Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hormones exert their actions by binding to specific receptor proteins, a process that induces conformational changes or compartmental redistribution of these proteins. The activated receptor is now capable of inducing positive (or negative) intracellular effects that ultimately are recognized as a physiologic response. The specificity of hormone action is determined by the affinity of hormones for different receptors, the cell-specific expression of the receptor, and the unique responses induced by ligand occupancy.
Since the early 2000s, our understanding of hormone action has advanced rapidly with the success of genomics and advanced molecular biologic techniques. This combined approach has led to the discovery and classification of an unexpectedly large number of receptors, some quite novel and others even unanticipated, that are members of large families of genetically conserved proteins. Moreover, our understanding of receptor action has been clarified by the identification and detailed characterization of postreceptor signaling proteins and signaling mechanisms. Four major receptor superfamilies have been identified that are distinguished by protein structure, cellular localization, and effector systems. These families include the G protein–coupled receptors (GPCRs), cytokine receptors, tyrosine kinase receptors (RTKs), and nuclear receptors ( Table 3.1 ). This chapter reviews major features of these important receptor families. Mutations influencing receptor function leading to endocrine disorders are also highlighted.
| Receptor Class | Hormone Receptors |
|---|---|
| G protein–coupled receptors | ACTH and other melanocortins, V2 vasopressin, LH, FSH, TSH, GnRH, TRH, GHRH, corticotropin-releasing factor, somatostatin, glucagon, oxytocin, gastric inhibitory peptide, type 1 PTH, free fatty acid, GPR54, orexin, ghrelin, melanin-concentrating, calcitonin, glucagon-like peptide-1, and calcium-sensing receptors |
| Type 1 cytokine receptors | Growth hormone, prolactin, and leptin receptors |
| Receptor tyrosine kinases | Insulin, IGF-1, and fibroblast growth factor receptors |
| Nuclear receptors | Thyroid hormone, vitamin D 3 , PPARγ, HNF4A, glucocorticoid, androgen, estrogen, mineralocorticoid, and DAX1 receptors |
A molecule that binds to a receptor is called a ligand . When ligand binding leads to activation of signaling processes inside cells that ligand is called an agonist . The response of a receptor to its ligand is generally assessed by two characteristics of the ligand: potency and efficacy. Potency describes the concentration of ligand needed to cause the biological effect by binding to the receptor. A potent agonist activates the receptors at a low concentration, typically in the nanomolar range. Efficacy describes the maximal effect induced by a ligand. Potency is generally described by using the EC 50 , which is the concentration of ligand that induces a half-maximal effect. When a synthetic agonist exceeds the efficacy of the natural ligand it is called a super agonist . When receptor ligands (natural or synthetic) do not induce full activation, they are called partial agonists . An antagonist is a molecule that blocks the natural ligand from binding to its receptor. If it binds to the same site as the natural agonist it is called an orthosteric antagonist . If it binds to a different site than the natural agonist, it is called an allosteric antagonist . The potency of the antagonist is described by the IC 50 , which is the concentration that causes half-maximal inhibition. A receptor that has constitutive basal activity can be bound by a ligand that inhibits the receptor’s activity in the absence of the natural ligand. In this case the ligand is called an inverse agonist . A scale has been formulated to express the continuity in receptor ligand function—from –1 (representing a full inverse agonist), to 0 (representing a neutral antagonist), to + 1 (representing a full agonist).
Receptors have multiple possible conformations that are constantly changing. Receptors exist in some conformations more than others, based on the free energy of each conformation. Agonists act as modulators that stabilize a given conformation of a receptor by reducing the energy needed to enter that conformation. This conformation is associated with activation of downstream signaling. Partial agonists stabilize the receptor in conformations less efficient at activation of downstream signaling. Super agonists stabilize the receptor in conformations more efficient at activation of downstream signaling. Conformational changes in a receptor can also affect the number and type of signals that a receptor generates. In the classic paradigm, a GPCR was believed to function as a binary switch that could be activated by agonist binding or inhibited by antagonist blockade of agonist binding. We now know that GPCR signaling is more complex than a simple binary switch model (i.e., “on” and “off”), and that different ligands can bind the same GPCR and selectively activate one downstream pathway versus another. This ability of different ligands to induce different confirmations of a receptor that activate (or inhibit) selective downstream signals is called biased signaling . Ligands that bind to the native site of a receptor but produce different signaling events are termed orthostatic ligands . An example of this is provided by the follicle-stimulating hormone (FSH) receptor, a classic GPCR. Fully glycosylated FSH is more acidic and acts as a full agonist at FSH receptors, activating Gs-coupled signaling and generation of cyclic adenosine monophosphate (AMP). Partially glycosylated FSH is more basic and acts as a partial agonist or a biased ligand as it activates both Gs and Gi pathways, which compete. Deglycosylated FSH has no effect on signaling but binds to the receptor and so acts as a competitive antagonist. These differently glycosylated variants exist in the circulation and are a means for fine-tuning the signal from the pituitary to the gonads. In certain cases, a neutral ligand can bind to the receptor at a different site than the natural ligand’s binding site and by doing so, influence the conformation of the receptor such that the ligand’s efficacy is increased, decreased, or biased. These are called allosteric modulators . They do not activate or inhibit the receptors on their own. Similarly, there are allosteric synthetic ligands for the FSH receptor that demonstrate signaling bias ranging from full agonists to reverse agonists.
Receptors are generally found associated with other proteins whether at the cell surface or in the cytoplasm. These associated proteins influence receptor conformation. An example is receptor activity modifying proteins or RAMPs. Calcitonin receptor and calcitonin-like receptor are GPCRs that can bind to several ligands (calcitonin, adrenomedullin, amylin, and calcitonin gene-related protein). RAMP1, RAMP2, and RAMP3 associate with both the calcitonin receptor and calcitonin-like receptor, and depending on which RAMP is associated determines selectivity for one of the aforementioned ligands.
Conformational fluidity of receptors and interactions with multiple ligands and modulators leads to greater complexity, specificity, and fine-tuning, as well as overall efficiency.
More than 1% of the genome of vertebrates encodes a large protein family of receptors that sense molecules outside the cell and activate signal transduction pathways and, ultimately, cellular responses. These receptor proteins are embedded in the plasma membrane and are coupled to intracellular signal generating systems by heterotrimeric G proteins (i.e., GPCRs). GPCRs are also known as seven-transmembrane domain receptors , 7TM receptors , heptahelical receptors , and serpentine receptors . They are called transmembrane receptors because they pass through the cell membrane, and they are called seven-transmembrane receptors because they have alpha helical regions that pass through the cell membrane 7 times. The human genome encodes roughly 950 GPCRs. GPCRs are involved in many diseases and are also the target of approximately 40% of all modern medicinal drugs. Approximately 150 of the GPCRs found in the human genome have unknown functions. Most GPCRs are odorant and pheromone receptors. Also important to note is that most hormones bind to GPCRs, and hence G protein–dependent signal transduction represents the most common mechanism for hormone action ( Table 3.2 ).
| Receptor | Germline Mutation | Endocrine Disorder |
|---|---|---|
| ACTH/melanocortin-2 receptor | Inactivating mutations (homozygous, compound heterozygous) | Familial glucocorticoid deficiency type 1 |
| Melanocortin-4 receptor | Inactivating mutations (most heterozygous, some homozygous) | Obesity |
| V2 vasopressin receptor | Inactivating mutations (most X-linked recessive, rarely X-linked dominant) | X-linked nephrogenic diabetes insipidus |
| LH receptor | Inactivating mutations (homozygous, compound heterozygous) Activating mutations (heterozygous) |
Males: types I and II Leydig cell hypoplasia Females: asymptomatic or hypergonadotropic hypogonadism with primary amenorrhea Males: male limited precocious puberty |
| FSH receptor | Inactivating mutations (homozygous, compound heterozygous) | Females: autosomal recessive hypergonadotropic ovarian dysgenesis or milder hypergonadotropic hypogonadism Males: variable impairment of spermatogenesis |
| TSH receptor | Inactivating mutations (most homozygous or compound heterozygous, rarely heterozygous) Activating mutations (heterozygous) |
Resistance to TSH Autosomal-dominant inherited nonautoimmune hyperthyroidism/toxic adenomas |
| GnRH receptor | Inactivating mutations (homozygous or compound heterozygous) | Isolated hypogonadotropic hypogonadism |
| TRH receptor | Inactivating mutations (compound heterozygous) | Central hypothyroidism |
| GPR54 | Inactivating mutations (homozygous, compound heterozygous) | Normosmic isolated hypogonadotropic hypogonadism |
| Ghrelin | Inactivating mutations (homozygous, possible heterozygous) | Short stature because of decreased growth hormone secretion |
| GHRH receptor | Inactivating mutations (homozygous/compound heterozygous) | Isolated growth hormone deficiency |
| Type 1 PTH receptor | Inactivating mutations (homozygous, heterozygous) Activating mutations (heterozygous) |
Blomstrand chondrodysplasia if homozygous and rarely if heterozygous; enchondromatosis if heterozygous Jansen metaphyseal chondrodysplasia |
| Calcium-sensing receptor | Inactivating mutations (heterozygous, homozygous) Activating mutations (heterozygous) |
Familial benign hypocalciuric hypercalcemia typical if heterozygous, neonatal severe hyperparathyroidism rarely if heterozygous, typical if homozygous Autosomal-dominant hypocalcemic hypocalciuria, Bartter syndrome type V |
The GPCR superfamily is divided into eight major classes. These receptors contain an amino-terminal extracellular domain that is frequently called the ectodomain or exodomain . These receptors also contain seven putative transmembrane spanning alpha helices (TM-I to TM-VII). The alpha helices are connected by three intracellular (i1–i3) and three extracellular (e1–e3) loops that are often collectively called the serpentine region ( Fig. 3.1 ). The carboxy-terminal intracellular region is usually referred to as the endodomain.
