Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter covers the rare tumors of childhood. No attempt has been made to consider the rarest of the rare tumors, and the focus is on the more common of the rare pediatric tumors that do not fall into other categories covered in this text. The chapter is generally organized from top to bottom, with cancers of the head and neck at the beginning and cancers of the gastrointestinal tract discussed later in the chapter. The tumors discussed afflict mostly adults, and some are actually common cancers in older patients. Many are carcinomas and are more familiar to the oncologist who treats adults. These cancers tend to increase in incidence as children reach adolescence.
Evaluation of Surveillance, Epidemiology, and End Results (SEER) data has shown that 9.2% of all childhood cancers are carcinoma, and 75% of these cases are diagnosed in children between the ages of 15 and 19 years. Of the cases in the study, 36% were thyroid carcinomas and 31% were melanomas. The next most common single carcinoma in children younger than age 19 years was nasopharyngeal carcinoma, which comprised 4.5% of these cases. Thus this chapter emphasizes discussion of melanoma, thyroid carcinoma, and nasopharyngeal carcinoma and reviews other rare tumors of childhood.
Oropharyngeal cancer of squamous cell histology in children remains uncommon. Most of the children who develop epithelial cancer of the nose, ears, and throat are diagnosed with nasopharyngeal carcinoma, which is considered separately in this chapter. The use of smokeless tobacco, cigarette smoking, and alcohol consumption are well established risk factors for the development of squamous cell carcinoma in adults, and the continued use of these substances in Western children warrants monitoring for the development of adult types of squamous cell carcinoma of the oropharynx in children. Although such habits ultimately lead to increased risk for a variety of cancers and early death from heart disease, it remains a theoretical concern that the use of smokeless tobacco by children, particularly by male adolescents, could increase the incidence of these tumors during adolescence and young adulthood.
Duration of smoking increases risk for tobacco-related morbidity and mortality, including several cancers, and significant public health efforts have been aimed at reducing adolescent initiation of tobacco use. Educational interventions have been shown to decrease the prevalence of cigarette and smokeless tobacco use in Massachusetts youth, but restrictions on tobacco sales have a mixed record, because even a complete ban on sales of smokeless tobacco in Finland did not lead to a decrease in its use by adolescents. Conversely, restrictions on smoking in public places have been demonstrated to decrease overall tobacco consumption (e.g., by up to 8% in Italy). Overall trends of cigarette smoking among adolescents have decreased steadily since the mid-1990s. Rates of smoking for white, non-Hispanic twelfth grade students are significantly higher in comparison to Hispanic and African-American students. Although smokeless tobacco use also declined during the late 1990s and early 2000s, recent data from Monitoring the Future and the National Youth Tobacco Survey have demonstrated increased use in recent years, with concurrent decreased reporting of perceived risk. These changes in trends are synchronous with aggressive marketing campaigns by tobacco companies of novel delivery forms of smokeless tobacco such as “snus” and dissolvable tobacco.
The increasing prevalence of human papillomavirus (HPV) infection in adolescents and young adults has raised new concerns about the development of HPV-associated oral squamous-cell carcinoma in younger patients. HPV has recently been detected in approximately 26% of head and neck squamous-cell carcinomas; HPV16 was highly prevalent among these tumors. Trends in adults show an increasing incidence in oral squamous-cell carcinomas despite declining use of tobacco in the United States because of the increasing prevalence of oral HPV infection. A recent study showed 1.7% of 14- to 17-year-old adolescents and 5.6% of 18- to 24-year-old adults had oral HPV; male sex, number of sexual partners, tobacco use, open-mouth kissing, and oral sex have been associated with infection. HPV is most often transmitted in adolescence and young adulthood; a quadrivalent vaccine for HPV that includes strains likely to cause oral cancers is now available and is recommended for both boys and girls.
Few existing studies report outcomes of pediatric squamous-cell carcinomas. A population-based study of 54 pediatric patients with oral-cavity squamous-cell carcinoma had a 5-year disease-specific survival rate of 75%. It is unlikely that many pediatric oncologists will be confronted by adult-type squamous-cell carcinoma of the head and neck, but it is likely that pediatric oncologists will be confronted with tobacco use in young adult and adolescent survivors of pediatric cancer. A St. Jude Children's Research Hospital study has found that 29% of surviving children treated on an acute myelogenous leukemia (AML) protocol were found to be cigarette smokers at follow-up examinations. Although survivors of childhood cancer may not be seen often in the pediatric oncology clinic, tobacco use and prevention still merit mention during such visits.
