Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Imaging is a significant but not independent component of the multifaceted evaluation of a patient with spine or limb pain. Imaging must be interpreted in concert with the history, physical exam, electrodiagnostic evaluation, and responses to image guided anesthetic or provocative procedures. Imaging alone should not be considered; it can only be understood in its proper context: the individual patient’s unique syndrome of pain or neurologic dysfunction.
Pain of spinal origin is extremely common. Low back pain is the second most common cause of symptomatic office visits in the United States. , The lifetime prevalence of back pain in the United States is approximately 75%, and one-third of United States adults experienced back pain in the previous three months. Advanced imaging is performed in such cases with ever-increasing frequency; the number of lumbar magnetic resonance imaging (MRI) scans among Medicare beneficiaries rose four-fold from 1994–2005. Over 40% of patients with acute low back pain underwent immediate imaging in a private insurance claims database study. In another Medicare-based study of low back pain patients without red flags for systemic disease, nearly 30% underwent imaging (radiography or advanced imaging) within 28 days. In a study in an emergency department environment, the use of advanced imaging (computed tomography [CT] or MRI) for low back pain tripled from 2002–2006. Approximately one-third of Medicare low back pain patients who undergo outpatient MRI studies have received no prior conservative therapy.
Despite this intensity of imaging and the downstream effects of increasing minimally invasive interventions and surgical procedures that flow from them, there is no evidence that patient outcomes are improving. Measures of physical functioning, work/school limitations, and mental health in United States adults with back or neck complaints were similar or worse in 2005 than in 1997. A regional study (North Carolina) demonstrated that the proportion of adults with chronic low back pain causing activity impairment rose from 3.9% in 1992 to 10.2% in 2006. A review article by Chou, Deyo, and Jarvik examined the evidence of inefficient utilization of imaging in back pain patients and the underlying causal forces and mechanisms for initiating improvements. The purpose of this chapter is to foster the rational decision making they advocate in the use of spine imaging, based on evidence, as we in the pain management community seek to improve patient outcomes.
The primary goal of imaging in patients with spine or limb pain is to identify patients suffering from an undiagnosed systemic process causal of the pain/dysfunction syndrome. This is an uncommon phenomenon. An analysis by Jarvik and Deyo suggests that 95% of low back pain is because of benign processes. In patients presenting to a primary care setting with low back pain, only 0.7% suffer from undiagnosed metastatic neoplasm. Spine infection, including pyogenic and granulomatous discitis, epidural abscess, or viral processes, is present in only 0.01% of subjects. Noninfectious inflammatory spondyloarthropathies, such as ankylosing spondylitis (AS), account for 0.3% of presentations. Osteoporotic compression fractures are the most common systemic pathologic process to present as back pain, accounting for 4% of patients. Imaging seeks to identify the approximately 5% of patients with back or limb pain with undiagnosed systemic disease as the etiology of their pain. A related imaging goal is to characterize and assist in therapy planning in the very small percentage of patients with neural compressive disease resulting in radiculopathy or radicular pain syndromes that fail conservative therapy and require surgical or minimally invasive intervention.
The low prevalence of systemic disease as a cause of back pain implies that most imaging studies primarily describe what are often, and inappropriately, termed “degenerative” phenomena. These may include anterior and lateral vertebral body osteophytes, loss of T2 signal in the intervertebral disc, and structural changes of facet arthrosis. Degeneration is a pejorative term implying disease; these changes have no relationship to pain syndromes and correlate only with age. They are best called age or age-related changes. These age changes are typically relatively uniform across the spine, although the lowest lumbar segments are over-represented. Evidence for the lack of specificity of such imaging findings for spine pain syndromes is evident in cadaver studies, imaging studies in asymptomatic populations, and population studies.
