue away longitudinally along the vein ( Fig. 45-5 , A ). The portal vein is then clamped as high in the hilum as possible and divided. Three Moynihan (tonsil) clamps are attached to the end of the portal vein and held straight up by the assistants. A surgeon who controls the portal vein with the left hand introduces the 28F to 36F wire-reinforced cannula (Edwards Lifesciences) with the right hand and keeps it in place as the first assistant secures it with tape (see Fig. 45-5 , B ).
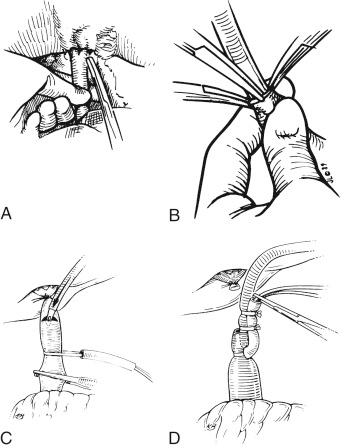
An alternative way to cannulate the portal vein is to ligate the portal vein up in the hilum and, after placing tape around the portal vein to control the vein where it emerges from the neck of the pancreas, make a transverse cut in the distal end of the portal vein to allow the portal vein cannula to be introduced (see Fig. 45-5 , C ). The cannula is secured with tape and tied down, thereby completing the portal vein cannulation, and the transverse incision of the portal vein is now completed to divide the portal vein in its entirety (see Fig. 45-5 , D ).
Venovenous bypass is now started ( Fig. 45-6 , A ). The entire cannulization process for venovenous bypass usually requires 5 to 10 minutes.
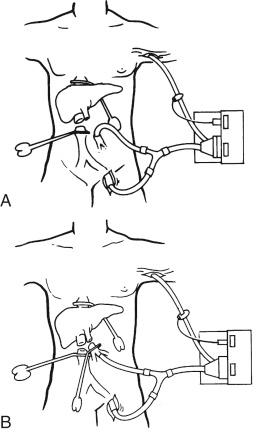
In cases in which the portal vein is injured or, even more important, when dense adhesions with large collateral veins are surrounding the liver, the inferior mesenteric vein can be used for bypass purposes. For the inferior mesenteric vein a No. 20 cannula is usually sufficient and needs to be introduced only 2 to 3 cm. If it is advanced further, the tip often goes out into the portal vein and makes portal vein clamping very difficult later (see Fig. 45-6 , B ).
In patients with organized portal vein thrombus, a formal thrombendvenectomy can be performed in the majority of situations to remove thrombus all the way down into the superior mesenteric vein (SMV) under manual control ( Fig. 45-7 ). In patients with portal vein thrombosis, partial or complete, utmost care should be taken to avoid any further traumatization of the portal vein. In cases in which avoidance of further trauma is impossible, portal vein venous conduits need to be used to ensure portal venous inflow to the liver ( Fig. 45-8 ). Patients with extensive portal vein repairs or conduits are administered low-molecular-weight dextran at 25 mL/hr for 1 or 2 days and then acetylsalicylic acid, 81 mg/day, for 3 months.
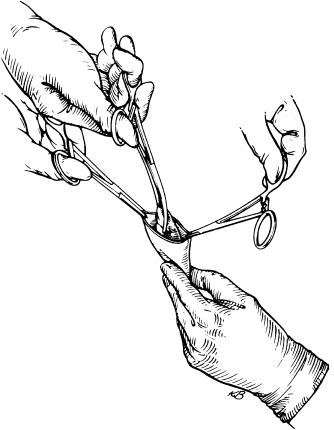
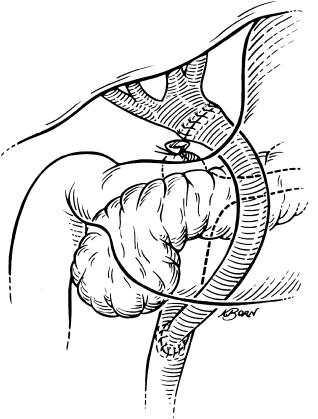
Vascular clamps are now placed on the suprahepatic vena cava and the lower infrahepatic vena cava ( Fig. 45-9 ). Care should be taken to place these clamps horizontally and with the liver in its normal anatomical position to prevent rotation of the vessels. The suprahepatic vena cava clamp should be placed on the very edge of the diaphragmatic reflection to avoid phrenic nerve injuries.