Mucoepidermoid carcinoma of the salivary glands is still another type of head and neck carcinoma from which children suffer and is commonly associated with prior therapy for cancer, including radiation. A study of childhood cancer survivors identified 23 cases of secondary head and neck malignancies; of the 14 secondary cancers that arose in the parotid gland, 10 were identified as mucoepidermoid carcinoma. Most children are cured with surgery and sometimes surgery and radiation therapy. Any mass lesion or presenting complaint potentially related to a mass lesion in the salivary glands, particularly if found in a previous radiation field, should prompt a thorough evaluation with suspicion for this tumor.
Nasopharyngeal carcinoma is a cancer of the epithelial lining of the nasopharynx. It shows varying degrees of differentiation but is a type of squamous-cell carcinoma. Most cases have undifferentiated histology; the undifferentiated type is associated with Epstein-Barr virus (EBV) infection of the nasopharyngeal epithelium, and virus is found in the tumor cells. It is the most common type of epithelial tumor of the head and neck in children, accounting for up to 50% of head and neck tumors in children. Incidence varies widely among populations and in relationship to exposure to EBV, environmental exposures such as preserved or salted fish, and geographic location. Rates in China are particularly high, and ethnic Chinese born in China who move to the West have a higher incidence of the disease than ethnic Chinese who are born in Western countries. In the United States, this cancer is more common in the South and more prevalent in African-American children, who have incidence rates of the disease that approach those of the Chinese. These findings suggest interplay of genetic, viral, and environmental factors in the carcinogenesis of nasopharyngeal carcinoma.
Analysis of incidence and survival rates from SEER data has demonstrated improved survival for Asian-American patients compared to Caucasian and African-American patients. However, nasopharyngeal carcinoma-specific mortality is worse for adults than for children and adolescents, and survival rates in the adolescent and young-adult age ranges tend to be similar among races.
Nasopharyngeal carcinoma is more common in Asia and is strongly associated with Chinese ethnic origin within Asia, with some parts of China having incidence rates from 15 to 30 cases per 100,000 population. There is also a higher incidence of the cancer in Turkey. A bimodal age distribution has been suggested in nonendemic areas, with an early peak in adolescents age 15 to 19 years. The actual cancer cells are infected by EBV, suggesting a possible role for immunosuppression in the pathogenesis of the tumor, or at least that immune augmentation could help prevent recurrence of nasopharyngeal carcinoma. In Greenland and other regions with similar native populations, high rates of nasopharyngeal carcinoma have been found. Additionally, high rates for other cancers associated with EBV infection, such as various forms of uterine cancer, are more common in families with EBV and nasopharyngeal carcinoma. Population-based screening based on EBV serology in endemic areas can help detect nasopharyngeal carcinoma early. However, this would be impractical in nonendemic areas.
The typical presenting signs and symptoms of nasopharyngeal carcinoma include evidence of tumor mass in the nasopharynx such as epistaxis, nasal obstruction and discharge, Eustachian tube dysfunction, palsy of the fifth and sixth nerves relating to skull-based extension, and neck masses. In children with epidemiologic factors making this disease more likely, clinicians must maintain a high index of suspicion for nasopharyngeal carcinoma and obtain imaging studies and consultation as necessary for these signs and symptoms. However, most children diagnosed with nasopharyngeal carcinoma in the United States are unlikely to have obvious epidemiologic associations for the cancer, and even in the United States EBV infection in childhood is common enough that screening based on evidence of EBV infection would be impractical.
Proper evaluation of patients suspected of having nasopharyngeal carcinoma includes computed tomography (CT) scan and/or magnetic resonance imaging (MRI) of the head and biopsy of appropriate tissue for diagnosis. A clinical examination is key to evaluate for any apparent lymph node spread. Although systemic evaluation, including laboratory testing, positron emission tomography (PET) scanning, whole-body anatomic scanning, and bone marrow biopsy can be considered, they have not been shown to improve staging accuracy in patients with nasopharyngeal carcinoma.
All nasopharyngeal carcinoma cases are considered squamous-cell cancers, but the World Health Organization (WHO) classification of nasopharyngeal carcinoma defines type 1 histology as keratinizing squamous-cell carcinoma, a form of cancer more common in adults, versus the type 2 histology, which does not show keratinization on light microscopy but does show some differentiation. Type 3 histology is nonkeratinized and nondifferentiated and is associated with endemic nasopharyngeal carcinoma. The majority of children with nasopharyngeal carcinoma have a presentation of undifferentiated or WHO type 2 or 3 histology.