Nathan described the presence of anterior and lateral osteophytes in 100% of cadavers at the age of 40 years, whereas posterior osteophytes are present only in a minority of cadavers at 80 years. Hult studied adults with spine radiographs and showed that by the age of 50 years, 87% will have radiographic evidence of age-related disc change (narrowing of the disc space, marginal sclerosis with osteophytes, vacuum phenomena). In a second study including a cohort of asymptomatic workers, Hult noted radiographic evidence of disc “disease” in 56% of those aged 40–44 years, which rose to 95% in subjects 50–59 years old. With the evolution of more sophisticated spine imaging techniques, this lack of specificity of degenerative findings has not improved. Hitselberger and Witten studied plain myelography of asymptomatic volunteers and noted that 24% showed abnormalities that would have been considered significant in the clinical context of back or leg pain. A study of lumbar spine CT in asymptomatic volunteers by Wiesel and colleagues showed that in patients older than 40 years, 50% had “significant” abnormalities. Similarly, Boden and colleagues evaluated MRI of the lumbar spine in asymptomatic volunteers. In patients older than 60 years, 57% had abnormalities considered significant in an appropriate clinical setting. Jarvik and colleagues studied a large patient population with MRI. Their study noted that only extrusions, moderate to severe central spinal canal stenosis, and direct visualization of neural compression were likely to be significant and would separate patients with pain from asymptomatic volunteers. Disc protrusions, zygapophysial joint (z-joint or facet joint) arthrosis, and anterolisthesis or retrolisthesis were virtually always asymptomatic findings. Imaging studies of asymptomatic volunteers are compiled in Table 20.1 .
| Test | Author (reference), Date | Patients (N) | Age Range (mean) | Disc Herniation | Disc Bulge | Disc Degeneration | Central Spinal Canal Stenosis | Annular Fissure |
| X-ray | Hult 1954 |
1200 | 40–44 55–59 |
56% 95% |
||||
| X-ray | Hellstrom 1990 |
143 | 14–25 | 20% | ||||
| Myelogram | Hitselberger 1968 |
300 | (51) | 31% | ||||
| CT | Wiesel 1984 |
51 | (40) | 20% | 3.4% | |||
| MRI | Weinreb 1989 |
86 | (28) | 9% | 44% | |||
| MRI | Boden 1990 |
53 | <60 ≥60 |
22% 36% |
54% 79% |
46% 93% |
1% 21% |
|
| MRI | Jensen 1994 |
98 | (42) | 28% | 52% | 7% | ||
| MRI | Boos 1995 |
46 | (36) | 76% | 51% | 85% | ||
| MRI | Stadnik 1998 |
36 | (42) | 33% | 81% | 56% | 56% | |
| MRI | Weishaupt 1998 |
60 | (35) | 60% | 28% | 72% | 20% | |
| MRI | Jarvik 2001 |
148 | (54) | 38% | 64% | 91% | 10% | 38% |
A study by Kanayama in 200 healthy adults (mean age 40 years, with no current complaint or therapy for back pain nor any history of lumbar surgery) segregated lumbar MRI findings by segmental level ( Table 20.2 ). Asymptomatic T2 signal loss and disc herniations were most common at the L4 and L5 segmental levels; this series also had a high prevalence of asymptomatic high intensity zones (HZ) (24% at L4 and L5). More recent studies have addressed the prevalence of disc “degenerative” imaging findings (T2 signal loss, loss of disc space height) in younger populations, primarily in Scandinavian countries; these are MRI population-based studies without regard to symptomatology. Kjaer and colleagues, studying children aged 13 years, found a 21% prevalence of disc “degeneration.” In a study of adolescents, Salminen and colleagues found a 31% prevalence of disc “degeneration” in 15-year-olds, which rose to 42% in 18-year-olds. Takatalo and colleagues evaluated 558 young adults aged 20–22 years. Using the five-point Pfirrmann classification of disc degeneration, they noted disc degeneration of grade three or higher in 47% of these young adults. There was a higher prevalence in males (54%) than in females (42%). Multilevel degeneration was identified in 17%.
| Segment | Disc Herniation | Nuclear T2 Signal Loss | Modic Change | HIZ |
| L1 | 0.5% | 7% | 1% | 0% |
| L2 | 3.5% | 12% | 3% | 4% |
| L3 | 16.5% | 15.5% | 4% | 5% |
| L4 | 25% | 49.5% | 11% | 23.5% |
| L5 | 35% | 53% | 10% | 24% |
A 2015 systematic literature review included 33 articles and again found a high prevalence of degenerative imaging findings among asymptomatic individuals, which increases with age. For instance, disc protrusions were found in 29% of asymptomatic individuals who were 20 years old and 43% of those 80 years old. Meanwhile, in the same year, a meta-analysis of 14 different studies meeting inclusion criteria provided some conflicting evidence to the concept that degenerative findings on advanced imaging are likely not clinically relevant. For patients 50 years old and younger, Brinjikji and colleagues found that several MRI findings were statistically significantly more prevalent in those with low back pain than those who are asymptomatic. These included disc bulges, degeneration, extrusions, protrusions, and Modic one changes. Spondylolysis demonstrated a similar higher prevalence in patients suffering from back pain. Panagopoulos and colleagues similarly demonstrated that a change over time in some findings (specifically annular fissure, disc herniation, and nerve root compromise) was twice as common in subjects with low back pain than in pain free controls. However, this higher frequency was not statistically significant. However, as acknowledged by the authors of both studies, such association neither is evidence of causation, nor does it provide prognostic information or specific treatment strategies.
