Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
▪ Radiation therapy ▪ X-ray therapy ▪ Radiation treatment
Radiotherapy is an important modality in the treatment of patients with skin malignancies
Radiotherapy may reduce morbidity (as compared to surgery), decrease the risk of recurrent disease, and even be life-saving
Although most patients with skin cancer are adequately treated by modalities other than radiotherapy, there are factors that may favor its recommendation (e.g. comorbidities, location)
Modern radiotherapy has an established role as a definitive, adjuvant, or palliative treatment
Treating benign skin disease with radiotherapy must always be carefully considered in light of the risk of radiation-induced malignancy, especially in younger patients
In dermatology, the primary role of radiotherapy is to treat basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC; see Ch. 108 ) . It is also used to treat less common, but potentially aggressive, cutaneous malignancies and, occasionally, benign diseases. The former include Merkel cell carcinoma, Kaposi sarcoma (AIDS- and non-AIDS-related), angiosarcoma, adnexal carcinoma, lymphoma, and melanoma (in particular lentigo maligna).
While the majority of skin cancers are amenable to excision or other treatment modalities (e.g. electrodesiccation and curettage) which are often more cost-effective, there are clinical settings where radiotherapy is favored. For example, definitive radiotherapy is often recommended when the outcome (functional and/or cosmetic) is likely to be better with radiotherapy than with surgery, especially in the situation where the clinician is constrained by the site or size of the tumor. Likewise, elderly patients with advanced lesions for whom complex surgery is best avoided are also excellent candidates for radiotherapy. The aim of adjuvant radiotherapy is to reduce the risk of recurrence, whereas palliative radiotherapy plays an important role in patients with advanced and/or incurable disease.
X-rays are a powerful form of ionizing electromagnetic radiation and the lethal damage they produce within double-stranded DNA represents the basis for radiation therapy . When X-rays (often referred to as photons) are absorbed by biological matter, an electron may be ejected from an atom with the local release of large amounts of energy. The result is that replicating, i.e. more rapidly dividing, malignant cells undergo a mitotic death. Although normal adjacent tissues maintain mechanisms for repair following sublethal damage, there are limitations as to the dose of ionizing radiation normal tissue can tolerate, beyond which late reactions arise (see below). Enhancement of the difference in the response of malignant cells versus normal tissue is accomplished via delivery of the radiation in small divided doses (referred to as fractions).
In the modern era of radiation oncology, high-energy megavoltage (mV) photons (6–25 mV) are generated by a linear accelerator (LINAC) located within a heavily shielded concrete bunker. One of the advantages of megavoltage radiotherapy is its ability to treat deep-seated internal malignancies with relative skin sparing. However, in general, when treating cutaneous lesions, deep beam penetration and skin sparing are not desirable. The delivery of low-energy kilovoltage (kV) photons (50–300 kVp) by a superficial/orthovoltage machine is preferable, as it avoids both skin sparing and the irradiation of deeper tissues. With low-energy photons, tissue penetration is measured in millimeters to centimeters. Shielding is achieved by applying 3–4 mm of lead either directly onto the skin or by using an internal (e.g. eye or mouth) shield.
The International Unit of radiotherapy is a Gray (Gy) . One Gy is equivalent to 100 cGy or 100 rads (in the older terminology). A fraction of radiotherapy refers to the dose delivered in one treatment. A typical course of radiotherapy for a cutaneous malignancy in an otherwise healthy patient is 50 Gy delivered in 20 daily (Monday through Friday) fractions (2.5 Gy/fraction). Historically, patients were often administered only two or three fractions per week. At the very least, this latter schedule simply protracted the overall treatment period, but in some cases may have resulted in decreased local control. The daily outpatient irradiations are only minutes in duration and treatment regimens usually consist of 10 to 30 weekday visits.
Hypofractionation refers to the delivery of larger doses per fraction (4–7 Gy). However, when the dose per fraction is increased, the total dose delivered is then decreased. For example, commonly prescribed dose fractionation schedules are 45 Gy in 15 (3 Gy) fractions, 40 Gy in 10 (4 Gy) fractions, and 35 Gy in 5 (7 Gy) fractions. Using a 2 Gy per fraction equivalent, this variation in the total dose results when the biological effective dose (BED) needed for eradicating a tumor is calculated . Despite this, the data suggest that, at least for small (≤2 cm) SCCs and BCCs, there is not a marked dose–response, and local control is similar whether a patient receives 40 Gy in 10 fractions (47 Gy BED) or 50 Gy in 20 fractions (52 Gy BED). However, when the tumors are larger or more infiltrative, better local control is achieved with a BED of around 60 Gy. Of note, late effects increase as the total dose and dose per fraction increase and it is these late effects that will impact on cosmesis and late tissue damage (e.g. fibrosis, atrophy, ulceration due to necrosis).