The American Joint Commission on Cancer (AJCC) tumor-nodes-metastasis (TNM) staging system (2010) is considered the most relevant for patients in the Western countries. The WHO staging system has fewer local (T) staging categories but seems to be adequate for patients in endemic areas. The latest AJCC staging system is shown in Tables 65-1 and 65-2 .
| Primary Tumor (T) | Regional Lymph Nodes (N) | Distant Metastasis (M) |
|---|---|---|
|
|
|
* The distribution and prognostic impact of regional lymph-node spread from nasopharynx cancer, particularly of the undifferentiated type, are different from those of other head and neck mucosal cancers and justify the use of a different regional lymph-node classification scheme.
† Parapharyngeal extension denotes posterolateral infiltration of tumor.
‡ Midline nodes are considered ipsilateral nodes.
§ Supraclavicular zone or fossa is relevant to the staging of nasopharyngeal carcinoma and is the triangular region originally described by Ho. It is defined by three points: 1) the superior margin of the sternal end of the clavicle; 2) the superior margin of the lateral end of the clavicle; and 3) the point where the neck meets the shoulder. Note that this would include caudal portions of levels IV and VB. All cases with lymph nodes (whole or part) in the fossa are considered N3b.
| Stage | Groupings |
|---|---|
| 0 | Tis, N0, M0 |
| I | T1, N0, M0 |
| II | T1, N1, M0 |
| T2, N0, M0 | |
| T2, N1, M0 | |
| III | T1, N2, M0 |
| T2, N2, M0 | |
| T3, N0, M0 | |
| T3, N1, M0 | |
| T3, N2, M0 | |
| IVA | T4, N0, M0 |
| T4, N1, M0 | |
| T4, N2, M0 | |
| IVB | Any T, N3, M0 |
| IVC | Any T, any N, M1 |
* Results of radiation therapy for nasopharyngeal carcinoma (locoregional control and survival) are usually reported by T stage and N stage separately or by specific T and N subgroupings rather than by numerical stages I to IV. Outcome also depends on various biologic and technical factors related to treatment.
Although nasopharyngeal carcinoma can be treated by radiation therapy only, it has been shown to be sensitive to chemotherapy in adults and children. Using chemotherapy may ultimately allow for curative radiotherapy at lower doses with less risk for late effects from head and neck radiotherapy. A Pediatric Oncology Group (POG) study used four cycles of preirradiation chemotherapy with methotrexate with leucovorin, 5-fluorouracil (5-FU), and cisplatin to treat children with AJCC stage III or IV nasopharyngeal carcinoma. Children with stage I or II disease were treated with irradiation only. The 4-year event-free and overall survival rates were 77% ± 12% and 75% ± 12%, respectively. Other agents used to treat nasopharyngeal carcinoma include bleomycin and doxorubicin. More recently, the use of neoadjuvant chemotherapy with concomitant chemoradiation has demonstrated improved outcomes. Xerostomia and dental problems are common adverse effects of treatment. Other long-term morbidities include sensorineural hearing loss, endocrinopathies, trismus, and recurrent infections. Sensorineural hearing loss incidence has been noted to increase with the use of concomitant chemoradiotherapy. Although both children and adults with nasopharyngeal carcinoma are at risk of developing secondary malignancies after treatment, the risk for children is 4.3 times the general population, compared with 1.4 for adults. Current treatment strategies are investigating the use of intensity-modulated radiation therapy (IMRT) and amifostine to preserve salivary function and to decrease acute and chronic sequelae of therapy.
This section summarizes the clinical-pathologic and therapeutic aspects of differentiated thyroid carcinoma in children and adolescents. Medullary thyroid carcinoma (MTC) and associated multiple endocrine neoplasia (MEN) syndromes are not discussed.