As in the lumbar region, age changes in the cervical and thoracic spine are common, asymptomatic, and increase in prevalence with age. Matsumoto studied nearly 500 asymptomatic patients with MRI; he noted a loss of T2 signal within cervical discs in 12%–17% of patients in their 20s, but in 86%–89% of patients older than 60 years old. Asymptomatic cervical cord compression was observed in 7.6% of patients, largely over the age of 50 years. Similarly, Boden studied 63 asymptomatic subjects with MRI and noted cervical disc “degeneration” in 25% of those younger than 40 years and in excess of 60% of patients older than 40 years. Asymptomatic subjects older than 40 years had a 5% rate of disc herniations and a 20% rate of foraminal stenosis. Teresi studied 100 asymptomatic subjects with MRI and noted asymptomatic cervical cord compression in 7% and either disc protrusion or annular bulge in 57% of subjects older than 64 years. In the thoracic spine, Wood studied 90 asymptomatic patients with MRI. In this population, 73% of the patients had positive thoracic imaging findings, 37% had disc herniations, 53% demonstrated disc bulges, and 29% had asymptomatic cord deformity.
The evidence is deep and overwhelming. The structural spine imaging findings most commonly referred to as “degenerative changes” or “degenerative disc disease,” including anterior and lateral osteophytes, loss of T2 signal in the disc, loss of disc space height, disc bulges and protrusions, and facet arthrosis, are ubiquitous and unassociated with pain syndromes; their only association is with age. They can only be avoided by a youthful death. They are not a disease state and are best referred to as normal age change or age-related change. Spondylosis (or spondylotic changes) is an alternative term for these maturational changes frequently used in radiology reports.
A consequence of this high prevalence of asymptomatic age-related changes is that the imager must know the nature of the pain syndrome if he or she is to properly focus on findings significant to the unique patient under consideration. There must be concordance of the imaging finding and the pain syndrome it is postulated to elicit. Imaging cannot prove causation; hence, there is a need for anesthetic and provocative procedures. Communication regarding the nature of the pain syndrome is essential, whether this occurs through a robust electronic medical record, an intake document at the imaging site, or direct interaction of the imager with the patient.
There is also a major sensitivity fault associated with spine imaging. Most patient symptoms referable to the spine occur in axially loaded positions, either sitting or standing. A substantial portion of radiographs and most advanced imaging (CT and MRI) are obtained in a recumbent position, removing the effects of axial load and physiologic posture. This may fail to reveal the lesion responsible for the index pain.
There is ample evidence of the effect of axial load and physiologic posture on the biomechanical and structural characteristics of the spine, derived from biomechanical, cadaver, and imaging studies. Lumbar intradiscal pressures are higher when sitting or standing than when in a recumbent position. The cadaver study of Inufusa demonstrated a reduction in the cross-sectional area of the lumbar central spinal canal and lateral recesses in extension, with an increase in flexion. Lumbar neural foraminal cross-section is also diminished in extension and increased in flexion. Fujiwara noted a reduction in the cadaveric lumbar neural foraminal area with side bending or rotation toward the index foramen; an increase in area was observed with side bending or rotation away from the foramen. Studying normal volunteers, Schmid observed a 40 mm 2 reduction in the cross-sectional area of the dural sac at the L3–L4 level with movement from flexion to extension. The lumbar neural foraminal cross-sectional area was reduced by 23% in moving from an upright neutral to an upright extended position. Danielson noted a significant decrease in the dural sac cross-sectional area with axial loading in 56% of subjects, most commonly at L4–L5; this was more common with increasing age. Dynamic reduction in dural sac area with loading was less frequent in normal volunteers than in a population of patients with neurogenic intermittent claudication. Hansson and colleagues identified the ligamentum flavum as the most important structure resulting in a dynamic reduction in the lumbar central spinal canal area under physiologic loading. Physiologic posture (lumbar lordosis) is likely more important than axial load. Multiple studies in patients with upright imaging have demonstrated the enlargement of lumbar disc bulges or protrusions with axial load, which may be further exacerbated with extension. , Synovial cysts, which may be provocative of radicular pain syndromes or contribute to neurogenic intermittent claudication, may be undetectable on recumbent imaging when synovial fluid remains in the facet joint space. Upon the assumption of axial load and apposition of the facet articular surfaces, the fluid is forced from the joint space into the cyst, where it may act as a neural compressive lesion.