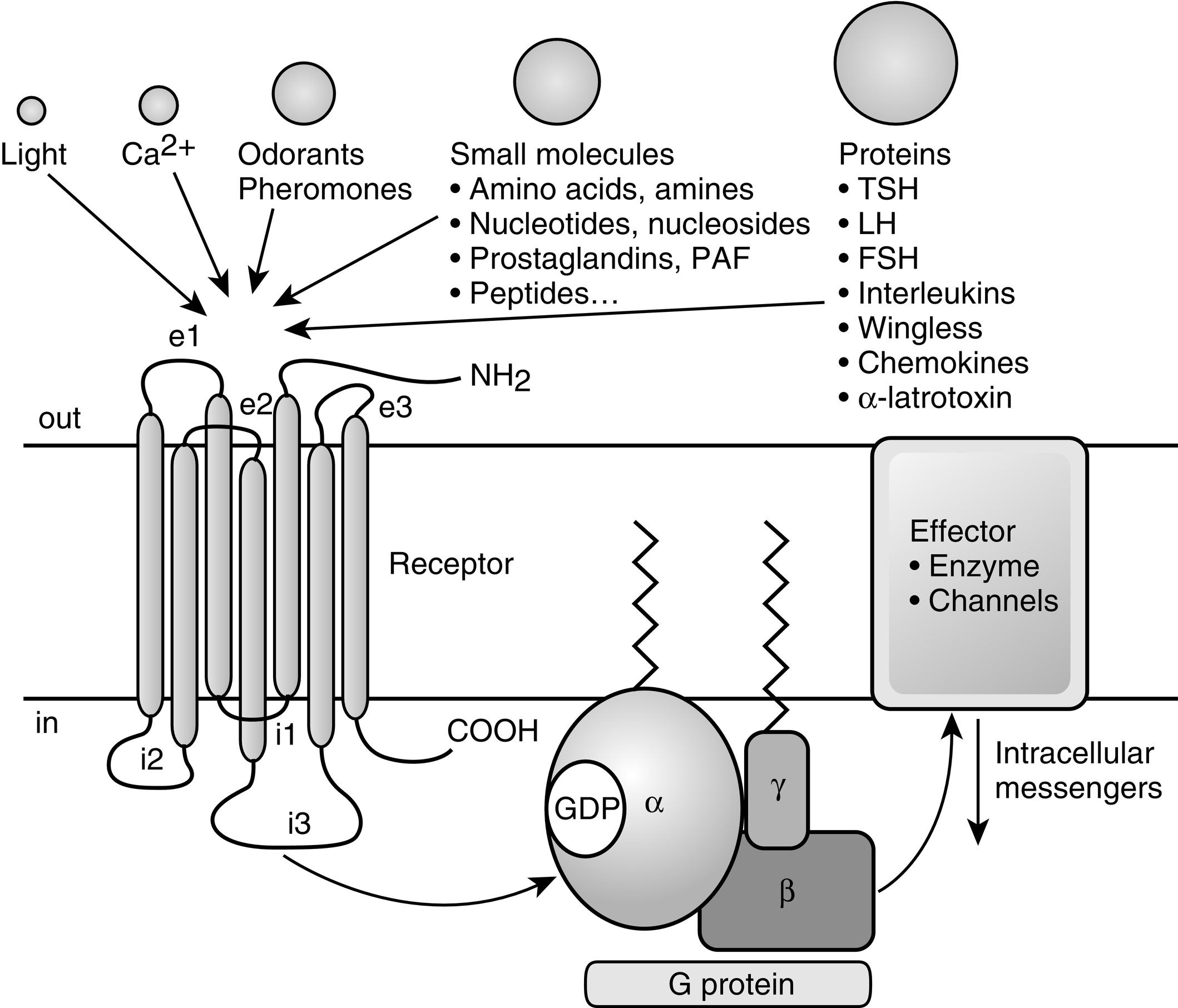
GPCRs are activated by a wide variety of signals, including proteins, nucleotides, amino acid residues, Ca 2 + , light photons, and odorants (see Fig. 3.1 ). It is postulated that ligand binding alters the conformation of transmembrane domains and intracellular loops, increasing the affinity of the receptor for specific heterotrimeric guanosine nucleotide binding proteins (G proteins) (see Fig. 3.1 ). G proteins share a common heterotrimeric structure consisting of an α subunit and a tightly coupled βγ dimer. The α subunit interacts with detector and effector molecules, binds guanosine 5’-triphosphate (GTP), and possesses intrinsic GTPase activity. There are 16 genes in mammals that encode some 20 different α chains. The Gα subunits are categorized in four classes and include Gsα (G stimulatory), Giα (G inhibitory) and Goα (G other), Gq/11α, and G12/13α. They behave differently in the recognition of the effector but share similar structures and mechanism of activation. The Gα subunits consist of two domains: a GTP-binding domain and a helical insertion domain. The GTP-binding domain is homologous to Ras-like small GTPases and includes switch regions I and II, which change conformation during activation. The switch regions are loops of alpha helices with conformations sensitive to guanine nucleotides. The helical insertion domain is inserted into the GTP-binding domain before switch region I and is unique to heterotrimeric G proteins. This helical insertion domain sequesters the guanine nucleotide at the interface with the GTP-binding domain and must be displaced to enable nucleotide dissociation.
The α subunits associate with a smaller group of β(5) and γ(12) subunits. Combinatorial specificity in the associations between various G protein subunits provides the potential for enormous diversity and may allow distinct heterotrimers to interact selectively with only a limited number of GPCRs and effector proteins.
There are two principal signal transduction pathways involving the GPCRs: the cyclic AMP signal pathway and the phosphatidylinositol signal pathway. G protein–induced signal generation is regulated by a “molecular timer” that is determined by the rate of GTP exchange and hydrolysis. In the inactive state, G proteins exist in the heterotrimeric form with guanosine 5’-diphosphate (GDP) bound to the α chain. Interaction of a ligand-bound receptor with a G protein leads to release of GDP, with subsequent binding of GTP to the α chain. The binding of GTP to the α chain leads to dissociation of the α chain from the βγ dimer, allowing the now free α-GTP chain to interact with target enzymes and ion channels. The βγ dimers also participate in downstream signaling events through interaction with an everwidening array of targets, including certain forms of adenylyl cyclase and phospholipase C, potassium channels, and GPCR kinases.
G protein signaling is terminated by the hydrolysis of α-GTP to α-GDP by an intrinsic GTPase. A group of proteins, called regulators of G protein signaling (RGSs), acts as GTPase-activating proteins (GAPs), specific for Gα subunits. These proteins accelerate hydrolysis of GTP to GDP and terminate the transduced signal. In some cases, the effector itself may possess intrinsic GAP activity, which helps deactivate the pathway. This is true in the case of phospholipase C β, which possesses GAP activity within its carboxy-terminal region. This is an alternate form of regulation for the Gα subunit. However, it should be noted that the GAPs do not have catalytic residues to activate the Gα protein. Rather, GAPs reduce the required activation energy for the reaction to take place. After hydrolysis of GTP, the Gα-GDP chain reassociates with the βγ dimer; the reassociated heterotrimeric G protein is now capable of participating in another cycle of receptor-activated signaling.
Specificity in ligand binding is conferred by variations in the primary structures of the extracellular and intracellular domains. Specificity of effector responses is conferred by the variations in the primary structure of intracellular domains and isoforms of the Gα subunits of G proteins. Some GPCRs couple predominantly with Gα i /Gα o subunits that act primarily to decrease adenylyl cyclase activity. Other GPCRs couple predominantly with Gα s subunits that increase adenylyl cyclase activity or Gα q /Gα subunits that increase phospholipase C activity.
Interestingly, data show that cytoskeletal proteins may modulate receptor–G protein coupling. For example, the erythrocyte membrane cytoskeletal protein 4.1G can interfere with A1 adenosine receptor signal transduction. 4.1G also influences metabotropic glutamate receptor 1α-mediated cyclic AMP accumulation, increases the ligand-binding ability of metabotropic glutamate receptor 1α, and alters its cellular distribution. 4.1G may also play a role in receptor-receptor dimerization.
Receptor agonist-independent and agonist-induced homo- and heterodimerization have increasingly been recognized as important determinants of GPCR function. For example, the GPCR somatostatin receptor 5 (SSTR5) primarily exist as monomers in the absence of an agonist. However, they form homodimers in the presence of an agonist. Furthermore, it has been shown that SSTR5 can form heterodimers with type 2 dopamine receptors (DRD2)—another GPCR—in the presence of hsst2 agonist or dopamine. Agonist-induced activation of SSTR5-DRD2 heterodimers in Chinese hamster ovary (CHO) cells expressing SSTR5 and DRD2 is increased, when compared with agonist-induced activation of monomers and homodimers in CHO cells expressing only SSTR5 or DRD2. Heterodimerization of receptors may also lead to inactivation of one of the receptors in the complex. For example, heterodimerization of somatostatin receptor 2A (sst2A) with somatostatin receptor 3 (SSTR3) appears to lead to inactivation of the heterodimerized SSTR3, without inactivating the heterodimerized SSTR2.
GPCRs can form heterodimers with nonreceptor transmembrane proteins. Both the calcitonin receptor (CALCR) and the calcitonin receptor-like protein (CALCRL) can form heterodimers with three different accessory proteins that are termed “ RAMPs ”: RAMP1, RAMP2, and RAMP3. Whereas CALCRs can be activated by ligand in the absence of heterodimerization with a RAMP, CALCRLs are only activated by ligand if heterodimerized with a RAMP. RAMPs alter the ligand specificity of the heterodimerized receptor.
CALCRs that are not in heterodimers with RAMPS are activated by calcitonin and thus constitute the classic CALCR. However, CALCRs heterodimerized with RAMP1, RAMP2, and RAMP3 bind amylin and constitute amylin1, amylin2, and amylin3 receptors, respectively. CALCRLs dimerized with RAMP1 bind calcitonin gene–related peptide and constitute the calcitonin gene–related peptide receptor. CALCRLs dimerized with RAMP2 and RAMP3 bind adrenomedullin and constitute adrenomedullin1 and adrenomedullin2 receptors, respectively. RAMPS alter function of other GPCRs that transduce hormone action. The distribution and function of parathyroid hormone 1 and 2 receptors are altered by binding to RAMP2 and RAMP3, respectively. The distribution and function of the glucagon receptor is altered by binding to RAMP2. Dimerization/heterodimerization may occur in the endoplasmic reticulum (ER), shortly after protein synthesis occurs. The ER plays a role in determining whether or not a protein will be expressed elsewhere in the cell, thus protecting the cell from misfolded and (likely) mutant proteins. The nonheterodimerized CALCRL is an orphan receptor because the CALCRLs cannot leave the ER for the cell membrane, unless heterodimerized with RAMPs.