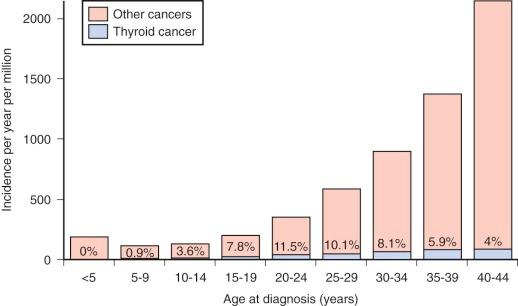
The prevalence of palpable thyroid nodules in children is lower than that seen in adults (1.5% vs. 5%); despite this, thyroid nodules in children are five times more likely to be malignant than those in adults (25% vs. 5%). Of the estimated 56,000 cases of thyroid cancer diagnosed in 2012, only 1.8% occur in patients under 20 years of age. Furthermore, thyroid carcinoma accounts for 3% of all cancers in patients younger than 20 years, and 75% of these occur in patients between the ages of 15 and 19 years ( Fig. 65-1 ). Epidemiological studies suggest that the incidence of thyroid carcinoma has steadily increased over time and that it affects both low and high socioeconomic counties that were surveyed by the SEER registries. In pediatrics, an increasing rate has been documented in the SEER registry for the years 1973 through 2007, with the rates being higher in whites, those aged between 15 and 19 years, girls, and in registries with predominantly white or Hispanic populations.
Radioactive exposure to the neck is a well-established risk factor for the development of thyroid carcinoma. Survivors of childhood cancer have up to a 14-fold excess risk of developing thyroid cancer if their thyroid gland received 20 to 25 Gy, and this malignancy accounts for 10% of all subsequent neoplasm in the survivor population. A risk-prediction model for subsequent primary thyroid carcinoma in survivors of childhood cancer has been developed. This model incorporates several variables including radiation and therapy information, age, sex, and a history of a thyroid nodule; the software to compute risk projections can be downloaded at http://dceg.cancer.gov/tools/riskassessment .
Exposure to radioactive iodine and cesium isotopes after the Chernobyl accident resulted in a markedly increased rate of thyroid cancer in children; the estimated relative excess risk was estimated to be 5.6 per Gy of exposure in subjects with estimated doses of less than 1 Gy. Other risk factors associated with the development of pediatric thyroid cancer include various genetic syndromes such as familial adenomatous polyposis (FAP), Cowden disease, Carney syndrome, and immune thyroid disorders such as Hashimoto thyroiditis.
More than 90% of pediatric thyroid carcinomas have well-differentiated histology; papillary histology accounts for over 70% of cases, and follicular histology represents about 20% of cases. Medullary thyroid cancer is seen in fewer than 10% of pediatric cases of thyroid cancer and can be found in association with MEN syndrome types 2A and 2B. The distribution of histologies varies slightly in cases with known radiation carcinogenesis; in a study of 740 children with thyroid cancer, of whom 92% were exposed to radiation at Chernobyl, 90% of cases were papillary, about 5% were follicular, and 0.4% were medullary.
RET-PTC rearrangements have been documented in 50% to 60% of pediatric cases of papillary thyroid carcinoma and in up to 70% of radiation-induced pediatric papillary thyroid carcinoma. NTRK1 rearrangements and AKAP9-BRAF fusions have also been described in a small number of radiation-induced papillary tumors. Unlike in adults, BRAF mutations are rarely seen in pediatric papillary tumors. Follicular tumors are characterized by RAS mutations and peroxisome proliferator–activated receptor γ (PPARγ) rearrangements, whereas MEN-associated medullary thyroid carcinomas are characterized by germline-activating mutations of RET.
Most patients with thyroid carcinoma are older than 10 years and initially have an asymptomatic palpable thyroid nodule. Younger age (<15 years), large fixed nodules, palpable adenopathy, history of radiation exposure, and diseases associated with an increased incidence of thyroid carcinoma such as FAP, Carney complex, Cowden syndrome, MEN types 2A or 2B, pheochromocytoma, and hyperparathyroidism should raise the suspicion for the presence of malignancy.