The cervical spine similarly exhibits dynamic physiologic change with posture and load. Cadaveric studies have demonstrated increased disc bulging and buckling of the ligamentum flavum in cervical spine extension; the ligamentum flavum effect was most significant. MRI studies of patients , noted an increase in central spinal canal stenosis with both extension and flexion of the cervical spine relative to neutral posture; the decrease was most marked in extension. Cervical neural foramina diminish in cross-section, width, and height in extension and increase in all these parameters in flexion.
In summary, physiologic extension and axial load reduce the area of all lumbar spine compartments; these are increased in flexion. The cervical central spinal canal is diminished in areas in extension more so than in flexion; it is maximal in the neutral position. Cervical foramina increase in all dimensions in flexion and diminish in extension. These dynamic changes constitute the greatest sensitivity fault in imaging: conventional supine imaging may fail to reveal a lesion causal to the patient’s symptoms when that lesion is only expressed in physiologic postures.
Several methods have been devised to overcome this sensitivity fault. Radiographs should always be obtained upright; this allows assessment of sagittal and coronal balance in a physiologic posture. Flexion-extension radiographs may detect instability not observed on neutral, upright views, but the yield of diagnostic information is very low. In studies in both the lumbar and cervical spine segments, less than 1% of flexion-extension radiographic studies provided information that was noted on static upright radiographs. , The cost and radiation exposure are best deferred to a pre-surgical setting, and not during the initial evaluation of the patient’s back or neck pain.
Advanced imaging can be performed with axial loading devices on conventional CT or MRI scanners. These devices can improve the sensitivity to the detection of clinically significant central spinal canal compromise. The 2011 North American Spine Society’s evidence based guidelines on evaluation and treatment of spinal stenosis suggest axially loaded imaging in the setting of suspected neurogenic intermittent claudication and stenosis unconfirmed by conventional imaging, with a canal diameter of less than 110 mm. , Willén and colleagues demonstrated that surgical results of cases of occult lumbar spinal stenosis detected only by axially loaded MRI were comparable to those of stenosis observed in unloaded MRI examinations.
MRI scanning in an upright position—sometimes referred to as dynamic, positional, or kinetic MRI—is now commercially available and widely marketed. Depending on the specific open MRI system, images can be obtained in the standing or seated weight bearing position or during flexion, extension, rotation, bending, or even real-time spinal movement. Performing MRI in a physiologically relevant position offers the possibility of improved sensitivity to detecting central spinal canal, lateral recess, or neuroforaminal stenosis, including spondylolisthesis, as recently reviewed by Baker and MacKay as well as Botchu and colleagues. , However, studies confirming the clinical and prognostic relevance (specificity) of such increased detection remain lacking. Indeed, no existing literature has demonstrated improved clinical outcomes for treatments based on weight bearing MRI evaluation. A practical challenge is that currently available systems are of low field strength (0.25–0.6 T), significantly lower than standard clinical field strengths (1.5 or 3 T), with an unavoidable reduction in image quality. This reduction in image quality can have important consequences for clinical image interpretation. If current low field strength dynamic systems were applied selectively to cases where conventional imaging failed to demonstrate a correlative lesion causal of the patient’s pain, the patient would likely benefit. However, all too often, the cost of these systems or promotion as the best available imaging tool for all spine conditions results in their routine use. This practice could harm patients, as diminished image quality reduces sensitivity to the detection of sinister lesions, which is the primary goal of imaging the back pain patient ( Fig. 20.1 ).