The melanocortin receptors also use accessory proteins. Circulating adrenocorticotropin hormone (ACTH) binds to five different forms of the melanocortin receptor (types 1–5), but only the melanocortin 2 receptor (MC2R) in the adrenal cortex leads to release of adrenal steroids. MC2R interacts with Gs, which leads to activation of adenylyl cyclase and formation of cyclic AMP. The MC2R is the smallest GPCR known to date and belongs to a family of melanocortin receptors (types 1–5) that bind to various derivatives of proopiomelanocortin, especially α-melanocyte-stimulating hormone (α-MSH). The accessory protein melanocortin 2 receptor-associated protein (MRAP) is required for MC2R function, as it is critical for the translocation of the receptor from the ER to the cell surface. Moreover, MRAP facilitates signaling of the MC2R. Loss of function of MRAP thus prevents membrane expression of MC2R and completely prevents ACTH signaling. MRAP-deficient mice die at birth, unless rescued with glucocorticoids, but have normal mineralocorticoid and catecholamine production. The adrenal glands of MRAP-deficient adult mice are small with abnormal adrenal morphology, abnormal cortex zonation, and abnormal adrenal progenitor cell differentiation. Interestingly, MRAP forms a unique antiparallel homodimer in close proximity to the MC2R. The MRAP accessory protein can also interact with other melanocortin receptors, particularly MC5R, but exerts negative effects on their signaling. Expression of MRAP was shown to be predominantly present in the zona fasciculata in the rat adrenal gland, consistent with its facilitating role in glucocorticoid production. Hence mutations in MC2R or MRAP can lead to familial glucocorticoid deficiency secondary to ACTH resistance. In contrast, MRAP2, a protein with 39% amino acid homology to MRAP, shares the MC2R-trafficking function of MRAP but does not appear to play a major supportive role in adrenocortical ACTH signaling. On the contrary, in vitro studies have shown that overexpression of MRAP2 can suppress MC2R activation. MRAP2 appears to play a role in energy homeostasis as MRAP2 KO mice develop obesity. MRAP2 interacts with MC4R in the paraventricular nucleus of the hypothalamus (PVN) where PVN-specific MRAP2 KO duplicates the global MRAP2 KO phenotype.
Failure of the ER to export mutant GPCR homodimers and mutant GPCR wild-type GPCR heterodimers to the cell membrane has been found to be the cause of dominant negative endocrine conditions. A dominant negative mutation is a heterozygous mutation that results in a phenotype that would be expected by a loss of function in both alleles. Some heterozygous MC4R mutations cause dominantly inherited obesity because of interaction of wild-type MC4R with the mutant receptor, and this specific effect of protein-protein interaction results in a dominant-negative effect. In addition, some heterozygous mutations in the gene encoding the V2 vasopressin receptor cause nephrogenic diabetes insipidus via production of mutant proteins that interfere with transit of normal receptors to the cell's membrane. These mutant receptors interfere with cell-surface expression of wild-type receptors by forming heterodimers with the wild-type receptors that cannot be exported from the ER to the cell membrane. This finding explains why females heterozygous for these V2 vasopressin receptor gene mutations do not concentrate their urine with even high doses of desmopressin, a synthetic V2 vasopressin receptor agonist, in spite of being able to produce wild-type V2 vasopressin receptors. A similar phenomenon explains dominant transmission of partial thyroid-stimulating hormone (TSH) receptor resistance in patients heterozygous for some inactivating TSH receptor mutations. In these patients, mutant TSH receptors form oligomers with wild-type receptors and prevent export of wild-type receptors from the ER to the cell membrane.
Similarly, misfolding and misrouting of some mutant gonadotropin-releasing hormone (GnRH) receptors in the ER (as well as oligomerization of these mutant GnRH receptors with wild-type GnRH receptors) decrease cell membrane expression of wild-type GnRH receptors. This phenomenon, however, has not been found to have clinical implications in patients who are heterozygous for mutations that cause autosomal recessive isolated hypogonadotropic hypogonadism (IHH), as the heterozygous individuals demonstrate an intact GnRH-gonadotropin axis and do not have clinical signs of IHH. Thus in these individuals, enough wild-type GnRH receptors do not oligomerize with mutant GnRH receptors and are transported to the cell membrane to maintain sufficiently normal GnRH-GnRH receptor interactions to avoid development of IHH.
Most GPCRs activate G proteins at very low levels in the absence of ligand binding. Some GPCRs have much higher constitutive (i.e., ligand-independent) activity, such as luteinizing hormone, TSH, thyrotropin-releasing hormone (TRH), glucagon-like peptide-1, melanocortin, and cannabinoid receptors. These receptors can activate G proteins in the absence of ligand binding, demonstrating constitutive activity that increases linearly with increased cell-surface expression of the receptors. As described earlier, inverse agonists decrease the activity of these receptors. In receptors without constitutive activity, genetic mutations that lead to substitution of a single amino acid can also greatly increase the interaction rate of the unliganded receptor for its G protein. It is possible that inverse agonists may play a role in treating medical conditions caused by GPCR mutations that lead to increased constitutional activation of the receptor.
Receptor desensitization and resensitization play a role in GPCR activity. Three processes for receptor desensitization have been described. The first receptor desensitization process is rapid uncoupling of the G protein from GPCRs. This process occurs within seconds to minutes after initiation of the process and occurs as a result of phosphorylation of GPCRs. G protein receptor kinases (GRKs) have been increasingly recognized as playing a major role when this process involves homologous desensitization. GRK-mediated phosphorylation of serine and threonine residues in the third intracellular loop, or the carboxy-terminal intracellular domain leads to activation of β-arrestins, which in turn inactivate adenylyl cyclase ( Fig. 3.2 ). Second-messenger–dependent protein kinases also contribute to receptor desensitization when this process involves homologous desensitization, but they also participate in receptor desensitization when desensitization involves heterologous desensitization. Heterologous or agonist-independent desensitization occurs as a result of activation of a different receptor from the one that is desensitized.
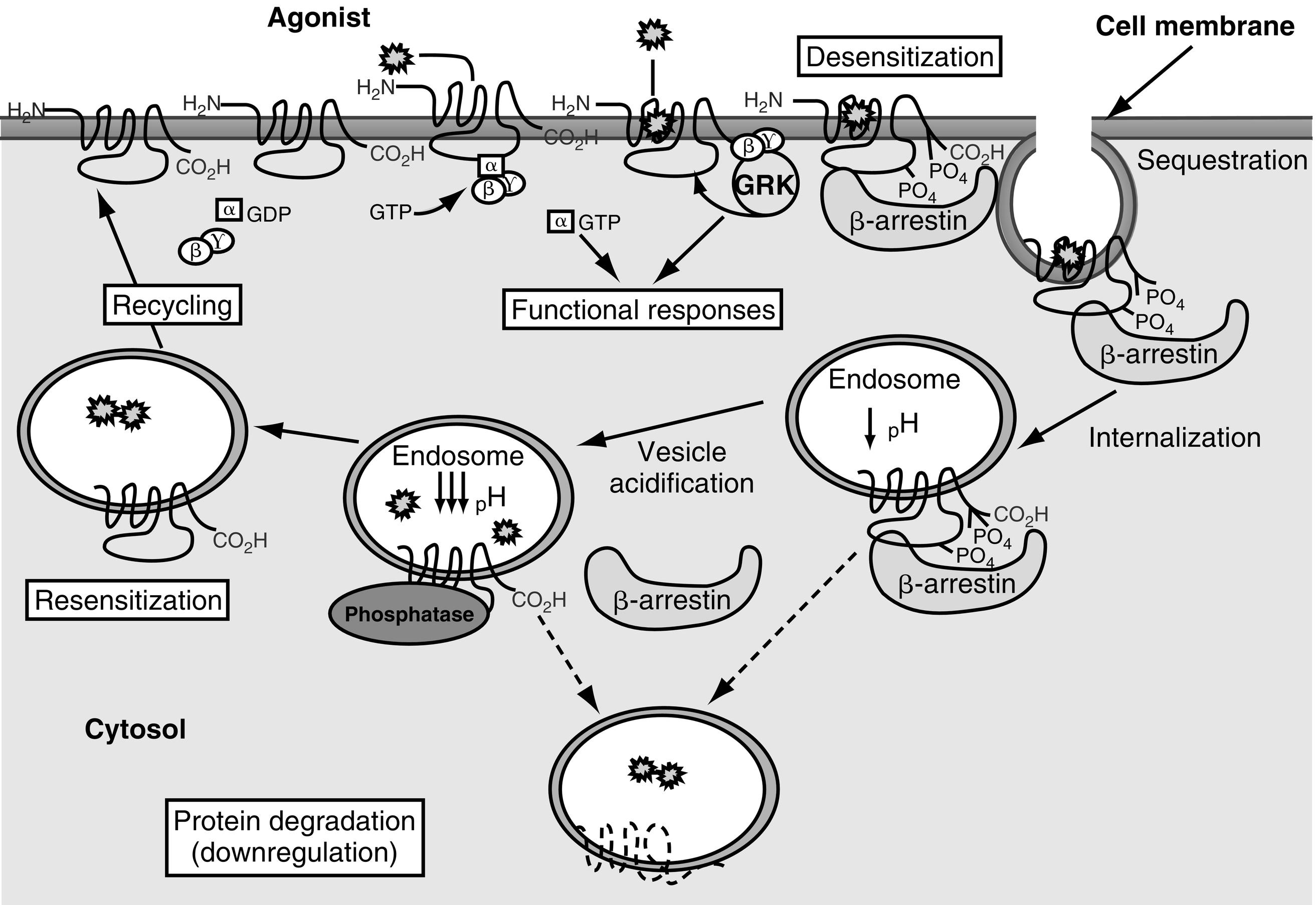
The second receptor desensitization process is internalization/sequestration of GPCRs. This process is slower than receptor phosphorylation-induced uncoupling of the G protein from GPCRs and occurs within minutes to hours after initiation of the process. In addition to phosphorylation, both the GPCR and β-arrestins are modified posttranslationally in several ways, including by ubiquitination. This process is reversible because the receptors can be recycled to the cell surface (see Fig. 3.2 ). GRKs and β-arrestins play a role in initiating internalization/sequestration of β2-adrenergic, luteinizing hormone (LH), FSH, TSH, TRH, vasopressin V2, angiotensin II type 1A, and other GPCRs in clathrin-coated vesicles (see Fig. 3.2 ). Dephosphorylation of the sequestered receptor, followed by disassociation of the receptor from β-arrestin, is necessary for the receptor to be recycled to the cell membrane and resensitized (see Fig. 3.2 ).