The qualities and “hardness” of a superficial/orthovoltage photon beam are determined by the placement of filters within the radiation beam . In radiation physics, a standard term is the “half value layer” (HVL) which refers to the thickness of a material (often copper [Cu] or aluminum [Al]) that is required to reduce the beam intensity by 50%. The filter serves to remove the softer photon component and improve the penetration quality of the beam. A second concept is half value depth (  ) and here filters are used to achieve radiation with a
) and here filters are used to achieve radiation with a  that corresponds to tumor depth. For each superficial and orthovoltage beam, at least one to two filters should be available.
that corresponds to tumor depth. For each superficial and orthovoltage beam, at least one to two filters should be available.
A typical 100 kVp superficial photon beam with an HVL of 7 mm of aluminum will deliver 100% of prescribed dose to the surface, 85% at 5 mm, and 70% at 10 mm. A 250 kVp orthovoltage beam with an HVL of 2.5 mm of copper will deliver 95% of prescribed dose at 5 mm and 90% at 10 mm. Most superficial lesions (<5 mm depth) will be adequately treated by a 100 kVp beam with an HVL of 6–8 mm aluminum (or similar). Deeper, more invasive lesions (5–10 mm in depth) are adequately encompassed by a 250 kVp orthovoltage beam with an HVL of 2–4 mm copper.
Some clinicians estimate tumor thickness and then choose an appropriate beam energy and HVL that will achieve 90–95% of the prescribed dose at the depth of the tumor. However, this latter approach requires an accurate estimate of tumor thickness.
The photoelectric effect is a phenomenon whereby underlying bone or cartilage receives a higher dose of radiation when low-energy photons are used. This is not the case with electrons (see below) and the latter is used by some clinicians as a justification for the use of electrons when treating lesions in close proximity to bone or cartilage. However, there are no convincing data that the late effects in these tissues are markedly increased when low-energy photons are used instead of electrons .