Spine imaging must be undertaken to fully understand the specificity and sensitivity faults inherent in its use. The ultimate question is one of validity: Does performing an imaging study of the spine segment in question result in improved patient outcomes through a more timely and accurate diagnosis of the process causing the patient’s pain? This is well studied, particularly in the application of imaging to the acute presentation of back pain. Chou and colleagues performed a meta-analysis of the six randomized controlled trials (n = 1804) examining the role of imaging in the acute presentation of back or limb pain with no clinical features suggesting systemic disease. They identified no benefit in pain, function, quality of life, or patient-rated improvement in patients undergoing imaging (radiographs, CT, or MRI) at presentation versus those undergoing clinically directed conservative care. Although routine imaging might have been expected to provide reassurance, imaged patients did not have better psychological outcomes.
Carragee and colleagues elegantly demonstrated the lack of utility of imaging in the acute setting in a five-year prospective observational study. A cohort of asymptomatic subjects deemed to be at-risk for back pain resulting from labor-intensive vocations underwent lumbar spine MRI. This patient cohort was followed periodically over the subsequent five years; a subset of these subjects presented to a medical care provider with acute back or leg pain during this five-year period, and a second lumbar MRI was obtained. Less than 5% of the MRI scans obtained during acute presentations with back or leg pain showed clinically relevant new findings; virtually all of the “positive findings” noted on the images at the time of presentation with back/leg pain had been present in the baseline data obtained when the patient was asymptomatic. Only direct evidence of neural compression in patients with a corresponding radicular pain syndrome was considered useful imaging information. Of particular note, psychosocial factors, not the morphology seen on imaging, were the best predictors of the degree of functional disability caused by back or leg pain.
A study by Modic, also in the acute presentation of low back pain or radiculopathy, showed no relationship between the extent of disc herniations and presenting signs or symptoms. The type, size, or location of a herniation at presentation, or change in size or type over time, did not correlate with clinical outcomes. MRI imaging characteristics did not have measurable value in planning conservative care. The study emphasized that surgical decisions must be made on clinical grounds, given the inability of imaging to predict outcomes. The Modic study, like that of Carragee, demonstrated that psychosocial factors predict functional disability better than imaging parameters.
Although the depth of evidence regarding the inability of imaging to improve outcomes in back and limb pain patients is most profound in the acute setting, it applies more broadly. Chou and colleagues explored the seemingly counterintuitive finding that routine imaging does not lead to better back or limb pain outcomes. This lack of utility can be attributed to the favorable natural history of back and limb pain, the low prevalence of sinister disease as causal of back pain, the weak correlation between imaging findings and symptoms (the specificity fault), and the minimal impact of imaging on clinical decision making. Given the demonstrable modest utility of imaging, the decision to undertake this path must be a carefully reasoned one.
The decision to proceed with any medical test or procedure should be preceded by a consideration of likely benefit weighed against risk or actual harms. There are benefits to be derived from imaging. Foremost, imaging may suggest the diagnosis of previously unsuspected systemic disease. In patients with a radicular pain syndrome or radiculopathy that has not responded to conservative therapy, imaging may supply invaluable information that allows planning minimally invasive or surgical procedures. Negative imaging could also be valuable in providing reassurance that there is no sinister disease present and stopping further workup in appropriate circumstances. In patients with chronic pain syndromes, imaging may help to identify the structural or inflammatory cause of such pain. Only when a specific pain generator is identified can a specific therapeutic intervention plan be developed, whether conservative or invasive. Advanced imaging, specifically MRI, can also help plan minimally invasive procedures, such as epidural steroid injections. In this setting, information on anatomy and pathology obtained from MRI allows the interventionalist to rationally plan a procedure in an attempt to maximize its efficacy and minimize patient risk. Although, at a minimum, it is helpful on a case-by-case basis, debate exists as to whether such pre-epidural steroid injection MRI examinations are routinely needed. Based on a randomized controlled trial, the availability of MRI results to the proceduralist performing epidural steroid injections has not been conclusively shown to improve outcomes or significantly affect decision making. However, subgroup analyses of the study cohort suggest that both leg pain score and functional assessment at three months after injection demonstrated greater improvement for those who had injections at optimal levels compared to those in whom the injected location occurred at levels disparate from the MRI findings. Moreover, in one-third of patients for whom the MRI was withheld, an injection different than what would have been recommended if MRI results were available was performed. , We (the authors of this chapter) do support the use of preprocedural MRI when epidural steroid injections are being considered.