The third receptor desensitization process is downregulation. With downregulation, the number of intracellular GPCRs decreases because of increased lysosomal degradation and decreased synthesis of the receptors caused by alteration of transcriptional and posttranscriptional regulatory mechanisms (see Fig. 3.2 ). Downregulation is a slow process that occurs within several hours to days after initiation of the processes that lead to its development. For many GPCRs, receptor ubiquitination promotes degradation of agonist-activated receptors in the lysosomes. Other proteins also play important roles in desensitization, including phosphodiesterases, RGS family proteins, and A-kinase-anchoring proteins. Together, this intricate network of kinases, ubiquitin ligases, and adaptor proteins orchestrate the acute and prolonged desensitization of GPCRs.
One of the ways the Arg137His V2 vasopressin receptor mutation interferes with mutant receptor function and causes X-linked nephrogenic diabetes insipidus is by altering desensitization and recycling of the mutant receptor. In vitro studies have revealed that the mutant receptor is constitutively phosphorylated. Thus even in the absence of ligand binding, the mutant receptor is bound by β-arrestin—which in turn leads to sequestration of the mutant receptor within clathrin-coated vesicles. Recycling of the mutant receptor back to the cell membrane requires the mutant receptor to be dephosphorylated and disassociated from β-arrestin. However, the mutant receptor does not undergo dephosphorylation, while sequestered, and thus cannot be disassociated from β-arrestin and recycled to the cell membrane—thereby reducing cell membrane expression of the mutant receptor.
Of the eight classes of GPCRs, only classes A, B, and C contain receptors for mammalian hormones and neurotransmitters ( Fig. 3.3 ). Class A receptors contain the rhodopsin-like receptors and are divided into at least 15 groups. Four of these groups contain receptors activated by hormones. These are the peptide receptor, hormone protein receptor, GnRH receptor, and the TRH and secretagogue receptor groups.
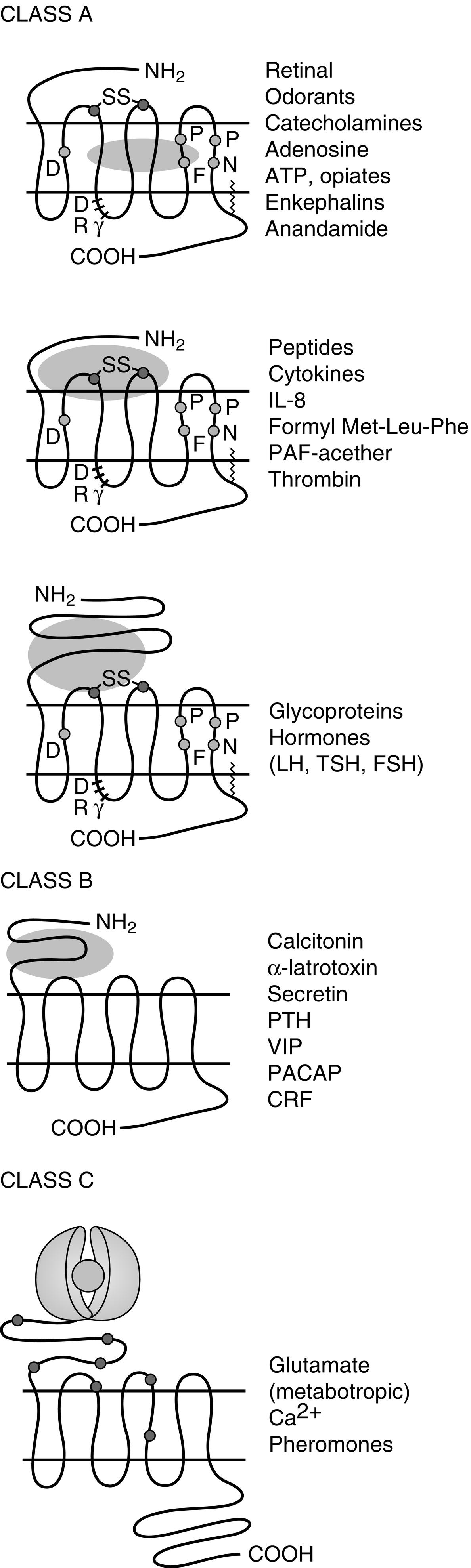
The peptide receptor group includes the angiotensin, ACTH/melanocortin, oxytocin, somatostatin, and vasopressin receptors. The hormone protein receptor group includes the receptors for glycoprotein hormones, including FSH, LH, and thyrotropin (TSH) receptors. These receptors have large extracellular amino-terminal domains and ligand-binding sites that include the first and third extracellular loops (see Fig. 3.3 ). There is also much similarity in amino acid sequence among these receptors (see Fig. 3.3 ). The GnRH receptor group only contains the GnRH receptor. The TRH and secretagogue receptor group includes the TRH receptor and the growth hormone (GH) secretagogue receptor.
Class B GPCRs are structurally similar to members of the hormone protein receptor group (see Fig. 3.3 ). However, unlike the glycoprotein hormone receptors, class B GPCRs do not share similar amino acid sequences. This family contains receptors for higher molecular weight hormones, including calcitonin, glucagon, gastric inhibitory peptide, parathyroid hormone (PTH), and corticotrophin-releasing factor (CRF).
Class C receptors have a large extracellular domain, with two lobes separated by a hinge region that closes on the ligand (see Fig. 3.3 ). This region has also been called the Venus flytrap domain or module because of the trapping mechanism of the hinge region. This family includes the calcium-sensing receptor (CASR).
Most GPCR-inactivating mutations can be classified into one of five classes. Class I inactivating mutations interfere with receptor biosynthesis. Class II inactivating mutations interfere with receptor trafficking to the cell surface. Class III inactivating mutations interfere with ligand binding. Class IV inactivating mutations impede receptor activation. Class V inactivating mutations do not cause discernible defects in receptor biosynthesis, trafficking, ligand binding, or activation, but may cause medical disorders. There are also inactivating mutations that interfere with receptor function via multiple mechanisms and thus cannot be placed into one class.
An alternative name for the ACTH receptor is melanocortin-2 receptor (MC2R) because the ACTH receptor is one of five members of the melanocortin receptor family of GPCRs, all of which couple to Gs to activate generation of intracellular cyclic AMP. For the purpose of clarity, when discussing interactions between ACTH and its receptor, the older name will be used for the remainder of this chapter. The ACTH receptor gene is located on small arm of chromosome 18 (18p11.2). The ACTH receptor has a small extracellular and intracytoplasmic domain. Adrenocorticotropin-induced activation of the ACTH receptor in the zona fasciculata and zona reticularis of the adrenal cortex stimulates G s , resulting in increased intracellular cyclic AMP levels that stimulate steroidogenesis by activating cyclic AMP-dependent kinases.
Hereditary isolated glucocorticoid deficiency, resistance to ACTH, and familial glucocorticoid deficiency (FGD) are the same names for an autosomal recessive syndrome that consists of glucocorticoid deficiency accompanied by normal mineralocorticoid secretion. FGD has been classified further as FGD types 1 and 2 and the triple A syndrome. Patients with FGD type 1 have biallelic MC2R mutations, resulting in ACTH receptors with abnormal function, and account for 25% of FGD cases. In contrast, patients with FGD type 2 have ACTH resistance caused by mutations in MRAP. Triple A (Allgrove syndrome) is an autosomal recessive syndrome characterized by ACTH-resistant adrenal insufficiency, achalasia, and alacrima—which is caused by mutations in the achalasia-adrenocortical insufficiency-alacrima syndrome ( AAAS ) gene encoding the protein ALADIN. ALADIN is thought to regulate nuclear pore complexes, and nucleocytoplasmic transport plays a role in modulating the oxidative stress response in adrenal cells.
The spectrum of disease in patients with FGD type 1 varies from presentation as a neonate with hypoglycemia, seizures, or circulatory collapse to presentation in childhood with fatigue and increased susceptibility to infection. Less commonly, patients may present with childhood asthma that resolves with treatment with physiologic doses of glucocorticoids. Hyperpigmentation thought to be caused by increased ACTH levels acting on the MC1R may be seen as early as the first month of life, but usually becomes apparent after the fourth month of life. There is one reported case of FGD type 1 without hyperpigmentation, despite elevated ACTH levels, in a patient with homozygous mutations in both the MC2R and the MC1R. The assumption that hyperpigmentation is caused by increased ACTH levels acting on the MC1R (both in FGD type 1 and Addison disease) was substantiated in this patient whose MC1R mutation had previously been implicated in red hair and pale skin phenotypes. Neonates with FGD type 1 may also suffer from jaundice. Tall stature accompanied by an advanced or dissociated bone age, in spite of normal age of onset of puberty, appears to be common in children with FGD type 1. The pathophysiology of tall stature has not been definitively elucidated. One theory is that the anabolic effects of GH are unopposed by cortisol.
Patients with FGD1 exhibit absent adrenarche, confirming the importance of ACTH in the induction and maintenance of adrenarche. At presentation, plasma cortisol, androstenedione, and dihydroepiandrosterone levels are low or low normal—and plasma ACTH levels are elevated. When supine, patients with FGD type 1 have renin and aldosterone levels that are near normal. Histologically, the zona fasciculata and zona reticularis are atrophied with FGD. However, demonstrating the lack of an essential role for ACTH in the embryologic development and maintenance of the zona glomerulosa, adrenal cortices in patients with all types of FGD contain zona glomerulosa cells.