A linear accelerator can also generate electrons and they are utilized in a variety of treatment settings. Unlike X-rays, electrons are a particulate radiation that carries a negative charge. Low-energy electrons (6–8 MeV [million electron volts]) offer an alternative to low-energy photons when treating cutaneous tumors, but the former do have some disadvantages . For example, electron beams are relatively skin-sparing ( Fig. 139.1 ), and, as a result, a tissue equivalent bolus needs to be placed on the skin surface ( Fig. 139.2 ). Also, utilizing electron beams for small treatment fields (<4 cm 2 ) may result in tumor underdosing at the deepest extent of the tumor.
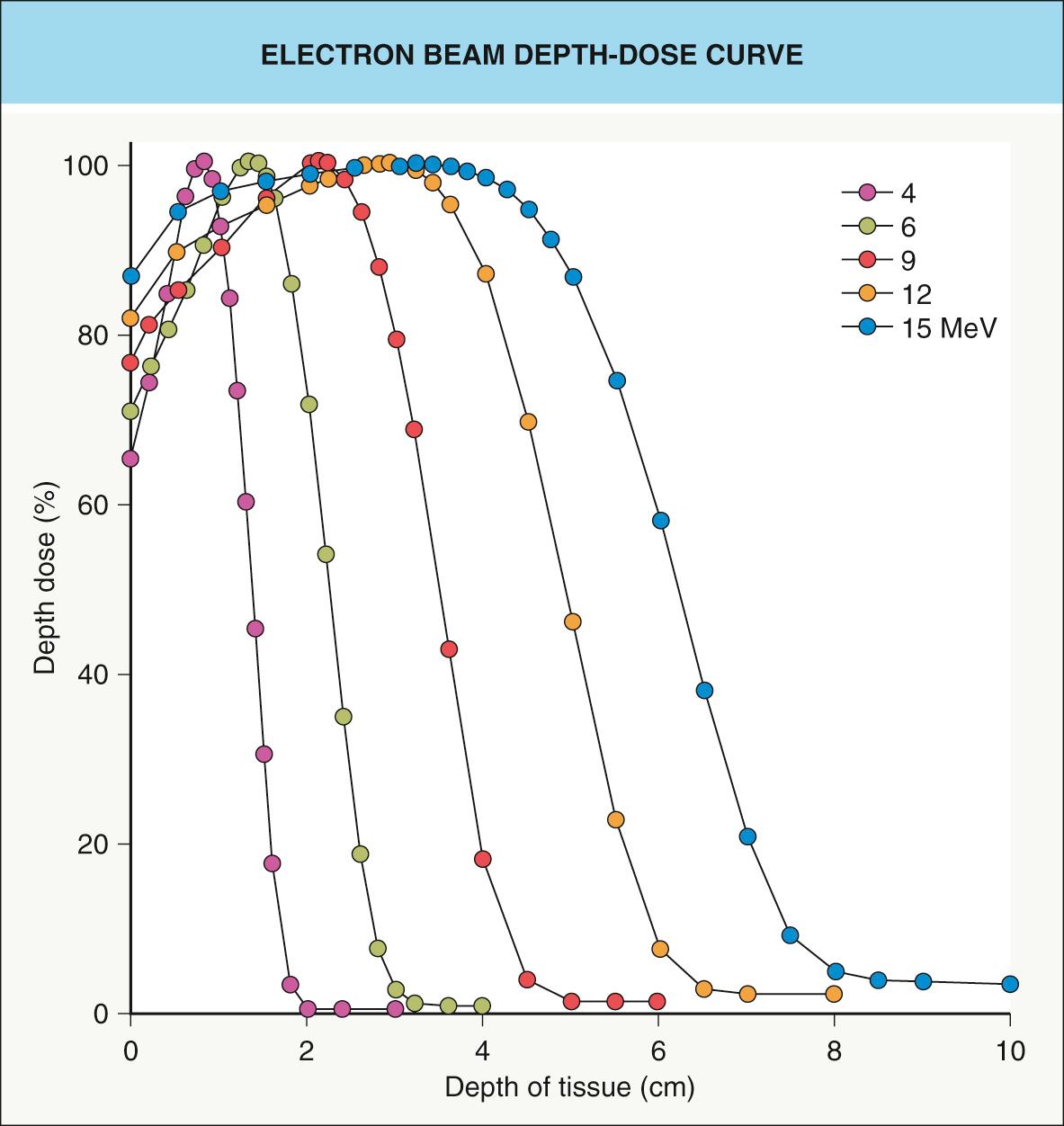
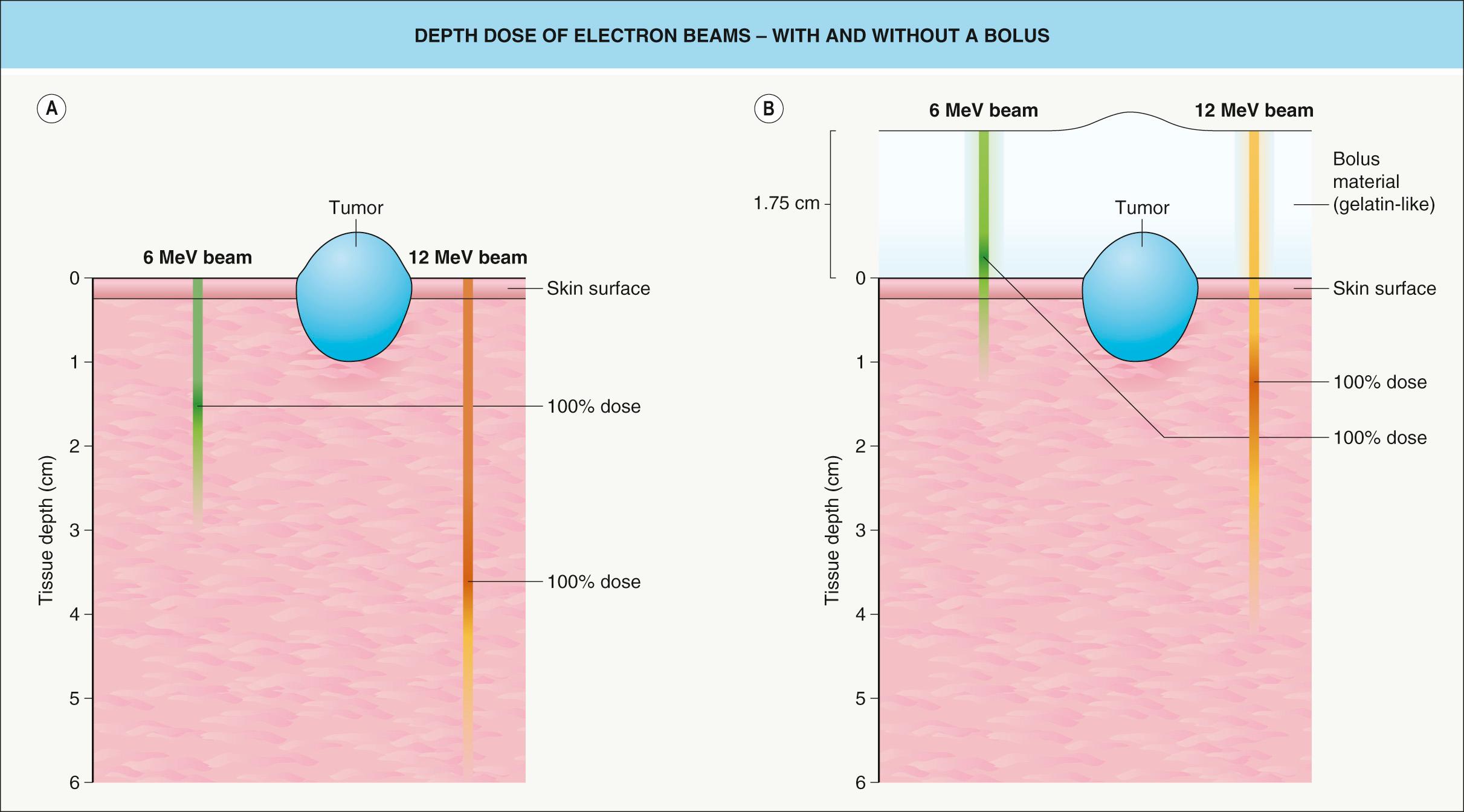
Electrons offer the advantage of a rapid linear decline in dose beyond a well-defined tissue depth that is a function of the selected beam energy (see Fig. 139.1 ). For example, a 6 MeV electron beam provides some of its dose (~70–75%) at the surface, but deposits 100% of the specified dose at a depth of approximately 1 cm. Using a higher-energy beam will result in deposition of a higher percentage of the dose at the skin surface (see Fig. 139.1 ), but with a higher dose to subcutaneous tissues as well as the skin surface (see Fig. 139.2A ). On the other hand, if a tissue equivalent bolus of 1 cm is applied in conjunction with a 6 MeV beam, close to 100% of the desired dose will be obtained at the skin surface (see Fig. 139.2B ). All of the deposition of dose will be brought 1 cm closer to the skin surface, further minimizing the dose to underlying structures.
In addition, tumors treated with electrons require 10–20 mm margins as a consequence of a wider penumbra (dose drop-off at the field edge). Electrons also have a lower relative biological effectiveness (RBE) in comparison to photons, leading some clinicians to recommend a 10–15% increase in total dose to compensate.
In summary, there are data to suggest that local control of skin tumors may not be as good with electrons when compared to low-energy photons. However, other studies suggest no differences and, in part, the discrepancy may reflect an under-appreciation by some clinicians of the intricacies of using small field electrons to treat skin cancers.