There are direct harms and potential risks associated with imaging, which must be balanced against potential benefits. These include radiation dose, cost, the labeling effect, and the downstream risk of provoking minimally invasive or surgical interventions of dubious efficacy.
Radiation dose in radiography, CT, or CT/myelography, and nuclear medicine studies, constitutes direct patient harm. Radiation exposure from radiographs, CT, and nuclear medicine studies carries a cumulative risk of neoplasm induction. This risk becomes particularly problematic when serial studies are performed. The biologically effective absorbed radiation dose is measured by the Sievert (Sv); in North America, the average annual natural background exposure is approximately 3 mSv. A frontal and lateral chest radiograph is often considered the common currency of radiation exposure, incurring a dose of approximately 0.1 mSv. A three-view lumbar spine radiographic series is then worth approximately 15 chest radiographs or 1.5 mSv; cervical spine radiographs incur a dose of 0.2 mSv. A dose of 6 mSv is typical for a lumbar spine CT scan, a value of 60 chest radiographs. Cervical spine CT incurs 2 mSv or 20 chest radiographs. A technetium bone scan has a dose of 6.3 mSv. The cumulative radiation exposure creates real harm; the 2.2 million lumbar spine CT scans performed in the United States in 2007 were projected to result in 1200 future cancers. Although less radiation intensive, lumbar radiography is performed much more frequently than CT and contributes nearly five-fold the cumulative radiation burden to the United States population. The average annual radiation exposure from lumbar radiographs is 75 times that of chest radiography. Radiation risks of spine imaging are made more significant by the necessary inclusion of radiosensitive tissues, the gonadal structures in the pelvis, and the thyroid in the neck.
Imaging studies of the spine are costly. In the United States, the medical imaging community incurs more than $100 billion of societal cost per year. The 2020 Medicare reimbursements for lumbar spine imaging include radiographs: $42; non-contrast CT: $172; CT-myelogram: $504; non-contrast MRI: $241; whole-body positron emission tomography (PET)/CT: $1621; and bone scan with single-photon emission CT (SPECT): $340. Nominal fees are typically three to five times the Medicare reimbursements. It is easy to appreciate how quickly imaging costs can accrue.
The labeling effect refers to the inevitable identification of age-related change, usually described as “degenerative change” or “degenerative disc disease” on imaging studies obtained in the evaluation of back or limb pain. The patient may then perceive that he or she suffers from a degenerative spine condition; the term “degenerative” has only negative connotations. Fearing further damage to their “degenerative” spine, they may give up favorite activities and exercise, resulting in deconditioning and contributing to depression. These fear-avoidance behaviors can be a major impediment to recovery. In a study in which back pain patients were randomized to either receiving or being blinded to the results of their MRI imaging, those who were privy to the results (which were all benign) had a lesser sense of wellbeing. A study of subacute or chronic back pain patients showed that those who underwent spine radiography reported more pain, had a diminished global health state, and consumed more follow up care than those who were not imaged. These findings emphasize the need for patient education regarding the insignificance of age-related imaging findings; imaging professionals should carefully choose descriptive language in imaging reports and avoid the use of pejorative terms such as “degenerative change.”
Finally, and most ominously, imaging may precipitate interventions with little evidence of efficacy and expose the patient to harm. Jarvik and colleagues documented that obtaining advanced imaging (MRI) early in a patient’s spine pain syndrome leads to increased surgical interventions despite equivalent pain and disability profiles compared with un-imaged patients. Likewise, Lurie and colleagues examined the dramatic regional variation (12-fold) in the rate of surgical intervention for central spinal canal stenosis. These investigators noted that the rate of surgical intervention correlated directly with the intensity of CT and MRI use. In evaluating subjects with acute job-related back pain, Webster and colleagues noted that undergoing an MRI in the first month of symptoms was associated with an eight-fold increased risk for surgery and a five-fold risk for more medical care consumption than that noted for clinically matched, un-imaged subjects. Even in healthcare settings largely unaffected by fee-for-service incentivization, such as the United States Department of Veterans Affairs, early lumbar spine MRI is associated with increasing utilization, such as surgery. Many of the interventions directed toward spine pain, both surgical and minimally invasive, have only modest, if any, evidence basis. Unfortunately, as evidenced by a recent study by Jarvik and colleagues, even including spine imaging reports, epidemiologic data on the high prevalence of lumbar spine degenerative imaging findings seem unlikely to affect subsequent spine related utilization (further imaging, clinical evaluation, or procedure/surgery). However, subsequent opioid prescriptions may be slightly reduced. As noted by the authors, there may be unmeasured benefits from patients and their providers better being able to interpret the significance of imaging findings.