FGD type 2 is caused by mutations in MRAP1 as described earlier and accounts for 20% of all FGD cases. Patients with FGD type 2 present with severe symptoms at an earlier age than patients with FGD type 1 (median age 0.08 years vs. 2 years). Patients can present with severe neurologic disability, seizures, and microcephaly thought to be caused by unrecognized hypoglycemia. Patients with FGD type 2 have normal heights. This is thought to be caused by early treatment with glucocorticoids. Mutations are splice site or nonsense mutations predicted to produce proteins without the transmembrane domain, which is necessary for interaction with MC2R.
Abnormalities in ACTH receptor expression may be seen in other conditions. Evidence suggests that the ACTH receptor-Gα s -adenylyl cyclase-cyclic AMP cascade maintains differentiation of adrenocortical cells and that impairment of this cascade leads to dedifferentiation and increased proliferation of adrenocortical cells. Adrenocortical carcinomas from some patients have been found to have a loss of heterozygosity (LOH) for the ACTH receptor gene, resulting in markedly decreased ACTH receptor messenger ribonucleic acid (mRNA) expression. Growth of the tumors with LOH for the ACTH receptor gene also may be more aggressive than the other tumors. An activating mutation of G i2 that constitutively suppresses adenylyl cyclase activity has also been found in adrenocortical tumors. Thus decreased ACTH receptor activity may be associated with tumorigenesis.
Interestingly, many patients with ACTH-independent macronodular adrenal hyperplasia (AIMAH)—a cause of ACTH-independent Cushing syndrome caused by inactivating mutations of the putative tumor-suppressor gene ARMC5 —exhibit increased glucocorticoid levels in response to noncorticotropin hormones that do not normally induce glucocorticoid release. These hormones include gastric inhibitory peptide, exogenous arginine and lysine vasopressin, LH, human chorionic gonadotropin (HCG), angiotensin II, catecholamines, leptin, and serotonin receptor agonists. Increased expression of the receptors for these ligands in the abnormal adrenal glands has been implicated as a possible explanation for the abnormal induction of glucocorticoid release by these noncorticotropin ligands. However, receptors for some of these ligands are expressed in normal adrenal glands. Thus the mechanism for this phenomenon remains to be fully elucidated.
Murine studies reveal that melanocortin-3 receptor (MC3R), another of the five members of the melanocortin receptor family, regulates fat deposition, as mice with MC3R deficiency or partially inactive receptors have normal resting energy expenditure and increased fat mass and reduced fat-free mass, including decreased bone formation. The role of the MC3R in humans is less clear. More than 24 human MC3R variants have been identified without evidence of obesity. However, patients with these variants were not phenotyped for fat mass to assess whether they phenocopy the mouse model, which exhibits normal body weight but greater energy intake and altered energy partitioning that is biased toward lipid-accumulating cells' altered partitioning. Several coding variants (p.D158Y, p.T280S, p.I183N), which are likely pathologic because of significantly decreased cyclic AMP production, as well as noncoding variants, demonstrate early-onset obesity in all affected individuals. Homozygosity for a pair of single-nucleotide polymorphisms of the MC3R gene (p.T6K + p.V81I) that result in production of partially inactive MC3Rs was found to be associated with pediatric-onset obesity in Caucasian American and African American children. Subjects homozygous for the double mutation had higher body mass index (BMI)-z, fat mass and percent fat mass, as well as waist circumference. That is despite finding no differences in energy intake, resting energy expenditure, total energy expenditure, respiratory quotient, physical activity. A mouse model carrying the human MC3R T6K + V81I variants showed that the double mutant exhibited greater fat mass and feeding efficiency with reduced fat-free mass. These findings are similar to the MC3R knockout mouse.
The melanocortin-4 receptor (MC4R) is another member of the melanocortin receptor family and plays a role in controlling appetite and weight. The MC4R has baseline constitutive (i.e., ligand-independent) activity that can be inhibited by the inverse agonist agouti-related peptide (AgRP). Activation of the MC4R by its natural agonist α-MSH produces anorexigenic effects. More than 150 naturally occurring MC4R mutations have been identified, causing hyperphagic obesity, increased lean body mass, increased bone density, and increased linear growth. Patients with homozygous mutations appear to have more severe obesity than their heterozygous relatives, consistent with codominant inheritance.
MC4R mutations are thought to be the most common monogenic cause of human obesity. The prevalence of pathogenic MC4R mutations in obese populations varies widely, ranging from 0.5% to 5.8%, depending on the screening criteria and population. AgRP gene polymorphisms appear to be associated with anorexia nervosa.
Little is known about melanocortin-5 receptors (MC5Rs) in animals and humans. There is only weak evidence from a single linkage and association study of families in Quebec that suggests that MC5Rs may also play a role in regulating body weight and fat mass.
Another member of the melanocortin receptor family, the MC1R, controls skin and hair pigmentation. Activation of MC1Rs in skin and hair follicle melanocytes by the proopiomelanocortin (POMC)-derived peptides α-MSH and ACTH stimulates the synthesis of eumelanin, a brown-black pigment. Inhibition of MC1R baseline constitutive activity by agouti protein, or specific mutations, leads to release of pheomelanin, a red-yellow pigment, from the melanocytes.
Inactivating homozygous mutations of the POMC gene cause hypoadrenalism, red hair, fair skin, and early-onset obesity. Hypoadrenalism is characterized by glucocorticoid deficiency because of lack of ACTH production from the POMC precursor. Fair skin and red hair are caused by a lack of ACTH and α-MSH-induced melanocyte release of eumelanin that results from activation of MC1Rs. Of note, nonwhite patients with homozygous POMC mutations do not appear to have the fair skin and red hair phenotype. In white individuals, eumelanin synthesis appears to be dependent on POMC-derived peptides, whereas in darker individuals, other genes may control eumelanin synthesis. Obesity is caused by lack of α-MSH–induced anorectic effects, which normally result when α-MSH activates MC4Rs. Heterozygosity for POMC gene mutations has been associated with hyperphagia, early-onset obesity, and increased linear growth. Both homozygous and heterozygous mutations of prohormone convertase 1 cause obesity in humans. Prohormone convertase 1 acts on POMC, proinsulin, and proglucagon. Patients with prohormone convertase 1 deficiency also have neonatal enteropathy and postprandial hypoglycemia. The cause of enteropathy is unknown but hypothesized to be related to the processing of GLP-2 by prohormone convertase 1. GLP-2 is known to stimulate proliferation and repair of intestinal epithelium.
Nephrogenic diabetes insipidus (NDI) results from decreased responsiveness of the renal tubule to arginine vasopressin (AVP), with resulting excessive loss of free water. NDI is characterized by polydipsia and polyuria that is not responsive to vasopressin and vasopressin analogs. Vasopressin binds to the V2 vasopressin receptor (AVPR2), a Gs-coupled receptor, in the basolateral membrane of collecting duct principal cells in the kidney and activates translocation of aquaporin-2 (AQP2) water channels to the apical membrane, thereby inducing water permeability. X-linked NDI is caused by inactivating mutations of the V2 vasopressin receptor ( AVPR2 ) gene located at Xq28 and accounts for about 90% of genetically determined NDI. More than 200 AVPR2 mutations have been described, including missense, nonsense, insertions, deletions, and complex rearrangements. Mutations have been categorized into five classes based on mechanism, including abnormal transcription, mRNA processing, translation, aberrant folding and intracellular retention, loss of the G protein binding site, loss of the AVP binding site, and defects in intracellular trafficking. Some patients with X-linked NDI are responsive to high doses of desmopressin. Autosomal recessive NDI (ARNDI) is caused by loss-of-function mutations in the gene for the AQP2 water channel and accounts for about 10% of genetic forms of NDI. More than 50 known mutations cause ARNDI. Autosomal dominant forms of NDI are also caused by mutations in AQP2 that are functional, but fail to be transported to the apical membrane. Eleven mutations have been described accounting for less than 1% of genetic forms of NDI and generally have a milder phenotype than ARNDI or X-linked NDI.
Gain-of-function mutations in the V2 vasopressin receptor have also been reported. Deoxyribonucleic acid (DNA) sequencing of two patients’ V2R gene identified heterozygous missense mutations in both, with resultant changes in codon 137 from arginine to cysteine (p.R137C) or leucine (p.R137L). These mutations resulted in constitutive activation of the receptor and clinical features of inappropriate antidiuretic hormone secretion (SIADH), which was termed nephrogenic syndrome of inappropriate antidiuresis (NSIAD) . To date, around 30 cases of NSIAD have been reported, most of which are described in males as the condition is X-linked. Patients can present in infancy but sometimes do not present until adulthood. There have been several reports of female patients with NSIAD, some diagnosed in infancy and others in adulthood. Patients with the p.R137L mutation demonstrated the expected decrease in AVP levels with a water-loading test, but urine AQP2 levels remained inappropriately elevated.
The glycoprotein hormones include TSH, FSH, LH, and HCG. These hormones share common α subunits that dimerize with hormone-specific β subunits. TSH, FSH, and LH bind to the extracellular amino-terminal domain of the TSH, FSH, and LH receptors, respectively. The effects of HCG are mediated by the LH receptor, which is also known as the luteinizing hormone/choriogonadotropin receptor ( LHCGR ).
Glycoprotein hormone receptors have a large (350 to 400 residues) extracellular amino-terminal domain, also known as the ectodomain , that participates in ligand binding (see Fig. 3.3 ). The ectodomain includes leucine-rich repeats that are highly conserved among the glycoprotein hormone receptors. There is 39% to 46% similarity of the ectodomain and 68% to 72% similarity of the transmembrane or serpentine domain among the three glycoprotein hormone receptors.
The glycoprotein hormone receptors are coupled to G s , and hormone binding stimulates adenylyl cyclase, leading to increased intracellular cyclic AMP levels and protein kinase A (PKA) activation. Mutations leading to endocrine dysfunction have been reported for each of the glycoprotein hormone receptors.