Children and adolescents with thyroid carcinoma are more likely to have nodal regional involvement, extrathyroid disease, and pulmonary metastases and are less likely to die from their disease than adults. Ultrasound is a useful diagnostic technique, and most investigators prefer this method for evaluating thyroid nodules ( Fig. 65-2 ). Initial evaluation of a thyroid nodule should include measurements of serum thyroid-stimulating hormone (TSH), antithyroid peroxidase, antithyroid globulin antibodies, and a neck ultrasound that evaluates the contralateral thyroid lobe and cervical nodes. Screening for MTC using plasma calcitonin may not be cost-effective in children given its low prevalence in the pediatric population. If the TSH level is low, raising the possibility of a hyperfunctional nodule, a thyroid technetium or iodine scan should be performed. Because of the higher likelihood of malignancy in children, it is recommended that all hyperfunctioning nodules be surgically removed in children, with further evaluation and treatment should the pathology be consistent with malignant disease. Fine-needle aspiration biopsy is the most cost-effective and expeditious preoperative procedure to help distinguish benign from malignant thyroid nodules; a meta-analysis of 12 pediatric studies found that this procedure has a sensitivity of 94% and a specificity of 81% (see Fig. 65-2 ). Preoperative staging should include a chest x-ray and a comprehensive neck ultrasound to evaluate the whole thyroid and lymph nodes.
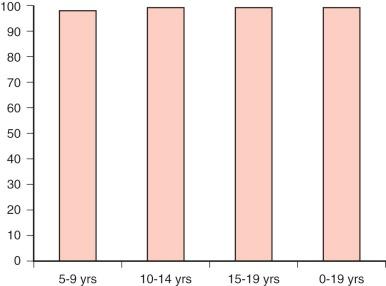
Factors associated with an increased risk of progression include capsular invasion, soft-tissue invasion, positive margins of resection, age younger than 10 to 15 years, male sex, nonpapillary histology, distant metastases, and residual disease after surgery. Survival for children with differentiated thyroid carcinoma approaches 100% ( Fig. 65-3 ).
Surgery is the mainstay of therapy for pediatric and adolescent thyroid carcinoma, and this procedure should be performed by a highly experienced thyroid surgeon. Total thyroidectomy is the initial treatment of choice. especially in younger patients (<10 years) or those with a history of radiation exposure or a family history of thyroid cancer. Many surgeons also advocate the upfront use of this procedure because pediatric thyroid carcinoma is associated with a high incidence of multifocal and metastatic disease, and follow-up screening with serum thyroglobulin is most useful after total thyroidectomy. Lobectomy and isthmectomy can be considered in patients who have a low risk for recurrence such as adolescents with localized small (<1 cm) lesions.
Upfront nodal dissection has been shown to decrease the risk of surgical reintervention and nodal recurrence in children with thyroid carcinoma. Current recommendations favor the use of central compartment neck dissection, reserving less extensive surgeries for patients with small tumors and no evidence of nodal involvement by ultrasound (see Fig 65-2 ).
In adults, the use of radioactive iodine treatment to ablate residual thyroid or iodine-avid metastatic disease decreases the risk of distant metastases. The goals for the use of this therapy in children includes ablation locoregional recurrence, elimination of residual sources of thyroglobulin production, and high remnant uptake that can obscure the presence of metastases and accurate detection of pulmonary metastatic disease. The use of this therapy in children has been associated with a reduced recurrence risk in the thyroid and nodes independent of the surgical approach. However, a recent study by Hay failed to show a significant difference in local and distant recurrence when this modality was used, and 73% of patients who died from a subsequent malignant neoplasm had received therapeutic irradiation postoperatively. Radioactive iodine treatment should be reserved for children at high risk for disease recurrence, and a suggested measured approach for the postoperative use of this agent is shown in Figure 65-2 . When radioactive treatment is prescribed, TSH should be greater than 30 µIU/mL. The dosing of this agent is not well established in children, and various centers follow the American Thyroid Association (ATA) guidelines for adults ( http://thyroidguidelines.net/revised/differentiated ). A posttherapy thyroid scan is usually obtained about 1 week after treatment, and this may identify additional sites of disease that were not apparent initially. Because of the risk of regrowth after TSH exposure, thyroid hormone suppression to achieve serum TSH levels between 0.1 and 0.5 µIU/mL is recommended in all pediatric patients with differentiated thyroid cancer.
A variety of “targeted” therapies have been developed for iodine-refractory differentiated thyroid carcinoma, and many of these agents have been approved for adult use. Most of these therapies target RET and the vascular endothelial growth factor (VEGF) receptors but some also target platelet-derived growth factor β receptors (PDGFRβs), FGFR, MET, and endothelial growth factor (EGF) receptors. Some of these agents include sorafenib, axitinib, motesanib, sunitinib, pazopanib, cabozantinib, and vandetanib. In children sorafenib has been successfully used in a patient with refractory papillary thyroid carcinoma, and in a phase I/II study, vandetanib produced two partial responses in children with MEN2b-associated medullary thyroid carcinoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here