Imaging is performed to identify the cause of a patient’s back or limb pain. Spine imaging has major specificity and sensitivity faults. The decision to initiate imaging must occur as a reasoned decision, weighing potential benefits against the real harm and risks. The evidence is clear there is no benefit to imaging in the acute presentation of uncomplicated back or limb pain in the absence of signs or symptoms of systemic disease. These principles are firmly based on evidence and have led to the imaging guidelines promulgated by several scientific societies. A 2015 study by Jarvik and colleagues is an example of a relatively recent investigation that confirms these principles. Based on a prospective cohort of over 5,000 patients aged 65 years or older with a new primary care visit for back pain from 2011–2013, early imaging (radiographs or MRI/CT) in the first six weeks after the index visit was not associated with better outcomes at 12 months.
These guidelines are not new. In 1994, the Agency for Health Care Policy and Research recommended against imaging patients with back pain within the first month of a pain syndrome without signs of systemic disease. The American College of Radiology (ACR) consensus practice guidelines were restated in 2016. Imaging is not indicated in the patient who presents with acute low back pain with or without radiculopathy except in the presence of “red flag” features raising the suspicion for serious underlying conditions, including:
Potential cancer or infection:
History of cancer
Unexplained weight loss
Immunosuppression
Urinary infection
Intravenous drug use
Prolonged use of corticosteroids
Back pain not improved with conservative management (up to six weeks of medical management and physical therapy)
Potential spinal fracture:
History of significant trauma
Minor fall or heavy lift in a potentially osteoporotic or elderly individual
Prolonged use of corticosteroids
Potential cauda equina syndrome or severe neurologic compromise:
Acute onset of urinary retention or overflow incontinence
Loss of anal sphincter tone or fecal incontinence
Saddle anesthesia
Global or progressive motor weakness in the lower limbs
A 2007 joint recommendation from the American College of Physicians (ACP) and the American Pain Society stated that imaging should not be obtained in patients with nonspecific low back pain. More recent guidelines/recommendations of other societies, including the Choosing Wisely initiative established by the American Board of Internal Medicine, continue to align with this 2007 recommendation. Imaging should only be performed when severe or progressive neurologic deficits are present or when serious underlying systemic disease is suspected. Furthermore, patients with signs or symptoms of radiculopathy or spinal stenosis should be imaged only if they are candidates for surgical or minimally invasive intervention (e.g. epidural steroid injection). In a further elaboration on these guidelines, the ACP, in its initiative to promote high-value medical care, provided more specific imaging recommendations based on clinical scenarios ( Table 20.3 ).
| Imaging Action and Clinical Situation | Suggestions for Initial Imaging |
| Immediate Imaging Radiography plus erythrocyte sedimentation rate* Magnetic resonance imaging |
Major risk factors for cancer (new onset of low back pain with history of cancer, multiple risk factors for cancer, or strong clinical suspicion for cancer). Risk factors for spinal infection (new onset of low back pain with fever and history of intravenous drug use or recent infection). Risk factors for or signs of the cauda equina syndrome (new urine retention, fecal incontinence, or saddle anesthesia). Severe neurologic deficits (progressive motor weakness or motor deficits at multiple neurologic levels). |
| Defer Imaging After a Trial of Therapy Radiography with or without erythrocyte sedimentation rateMagnetic resonance imaging |
Weaker risk factors for cancer (unexplained with weight loss or age >50 years). Risk factors for or signs of ankylosing spondylitis (morning stiffness that improves with exercise, alternating buttock pain, awakening because of back pain during the second part of the night, or younger age [20 to 40 years]). Risk factors for vertebral compression fracture (history of osteoporosis, use of corticosteroids, significant trauma, or older age [>65 years for women or >75 years for men]). Signs and symptoms of radiculopathy (back pain with leg pain in an L4, L5, or S1 nerve root distribution or a positive result on straight leg raise or crossed straight leg raise test) in patients who are candidates for surgery or epidural steroid injection. Risk factors for or symptoms of spinal stenosis (radiating leg pain, older age, or pseudoclaudication) in patients who are candidates for surgery. |
| No imaging | No criteria for immediate imaging and back pain improved or resolved after a one-month trial of therapy. Previous spinal imaging with no change in clinical status. |
There is evidence that of the small proportion of subjects with a sinister disease as the cause of their back or limb pain, virtually all have risk factors that trigger imaging under these guidelines. A retrospective study of 963 patients presenting with acute low back pain noted that the eight subjects with neoplasm all had clinical risk factors. In a prospective study of 1170 acute low back pain patients without clinical risk factors, no cases of neoplasm were found. No sinister disease was missed in the absence of clinical risk factors in a subsequent systematic review.