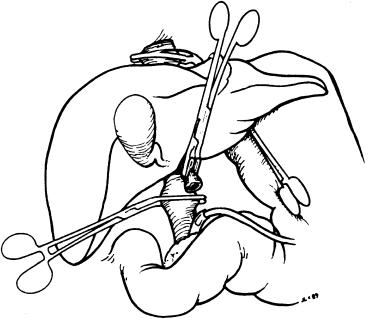
We now divide the vena cava while taking care to retain as much of the right, middle, and left hepatic veins as possible and pull the veins up by almost cutting into the liver substance ( Fig. 45-10 ). The lower cava is then divided as far proximal as possible. The previously ligated retrocaval tissue is divided and the liver removed.
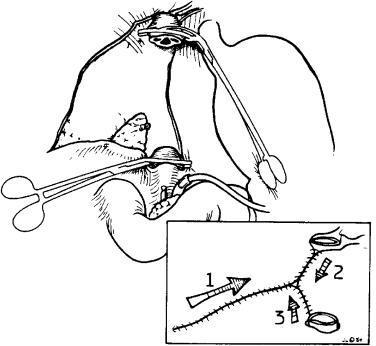
To achieve hemostasis in the hepatic fossa, the bare area can be reperitonealized, as shown in the inset of Figure 45-10 . With the use of 3-0 or 2-0 monofilament polypropylene suture (Prolene; Ethicon, Somerville, NJ), the bare area is sutured in the direction of the arrows and back to the starting point, as numbered 1, 2, and 3. Care should be taken to ensure that the needle passes superficially under the bottom of the entire wound to obtain hemostatic control. In many cases this can be done before liver removal.
In patients with difficult dissections the vena cava can be clamped above and below the liver before all the right-sided and retrohepatic dissection is performed ( Fig. 45-11 , A ). The dissection can now be concluded quickly (see Fig. 45–11 , B ). In such cases it is often expedient to retain the dorsal side of the vena cava to eliminate the need for elaborate hemostatic control of the retrocaval tissue.
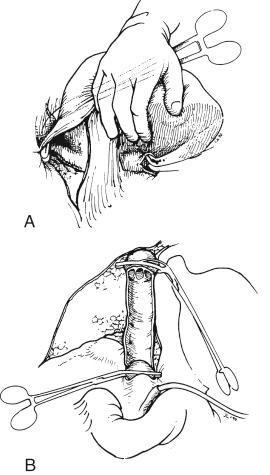
The suprahepatic vena cava is now prepared by opening the right, middle, and left hepatic veins into a common cloaca of the IVC ( Fig. 45-12 ). All phrenic vein ostia are oversewn with 4-0 polypropylene and the knots placed outside the suprahepatic vena cava cuff. The liver is now brought onto the field. By placing a lap in the hepatic fossa, the liver receives support from below to prevent it from sinking down too far into the wound; however, with oversized allografts, as is seen more frequently today, this is often not necessary.
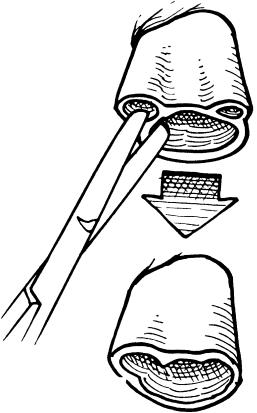
Corner stitches are placed on the two opposing ends ( Fig. 45-13 , A ), and a stay suture may be placed in the middle for retraction of the posterior wall. The suprahepatic vena cava anastomosis is performed with 3-0 polypropylene; care is taken to ensure perfect intimal adaptation. The dorsal suture is run to the right-sided stay suture and just one bite beyond and anterior to it. The midline stay suture is removed, and the front wall closure is completed in a simple running suture fashion.
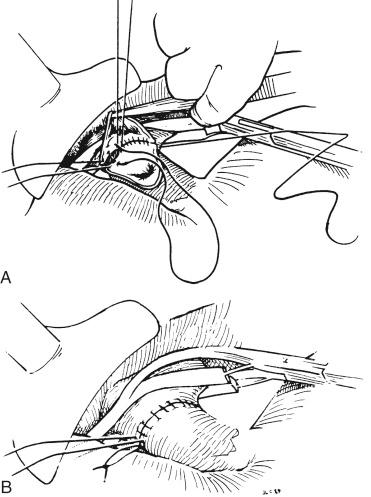
When the anastomosis is finished, a 1- to 1.5-cm “growth factor” is tied in. The lateral stay suture is tied down securely to prevent the anastomosis from separating as the growth factor is taken up by expansion of the vessel at the time of reperfusion (see Fig. 45-13 , B ).