Both inactivating and activating mutations of the LH receptor have been found in humans. The LH receptor gene is located in chromosome 2p21 and consists of 11 exons. Exon 1 encodes a peptide that directs the LH receptor to the plasma membrane. Exons 2 through 10 encode the ectodomain. The last exon encodes the transmembrane domains that are also known as the serpentine regions . Single nonsense mutations, amino acid changes, and partial gene deletions have been described that generate LH receptors with decreased activity. Single–amino-acid changes have also been found that lead to activation of G s in the absence of ligand binding.
Development of LH resistance requires biallelic mutations that inactivate the LH receptor gene, as one normal receptor allele is capable of producing adequate receptor protein to ensure physiologic signaling. In contrast, activating mutations of the LH receptor gene cause endocrine disorders in the heterozygous state.
In the fetus, LH receptors are primarily activated by HCG. Leydig cells begin to express LH receptors shortly after testicular differentiation at 8 weeks of gestation. Thereafter, androgen production, caused by activation of these receptors by HCG, plays an important role in the development of male genitalia and testicular descent. Thus male infants, with inactivating mutations of the LH receptor, may present with abnormally developed genitalia—including micropenis, cryptorchidism, and an XY disorder of sexual differentiation.
Males with mutations that completely inactivate the LH receptor exhibit failure of fetal testicular Leydig cell differentiation. This phenotype, which is known as type 1 Leydig cell hypoplasia , includes female external genitalia with a blind-ending vagina, absence of Müllerian derivatives, and inguinal testes with absent or immature Leydig cells. In addition, patients have elevated serum LH levels, normal serum FSH levels, and decreased serum testosterone levels that do not increase in response to HCG administration. Mutations that lead to this phenotype include a nonsense mutation (Arg545Stop) that results in a receptor that is missing TM4-7, an p.Ala593Pro change, and a TM7 deletion (p.Leu608del, p.Val609) that decreases cell-surface expression of the LH receptor. These mutant receptors are unable to couple to G s .
Males with mutations that do not completely inactivate the LH receptor present with type 2 Leydig cell hypoplasia, which is characterized by a small phallus and decreased virilization. A mutation that leads to this phenotype includes the insertion of a charged lysine at position 625 of TM7 in place of hydrophobic isoleucine that disrupts signal transduction. Another mutation (p.Ser616Tyr, found in patients with mild Leydig cell hypoplasia) is associated with decreased cell-surface expression of the LH receptor. Other deletion and nonsense mutations have also been found to cause mild Leydig cell hypoplasia.
Males with inactivating mutations of the LH receptor may also present with a phenotype intermediate in severity between type 1 and type 2 Leydig cell hypoplasia. A compound heterozygote patient with p.Ser616Tyr on one allele and an inactivating deletion (△exon 8) on the other allele, presented with Leydig cell hypoplasia, micropenis, and hypospadias. The Cys131Arg mutation has also been found in patients with Leydig cell hypoplasia, small phallus, and hypospadias. This mutation is located in the leucine-rich repeat segment of the LH receptor extracellular domain and interferes with high-affinity ligand binding.
Deletion of exon 10 of the LH receptor gene leads to an LH receptor that binds LH and HCG normally. Interestingly, whereas HCG binding can elicit normal transmembrane signaling, LH binding fails to activate the receptor. Because HCG is the principal hormone in utero that activates the LH receptor, and second-messenger response of the mutant receptors to HCG is not impaired, it is not surprising that a male patient found to be homozygous for the mutation was born with normal male genitalia. Pubertal progression and later gonadal function, however, are dependent on LH activation of the LH receptor.
Because deletion of exon 10 of the LH receptor gene results in a mutant LH receptor, with diminished intracellular signaling in response to LH, it is also not surprising that the patient homozygous for this mutation was found to have delayed pubertal development, small testes, and hypergonadotropic hypogonadism, when evaluated at the age of 18 years. Prolonged HCG therapy resulted in normalization of testicular testosterone production, increased testicular size, and the appearance of spermatozoa in semen. Similarly, inactivating mutations of the LH β subunit cause abnormal pubertal development, severe testosterone deficiency, and azoospermia, but normal external genitalia in males. In females, inactivating mutations of the LH β subunit are associated with normal pubertal development and menarche followed by oligomenorrhea, enlarged multicystic ovaries and infertility.
Females with loss of function mutations of the LH receptor may be asymptomatic or present with amenorrhea or oligomenorrhea. Females with complete inactivating LH receptor mutations commonly have primary amenorrhea, inability to ovulate, and decreased estrogen and progesterone levels, accompanied by elevated LH and FSH levels. Affected individuals may have signs of low estrogen levels, including a hypoplastic uterus, a thin-walled vagina, decreased vaginal secretions, and decreased bone mass, although pubertal breast development is normal. Homozygous LH receptor mutations (p.N400S, and p.Ala449Thr) have been associated with empty follicle syndrome, a disorder in which no oocytes are retrieved during in vitro fertilization.
Mutations that constitutively activate LH receptors cause male-limited precocious puberty (MLPP), also known as testotoxicosis —which may be familial or sporadic. Boys with this condition develop GnRH-independent precocious puberty before the age of 4 years, when p.Asp578Gly is present, and as early as the first year of life, when the p.Asp578Tyr mutation is present. Patients with this condition may also have an enlarged phallus at birth.
During the first 5 years of life, patients with MLPP have very low LH and FSH levels but have testosterone levels in pubertal range. During adolescence and adult life, testosterone levels do not increase above age-appropriate concentrations and gonadotropin levels normalize. Thus adolescents and adults with MLPP do not usually manifest signs of androgen excess (such as hirsutism or severe acne). Most mutations that cause MLPP are located in the TM6 and i3, regions that participate in receptor-Gs protein coupling. A milder phenotype was reported in a patient with a heterozygous activating mutation (p.C617Y) in TM7. This mutation was inherited from the patient’s mother who was apparently unaffected. Somatic activating mutations cause sporadic Leydig cell adenomas.
Activating mutations of the LH receptor do not appear to cause clinical disturbances in females. In prepubertal girls, this may be caused by low or absent LH receptor expression or because of insufficient aromatase expression in prepubertal granulosa cells. During puberty, activation of LH receptors on ovarian theca cells leads to the production of androgens that are converted to estrogens by aromatase in granulosa cells. LH, along with FSH, also plays a role in inducing the differentiation of follicles into Graafian follicles and triggers ovulation and release of the oocyte. Detailed phenotyping of the carrier mother of an MLPP male with the p.Asp578Gly activating mutation of the LH receptor failed to reveal any abnormalities in her menstrual cycles or fertility. LH dynamics, androgen, and FSH levels, as well as response to GnRH agonists, were normal.
Inactivating and activating FSH receptor mutations have been described, but they are far less common than LH receptor mutations. The FSH receptor gene is located in chromosome 2 at p21 and contains 10 exons. The last exon of the FSH receptor gene encodes the transmembrane and intracellular domains.
FSH is required in females for normal follicle maturation and regulation of estrogen production by ovarian granulosa cells. FSH is required in pubertal males for Sertoli cell proliferation, testicular growth, and the maintenance of spermatogenesis.
The first inactivating mutation of the FSH receptor was found in Finnish females with autosomal recessive hypergonadotropic ovarian dysgenesis (ODG). ODG is characterized by primary amenorrhea, infertility, and streak or hypoplastic ovaries in the presence of a 46XX karyotype and elevated gonadotropin levels. Twenty-two out of 75 Finnish patients with ODG were found to be homozygous for a c.C566T point mutation in exon 7 of the FSH receptor gene. This mutation leads to the production of an FSH receptor with an p.Ala189Val substitution in an area of the extracellular ligand-binding domain that is thought to play a role in turnover of the receptor or in directing the receptor to the plasma membrane. The mutated receptor demonstrates normal ligand-binding affinity but has decreased binding capacity and impaired signal transduction, when studied in transfected mouse Sertoli cells. Males homozygous for this mutation have variable impairment of spermatogenesis and low to low-normal testicular volume, but are not azoospermic and can be fertile. The c.C556T point mutation is uncommon outside Finland, where the carrier frequency is 0.96%. Other mutations that alter signal transduction but not receptor expression or binding include p.Ala189Val, p.Asn191Ile, p.Ala419Thr, and p.Phe591Ser. The p.Ala189Val mutation causes primary hypergonadotropic amenorrhea in women and no spermatogenesis in men in the homozygous state and secondary amenorrhea in the heterozygous state. The nearby Asn191Ile mutation also causes hypergonadotropic amenorrhea in the homozygous state, but no clinical phenotype in the heterozygous state. The Ala419Thr mutation was identified in a heterozygous woman with primary amenorrhea. The Phe591Ser mutation causes primary amenorrhea and premature ovarian failure (POF) in the homozygous state and a predisposition to sex cord ovarian tumors in the heterozygous state. Primary amenorrhea and POF have been described in women with homozygous mutations that totally impaired receptor binding to FSH or that resulted in reduced expression of the FSH receptor on the cell surface.
A more recently published series described 13 Chinese families with nonsyndromic premature ovarian insufficiency. They found three novel mutations and 11 previously described homozygous mutations and 3 previously described compound heterozygous mutations. Clinical manifestations ranged from primary amenorrhea to normal menarche with oligomenorrhea or secondary amenorrhea. It was the first study to identify a frameshift mutation (p.Lys140Argfs*16 in the ectodomain) and a missense mutation in the signal peptide of the FSH receptor (p.Gly15Asp). The other novel mutation was p.Pro504Ser in the transmembrane domain.
Compound heterozygosity for mutations that cause partial loss of FSH receptor function may cause endocrine dysfunction in women. Women may present with infertility, secondary amenorrhea, osteoporosis, and a history of normal or delayed onset of puberty, accompanied by elevated LH and FSH, low-normal plasma estradiol, low plasma inhibin B levels, slightly enlarged ovaries with immature follicles, and a small uterus. This may be caused by FSH receptor gene mutations that result in an p.Ile160Thr mutation in the extracellular domain that impairs cell-surface expression and an p.Arg573Cys mutation in e3 that interferes with signal transduction. Other women present with primary amenorrhea and very elevated gonadotropin, low plasma estradiol and inhibin B levels, normal-size ovaries with immature follicles, and a normal-size uterus. This condition is associated with an p.Asp224Val substitution in the extracellular domain, leading to impaired cell-surface expression and a p.Leu601Val substitution in e3 impairing signal transduction.