Brachytherapy represents radiotherapy that is delivered by the direct application of a radioactive source to involved tissues. In the vast majority of clinical settings, including dermatologic, radioactive molds and implants offer little advantage over the range of energies of external beam photons and electrons currently available. As a result, they are rarely used nowadays to treat BCCs or cutaneous SCCs. Nonetheless, some clinicians have advocated using radioactive molds for areas with poor vascularity and slow rates of healing, e.g. the anterior lower limb or dorsum of the hand .
A newer, non-isotope-generated form of brachytherapy, referred to as electronic (surface) brachytherapy, has been reported in short-term studies to offer an excellent outcome in patients with small, superficial skin cancers . Safety issues are less of a concern because of the absence of radiation-producing isotopes. However, as with any form of brachytherapy, suitable lesions need to be superficial (<3–4 mm in depth) and well-defined.
Office-based superficial radiotherapy was once the standard modality by which dermatologists treated skin cancer . Typical machines delivered photons of 70–100 kVp with a depth dose suitable for most patients with cutaneous malignancies. Over time, the cost of radiotherapy machines, combined with increased enthusiasm and training in dermatologic surgery, led to a significant decline in office-based radiotherapy. More recently, however, the emergence of newer superficial radiation therapy (SRT) machines has led to renewed interest in office-based radiotherapy for BCCs and cutaneous SCCs. In a retrospective analysis of 1715 non-aggressive tumors treated with superficial office-based radiotherapy (35 Gy in 5–7 fractions three times weekly), the raw recurrence rate was 2.6% (mean follow-up, 2.6 years), with good to very good cosmesis observed in all the patients . The estimated cumulative recurrence rate at 5 years was 5% (4.2%, BCCs; 5.8%, SCCs).
Given the role dermatologists play in diagnosing skin cancers and outlining therapeutic options, understanding the various types of radiotherapy and becoming familiar with the indications, treatment regimens, and long-term outcomes is important. Depending upon local regulations and practices, the radiotherapy may be administered by a radiation oncologist or an appropriately trained dermatologist.