A portal flush with normal saline or lactated Ringer’s solution and 25 g of albumin per liter is run through the portal vein during the completion of the infrahepatic vena cava ( Fig. 45-14 ). Such flushing is needed to empty the graft of air to prevent air emboli, as well as to remove the excessively high concentrations of intravascular potassium left from the preservation solution. When the organ is cooled down for preservation, the sodium-potassium pump is inhibited, allowing the high concentration of intracellular potassium to leak out into the vascular space. The infrahepatic vena cava is sewn in exactly the same manner as used for the suprahepatic vena cava, usually with 4-0 polypropylene, and a 1.5-cm growth factor is included.
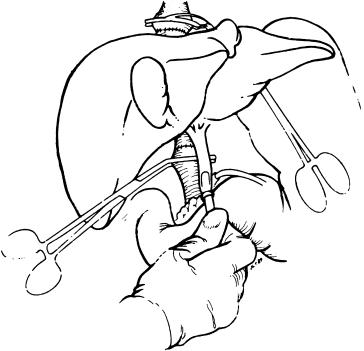
Care must be taken to prevent too long a vena cava. Excessive length of vena cava is the main cause of folding and kinking, which can cause formidable postoperative problems that require extraordinarily difficult reconstructions at a later stage.
If venovenous bypass is used, the portal bypass cannula is now clamped, and only the systemic venovenous bypass is continued. The cannula is removed from the portal vein, and an atraumatic clamp is carefully placed proximally on the portal vein ( Fig. 45-15 ). The donor portal vein is now shortened to the appropriate length. One should not cut the recipient portal vein shorter than 1 cm because length should be conserved in the event that retransplantation becomes necessary. A laparotomy pad is placed against the right hemidiaphragm to prevent kinking of the portal vein anastomosis. The portal vein is sutured with running 6-0 polypropylene, and the inverting stitch in the back wall also includes a growth factor that is three quarters of the portal vein diameter. If size must be adjusted to either the donor or the recipient portal vein, a fish-mouth reconstruction is recommended. There are several methods to reperfuse the liver after portal venous reconstruction: (1) reperfusion through the portal vein with or without vena cava venting, (2) opening of the IVC followed by portal vein reperfusion, and (3) reperfusion through the portal vein and hepatic artery simultaneously. To date no randomized trial has conferred superiority to any of these methods. Furthermore, a randomized trial conducted at the University of California at Los Angeles (UCLA) has shown that venting through the vena cava before portal vein reperfusion does not result in a lower incidence or decreased severity of reperfusion syndrome. However, venting through the intrahepatic IVC with 250 to 400 mL portal vein blood does ensure washout of the high K + -containing preservation solution. It is our preference to release the portal vein clamp slowly with an eye on the electrocardiogram to monitor the procedure. We usually open the portal vein in stages after any T-wave segment elevations have reversed. Once these elevations have normalized, the portal vein is completely opened. It usually takes three or four partial decompressions before the vein is completely open.
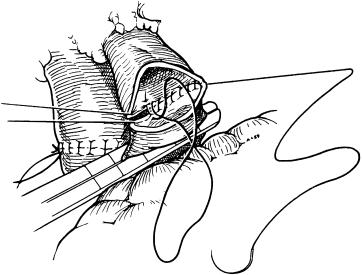
After the surgeon makes sure that there is no significant bleeding from the vena cava, portal vein, or its anastomoses, the systemic bypass is discontinued and the cannula removed from the groin to allow blood in the bypass system to be reinfused into the patient. When the portal vein is unusable, which is extremely rare because of the increased application of thrombendvenectomy, a portal venous conduit is used (see Fig. 45-8 ). In such cases, arterial reconstruction and reperfusion of the liver are performed before the portal conduit is sewn in. For the portal venous conduit, a donor iliac vein is the preferable conduit. The SMV is most commonly used for inflow and is accessed at the base of the colon mesenterium. After the SMV has been identified and side-occluded, the venous conduit, which should have already been anastomosed to the donor portal vein and pulled behind the stomach in front of the pancreas through a tunnel in the colon mesenterium toward the SMV, should now be anastomosed with running 6-0 polypropylene. It should be pointed out that when the side-occluding clamp on the SMV is released, there must be an outflow already established from the venous conduit. Otherwise, the anastomosis line may rupture completely and cause an immediate catastrophe. Sometimes, large collateral veins can be found in the subhepatic area, either along the minor curvature of the stomach or as a collateral to the liver, that can be used for the donor portal vein anastomosis instead of a mesenteric venous conduit.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here