Activating mutations of the FSH receptor have also been described. Surprisingly, a hypophysectomized male was found to be fertile and to have serum testosterone levels above 4.9 nmol/L (141 ng/dL) and normal testis volume, in spite of undetectable gonadotropin levels. This patient was found to be heterozygous for a c.A1700G mutation in exon 10 of the FSH receptor gene that resulted in a p.Asp567Gly substitution in an area of the third intracytoplasmatic loop that is highly conserved among FSH, LH, and TSH receptors. The same substitution in corresponding areas of the LH and TSH receptors also results in constitutively active receptors and is found in MLPP and thyroid adenomas, respectively. Other activating mutations have been identified to cause spontaneous ovarian hyperstimulation syndrome (OHSS). OHSS is a common complication of treatment protocols used to induce ova for in vitro fertilization and is characterized by multiple follicular cysts lined by luteinized cells, which can result in abdominal discomfort and distention, as well as ovarian enlargement and fluid sequestration. One such mutation is the p.Asp567Asn, which was found in a woman with recurrent spontaneous OHSS. The p.Thr449Ile and p.Thr449Ala mutations cause a conformational change that leads to loss of specificity for FSH, leading to sensitivity to HCG and TSH, causing spontaneous OHSS during pregnancy or with hypothyroidism. The p.Ile545Thr mutation caused spontaneous OHSS in a woman during the first trimester of pregnancy, despite a normal HCG level. This mutant receptor displayed detectable constitutive activity, as well as promiscuous activation by HCG and TSH.
The TSH receptor gene is located on chromosome 14 and contains 10 exons, with the first nine exons encoding the large extracellular domain and the 10 th exon coding the remainder of the receptor. At low extracellular TSH concentrations, TSH receptor activation leads to stimulation of Gα s —which activates adenylyl cyclase, resulting in increased intracellular cyclic AMP levels. At higher extracellular TSH concentrations, activation of the TSH receptor also stimulates the G q and G 11 proteins—activating phospholipase C and resulting in the production of diacylglycerol and inositol phosphate.
TSH receptors differ from the other glycoprotein hormone receptors in that they exist in two equally active forms. These are the single-chain and two-subunit forms of the TSH receptor ( Fig. 3.4 ). The single-chain form of the TSH receptor is made up of three contiguous subunits: the A subunit, C peptide, and B subunit. The A subunit begins at the amino-terminal of the extracellular domain and contains most of the extracellular domain. The C peptide is connected to the carboxy-terminal of the A subunit and continues the extracellular domain. The C peptide contains a 50-amino-acid sequence that is only found in TSH receptors. The B subunit is connected to the C terminal of the C peptide and contains the TMs and the carboxy-terminal cytoplasmic portion of the receptor. The two-subunit form of the receptor is missing the C peptide, which is cleaved from the protein during intracellular processing and consists of the A and B subunits attached by disulfide bonds. It is surprising that both receptor forms are activated equally by TSH because the C peptide and nearby regions of the A and B subunits participate in signal transduction.
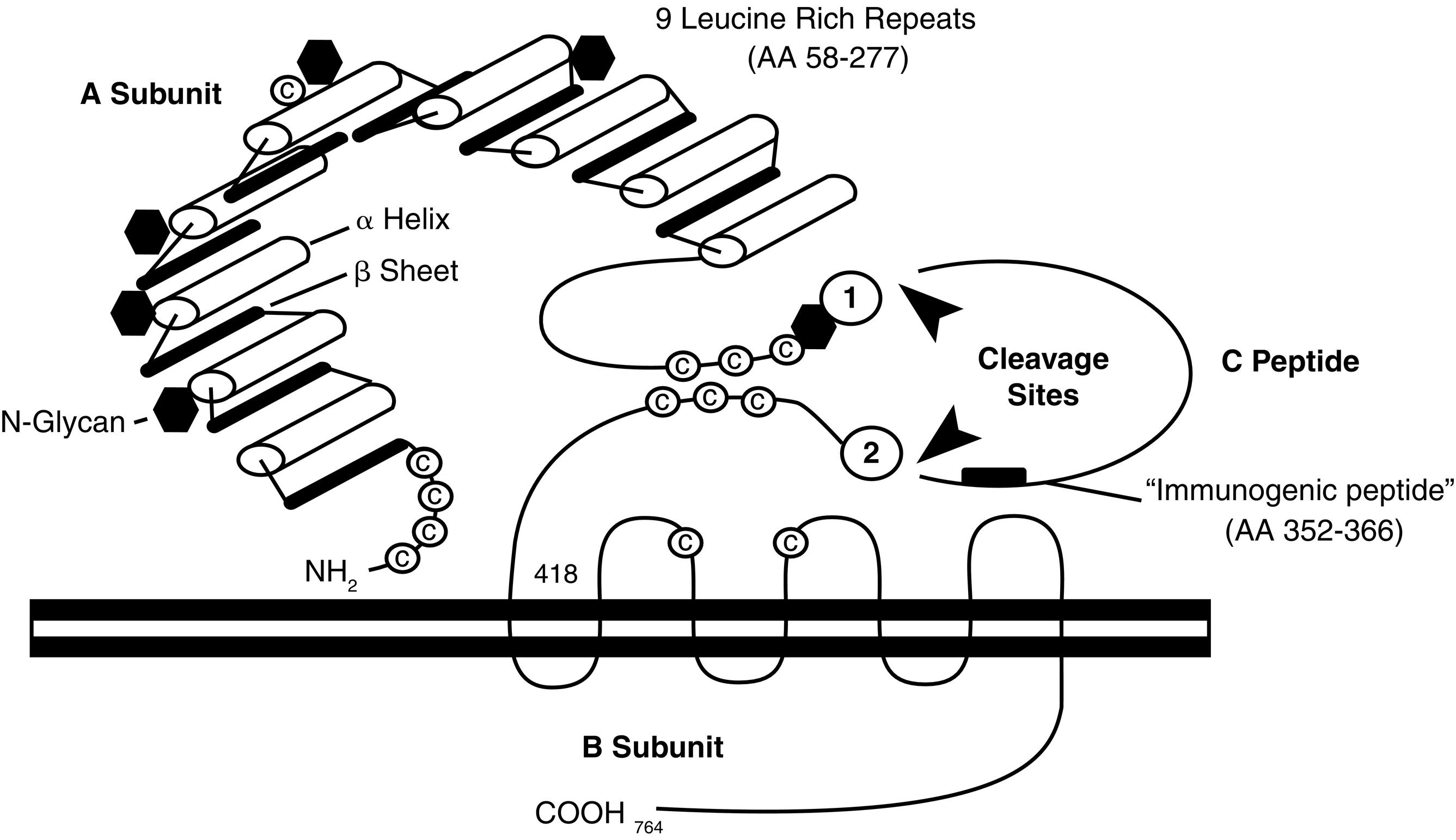
Missense mutations of the TSH receptor gene, leading to replacement of Ser-281 near the carboxy-terminus of the A subunit, with Ile, Thr, or Asn, result in a constitutively active TSH receptor that may cause intrauterine or congenital hyperthyroidism, or toxic adenomas. Activating somatic mutations that cause toxic adenomas have also been found in different transmembrane domains of the TSH receptor. More specifically, clusters of mutations are located in the i3 and TM6 regions—found to be involved with signal transduction in all glycoprotein hormone receptors. The prevalence of activating mutations of the TSH receptor in toxic adenomas has been estimated to range from 2.5% in Japan to 86% in Brazil.
Activating somatic mutations of the TSH receptor have also been found in multinodular goiters. Interestingly, different activating mutations have been found in separate nodules in the same individual. Some well-differentiated thyroid carcinomas have activating mutations of the TSH receptor. Somatic activating mutations of the GNAS gene encoding Gα s have also been found in some toxic adenomas and differentiated thyroid carcinomas. Activating germline mutations of the TSH receptor can cause sporadic or autosomal dominant inherited nonautoimmune hyperthyroidism that presents in utero, during infancy, during childhood, and in some cases in adulthood. These mutations have been found in the amino-terminal extracellular and transmembrane domains.
Patients with heterozygous mutations that lead to constitutively active TSH receptors typically develop hyperthyroidism. In contrast, biallelic loss of function mutations in the TSH receptor genes cause hypothyroidism. Most known loss-of-function TSH receptor mutations are located in the amino-terminal extracellular domain. A spontaneous p.Asp410Asn substitution, near the carboxy-terminus of the C peptide, results in a TSH receptor, with normal ligand binding affinity and impaired Gα s -mediated signal transduction. Patients who are homozygous for this mutation present with compensated hypothyroidism.
Patients homozygous or compound heterozygous for loss-of-function mutations of the TSH receptor present with the syndrome of resistance to TSH (RTSH). Loss-of-function mutations of the TSH receptor that cause RTSH have been identified in the amino-terminal extracellular domain, TM4, TM6, i2, e1, and e3. Clinical severity of RTSH may range from a euthyroid state accompanied by elevated TSH levels (fully compensated RTSH), to mild hypothyroidism unaccompanied by a goiter (partially compensated hypothyroidism), to congenital thyroid hypoplasia accompanied by profound hypothyroidism (uncompensated RTSH). In patients with uncompensated RTSH, a small bilobar thyroid gland is located at the normal site. Loss-of-function mutations of the TSH receptor are a rare cause congenital hypothyroidism more common in Japan and Taiwan (≤ 7% of children), where p.R450H is particularly frequent. Because expression of the sodium-iodide symporter is TSH dependent, thyroid gland uptake of iodine and 99m pertechnetate is diminished or absent in patients with RTSH. In rare cases, iodine uptake is high-normal. These compound heterozygous mutations of the TSH receptor had some Gα s activity and no G q activity, suggesting that iodine uptake is solely controlled by Gα s activity and not G q activity. Some families have been found to have an autosomal-dominant form of RTSH that is not caused by a mutation of the TSH receptor.