Radiotherapy offers the advantage of a non-surgical approach, thereby avoiding surgical morbidity, scarring, and the need for reconstruction. In situations where reconstruction with grafts or flaps is required, improved cosmesis may result from radiotherapy . Radiotherapy fields can also be tailored with generous margins in order to encompass “at-risk” areas (e.g. possible sites of clinically inapparent tumor extensions) that, if surgically approached, would require involved and complex surgery. In addition, standard oncologic excision margins in certain sites are often difficult to achieve without causing surgical morbidity; such sites include the midface triangle, i.e. the periorbital region (especially the medial canthus), the lower eyelid, nose (especially the ala nasi and nasal tip), nasolabial fold, lip, and chin.
Radiotherapy does have its disadvantages; in particular, most patients require a protracted course of treatment compared to what is often a simple outpatient excision. Patients should also be informed that because of the risk of serious late effects such as soft tissue and cartilage necrosis, a second course of definitive radiotherapy cannot be delivered to the same site. In addition, the reduced “quality” of irradiated skin may make subsequent surgery difficult if the tumor recurs or persists following radiation therapy. Over time, irradiated skin may also develop dyspigmentation, telangiectasias, and a shiny appearance (see Table 139.3 ). Lastly, individuals with xeroderma pigmentosum or basal cell nevus syndrome should not be irradiated owing to their marked predisposition for developing multiple and recurrent skin cancers, often at a young age.
Tumors that have deeply invaded cartilage or bone often do better with a combination of excision plus adjuvant radiotherapy, although definitive radiotherapy still remains an option (local control 60–70%). Radiotherapy of lesions located on the foot, anterior lower leg or dorsum of the hand, although not contraindicated, should be avoided if possible; lesions in such sites are best treated with excision. Unfortunately, radiotherapy to poorly vascularized, edematous tissues is often associated with poor healing. Skin cancers arising in sites of chronic ulceration, trauma, or thermal burns should not be irradiated if at all possible. These sites often have poor vascularity and consequently poor healing. Patients with systemic sclerosis and other causes of cutaneous fibrosis are also best treated by surgical means, as radiotherapy may enhance the fibrosis and lead to poor healing.
Radiotherapy has a very small (~1 in 1000) risk of causing a radiation-induced in-field malignancy (often soft tissue/bone sarcoma) 10–15 or more years after exposure. However, these data were obtained from studies involving many different malignancies treated by various techniques and dosing schedules. It is likely that with modern radiotherapeutic techniques as well as the smaller volumes being irradiated when treating skin cancers, a figure of 1 in 1000 represents an overestimate of the real risk. Nonetheless, for younger patients (<50–60 years of age), this is an important issue and patients need to be informed of the potential risk.
BCCs and cutaneous SCCs can also develop within radiation sites. For example, in a retrospective analysis of 612 radiation sites (average dose, 80 Gy), BCCs and SCCs were observed in 2% and 1.5% of sites, respectively, after a minimum follow-up of 10 years . In a case series of seven balding Caucasian men who had received electron beam radiotherapy for cutaneous malignancies of the scalp, multiple non-melanoma skin cancers (primarily in situ SCCs) arose within and immediately around the radiation sites. The authors postulated an interplay between UV damage and radiation although field cancerization could explain some of the immediately adjacent skin cancers.
The role of radiotherapy in treating benign cutaneous disorders has markedly diminished over the past several decades. It is no longer ethical to offer radiotherapy as a treatment for acne, warts, hirsutism, or tinea capitis. The role of radiotherapy in treating inflammatory skin conditions (e.g. dermatitis, psoriasis, lichen planus) is historical due to efficacious alternative treatments (e.g. corticosteroids, phototherapy, biologic immunomodulators), and the risk of exposing patients to ionizing radiation.
Recurrent keloids, often refractory to re-excision and intralesional corticosteroid injections, can have significant cosmetic, functional, and psychologic sequelae for patients, many of whom are young women. The addition of low-dose (12–16 Gy) adjuvant radiotherapy delivered in three to four fractions, beginning within 24–48 hours following surgery, can markedly and safely reduce the incidence of recurrence . A recent dose-response study involving 119 patients concluded that 20 Gy delivered in 5 fractions was the optimal postoperative regimen, when balancing relapse rate (11%) against adverse effects . A common site for keloids is the earlobe and patients can be treated with low-energy photons plus lead shielding of surrounding tissues. Young patients with keloids of the lower anterior neck (in close proximity to the thyroid gland) should not be irradiated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here