Because of its structural similarity with TSH, at very high concentrations, HCG can activate the TSH receptor. During pregnancy, HCG activation of TSH receptors leads to elevation in thyroid hormones after the ninth week of gestation—and decreases in TSH levels between the 9th and 12 th weeks of gestation. This phenomenon does not usually result in maternal hyperthyroidism (gestational thyrotoxicosis). However, when HCG levels are abnormally elevated because of gestational trophoblastic disease caused by a molar pregnancy or choriocarcinoma, hyperthyroidism may occur. The prevalence of thyrotoxicosis in gestational trophoblastic disease correlates with HCG levels. In one study of 196 patients treated with chemotherapy for gestational trophoblastic neoplasia, the prevalence of thyrotoxicosis was 7%. Biochemical thyrotoxicosis only occurred in patients with HCG levels > 10 5 and clinical thyrotoxicosis only occurred in patients with HCG levels greater than 10 6 . Serum TSH is consistently suppressed when HCG levels are above 4 × 10 5 mIU/mL.
A mother and daughter were identified with recurrent gestational hyperthyroidism and normal serum HCG levels. These individuals were found to be heterozygous for a missense mutation in the TSH receptor gene, resulting in a p.Lys183Arg substitution in the extracellular domain of the receptor. It is believed that this substitution increases sensitivity of the receptor to activation by HCG, causing gestational hyperthyroidism.
The GnRH receptor gene is located on 4q13 and includes three exons. Unlike glycoprotein hormone receptors, GnRH receptors lack an intracellular carboxy-terminal domain. In contrast to most GPCRs, the GnRH receptor is coupled to G q /G 11 and hence ligand-binding leads to stimulation of phospholipase C and not adenylyl cyclase. Phospholipase C cleaves phosphatidylinositol-4,5-diphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and diacylglycerol, leading to increased protein kinase C activity.
Some patients with IHH are homozygous or compound heterozygous for loss-of-function mutations in the GnRH receptor gene. Unlike patients with Kallmann syndrome (KS), they have a normal sense of smell. GnRH receptor mutations were found in approximately 5% of patients with normosmic congenital hypogonadotropic hypogonadism. GnRH receptor mutations that cause IHH result in decreased binding of GnRH or impaired GnRH receptor signal transduction, or decreased GnRH receptor cell membrane expression because of misrouting of GnRH receptor oligomers from the ER. Some mutations, including p.E90K, p.L266R, and p.S168R, that cause misfolding and retention, within the ER, exhibit a dominant negative effect because of retention of wild-type receptors.
Female patients with mutations that partially compromise GnRH receptor function may present with primary amenorrhea and infertility, associated with a normal breast development, normal or small uterus, and small ovaries with immature follicles. Males with the same mutations may present with incomplete hypogonadotropic hypogonadism (characterized by a delayed and incomplete puberty) or with complete hypogonadotropic hypogonadism (characterized by absent puberty).
Some patients with IHH caused by mutated GnRH receptors have partial or normal gonadotropin responses to exogenous GnRH. However, decreased amplitude in the pulsatile LH secretion can be observed in these patients. Females with a partial or normal gonadotropin response to exogenous GnRH are more likely than nonresponders to become fertile in response to pulsatile exogenous GnRH.
Activating mutations of the GnRH receptor have not been described in the germline or in pituitary adenomas.
Like the GnRH receptor, TRH receptor activation leads to increased phospholipase C activity. To date, only inactivating mutations that cause endocrine dysfunction have been reported for the TRH receptor. One patient was identified with central hypothyroidism caused by mutated TRH receptors. He presented during the ninth year of life with short stature (− 2.6 SD), accompanied by a delayed bone age (− 4.1 SD), a low plasma thyroxine level, and a normal plasma TSH level. Exogenous TRH did not induce an increase in plasma TSH and prolactin levels. He was found to be compound heterozygous for TRH receptor gene mutations, resulting in receptors that failed to bind TRH or induce IP3 production. Another family was identified with complete resistance to TRH because of a nonsense mutation in the TRHR (p.R17X) producing a TRH receptor that lacked the entire transmembrane domain. The proband was homozygous and presented with short stature, growth failure, and fatigue at age 11 years. He had a low free T 4 , with a low-normal TSH. TRH stimulation testing failed to stimulate TSH or prolactin. Surprisingly his 33-year-old sister who was also homozygous had escaped detection despite two normal pregnancies brought to term. She had no signs or symptoms of hypothyroidism but exhibited thyroid function tests similar to the proband. She breastfed normally. Both the proband and his sister had normal cognitive function. This report suggested that the TRH receptor is not essential for normal cognitive function or female fertility and lactation. The mouse model corroborates these findings.
At the time a new GPCR is discovered, the ligand for the newly discovered receptor is often unknown. Thus until a specific ligand is discovered, these GPCRs are known as orphan receptors . According to the Human Genome Organization (HUGO) Gene Nomenclature Committee, these G protein–coupled orphan receptors should be named alphanumerically GPR followed by a number, until their ligand is known. Once a specific ligand is identified, a more specific name is given the receptor.
The ligands for GPR40 were unknown when the receptor was first discovered. The HUGO Gene Nomenclature Committee changed the name of the receptor to free fatty acid receptor 1 (FFAR1) when the ligands were identified as medium- and long-chain fatty acids. With rare exceptions that are clearly identified, this chapter follows HUGO Gene Nomenclature Committee recommendations (see www.gene.ucl.ac.uk/nomenclature/index.html for more information on receptor nomenclature).
FFAR1 is one of several GRCRs for lipid mediators. Lipid mediators are intercellular lipid messengers that include sphingosine 1-phosphate, sphingosylphosphorylcholine, dioleoyl phosphatidic acid, lysophosphatidic acid, eicosatetraenoic acid, bile acids, and free fatty acids. FFAR1 is activated by medium- and long-chain fatty acids, whereas FFAR2 (formerly known as GPR43 ) and FFAR3 (formerly known as GPR41 ) are activated by shorter-chain fatty acids. There is now evidence that FFAR1 activation by medium- and long-chain fatty acids has endocrine implications. FFAR1 is expressed in human pancreatic β-islet cells. FFAR1 is involved in cholecystokinin secretion from I cells in response to fatty acids. It has also been implicated in fatty acid stimulated GLP-1 and GIP secretion from L and K cells. GPR120 is expressed in enteroendocrine cells and has a physiologic role in GLP-1 secretion. FFAR2 and FFAR3 are expressed in adipose tissue and FFAR3 has been implicated in leptin production.
Fatty acid–induced stimulation of FFAR1 in β-islet cells leads to activation of the Gα q -phospholipase C second-messenger pathway, which in turn leads to release of calcium from the ER that augments insulin-mediated increases in intracellular calcium concentrations because of glucose-induced activation of voltage-gated calcium channels. Because an increased intracellular calcium concentration induces insulin release, FFAR1-mediated augmentation of glucose-mediated increases in the intracellular calcium concentration leads to amplification of glucose-stimulated insulin release.
A variant in FFAR1 (p.Gly180Ser), found in a Sicilian population, resulted in obesity, impaired glucose tolerance, and lipid stimulated insulin secretion. Two other variants, p.Arg211His and p.Asp175Asn, are not associated with alterations in insulin release. TAK-875, an FFAR1 agonist, was shown to reduce hemoglobin A1c in patients with type 2 diabetes, in a phase 2 clinical trial. Wild-type mice placed on an 8-week high-fat diet develop glucose intolerance, insulin resistance, hypertriglyceridemia, and hepatic steatosis—whereas FFAR1 knockout mice on the same diet do not develop these conditions. The clinical relevance for patients is not yet clear. However, an Arg211His polymorphism in the FFAR1 gene may explain some of the variation in insulin secretory capacity found in Japanese men: Arg/Arg homozygotes had lower serum insulin levels, homeostasis model of insulin resistance, and homeostasis model of beta-cell function than His/His homozygotes.
Studies in animal models suggests that Kiss1-expressing neurons in the hypothalamus modulate GnRH expressing neurons to initiate puberty and modulate sex steroid feedback on GnRH release. Homozygous inactivating mutations in the gene encoding the KISS1 receptor (GPR54) were initially described in French and Saudi Arabian patients with IHH; in both cases the affected subjects came from consanguineous families. The Saudi patients carried a p.Leu148Ser mutation, whereas the French patients carried a 155bp deletion. Leu148 is highly conserved among class A GPCRs. The mutation does not affect expression, ligand binding, or association with G s , but impairs ligand-induced catalytic activation of G s . At the same time, an African American patient with IHH was described who was compound heterozygous for inactivating GPR54 mutations. Since publication of these initial reports, additional patients have been described. A boy with a Jamaican father and a Turkish-Cypriot mother, and with cryptorchidism and micropenis at birth, and undetectable LH and FSH levels at 2 months of age, was found to have compound heterozygous GPR54 mutations. Another missense mutation (p.Leu102Pro) that exhibits complete inactivation of GPR54 signaling has been identified. Surprisingly, patients with this mutation exhibited spontaneous pulsatile LH and FSH secretion with normal frequency, and a blunted amplitude and family members had partial pubertal development. Biallelic loss of function mutations in GPR54 are a rare cause of normosmic IHH. A study of genetic causes of normosmic congenital hypogonadotropic hypogonadism showed that KISS1R mutations accounted for 2% of the variants.
Unlike patients with KS, but similar to patients with GnRH mutations, patients with GPR54 mutations have an intact sense of smell. In contrast to patients with IHH caused by GnRH mutations, patients with GPR54 mutations increase serum gonadotropin levels in response to exogenous GnRH.
Ligands for GPR54 derive from a single precursor protein, kisspeptin-1. The longest derivative protein that acts as a ligand for GPR54 is metastin, so called because it is a metastasis suppressor gene in melanoma cells. Metastin consists of kisspeptin-1 69-121. However, shorter carboxy-terminal peptides derived from kisspeptin-1 bind and activate GPR54. Administration of metastin to adult male volunteers increases LH, FSH, and testosterone levels.
An activating mutation in GPR54 was identified in a patient with central precocious puberty. The adopted girl was found to have an Arg386Pro mutation, which led to prolonged activation of signaling in response to kisspeptin. Mutational analysis of 28 subjects with idiopathic central precocious puberty failed to find any variants in KISS1 or KISS1R. A larger study showed an association between several variants and central precocious puberty in Korean girls.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here