Despite these well-supported, evidence based guidelines, clinical practice in the United States remains greatly divergent from this ideal. By one estimate, between one-third and two-thirds of all advanced spine imaging is inappropriate when measured against existing guidelines. Utilization of spine imaging is accelerating despite a complete lack of evidence of its effectiveness in improving the outcomes of back and limb pain patients. Chou and colleagues have enumerated the causes of this overutilization: inappropriate patient expectations, direct and indirect financial incentives on the part of providers, defensive medicine, and provider time constraints. , , These issues present great challenges to imaging professionals and those who utilize imaging to improve the clinical state of their patients. Solutions will undoubtedly be multifactorial, but the education of the patient, the imaging professional, and imaging consumers would seem to be at the heart of the matter. It is hoped that the evidence presented here will assist in more rational decision making regarding imaging the spine pain patient ( Box 20.1 ).
The primary role of imaging is the identification of undiagnosed systemic disease.
Spine imaging has a significant specificity fault: a high prevalence of asymptomatic age-related “degenerative” findings.
Significance of imaging findings depends on concordance: the imager must know the pain/dysfunction syndrome.
Spine imaging may be insensitive to dynamic lesions.
No imaging is indicated in the acute presentation of back or limb pain in the absence of “red flag” features.
The decision to undertake imaging must be a reasoned harms and risk/benefit judgment.
Imaging correlates poorly with clinical presentation and course.
Clinical decision support tools represent an emerging array of applications for helping providers decide whether to order spinal imaging and, if so, what modality. Electronic templates can be and have been created to assist practitioners in various clinical scenarios, including acute low back pain. The use of such templates can decrease total imaging and MR imaging rates in ambulatory care and emergency department settings, though providers may still have valid reasons for over-riding the decision support tool’s recommendation not to image. , Feedback on evidence based guideline adherence (“report cards” for ordering providers) can reduce outpatient lumbar spine MRI ordering. Such relatively low cost health information technology tools can promote evidence based, high-value, and cost-conscious care for patients experiencing spinal pain. Hopefully, they will be increasingly utilized.
With the failure of clinically directed conservative care and having made a reasoned decision to initiate imaging of the spine pain patient, the choice of imaging modality should, according to the ACR, be based on the working diagnosis, the urgency of the clinical problem, and the comorbidities of the patient. Radiographs are not routinely recommended for acute nonspecific low back pain, because of their inherently low value in making a specific diagnosis. However, radiography is the initial modality of choice for patients with low back pain who have a history of low velocity trauma, osteoporosis, or steroid use, or for those who are elderly (i.e. for those who are suspected of having vertebral compression fractures). Such radiographs should ideally be upright, weight bearing, and include the appropriate spine segment. Radiography is also recommended to evaluate a young patient for AS. The ACR and the ACP are consistent in their recommendations that patients with “red flag” features of recent low velocity trauma, osteoporosis, age greater than 70 years, or chronic steroid use (i.e. possible vertebral compression fracture), or young patients with clinically suspected inflammatory spondyloarthropathy should initially undergo radiographs; advanced imaging (specifically, MRI as the first line) should be reserved for patients with progressive neurologic deficits or a strong clinical suspicion of infection or neoplasm or immunosuppression, or in those with persistent symptoms after six weeks of conservative management. Radiographs provide a modest sensitivity screen for sinister conditions, establish spine enumeration, and assess the sagittal and coronal plane balance when performed in physiologic positions.
Spine enumeration is a critical but underappreciated role of radiographs. The typical spine morphology of 24 mobile, presacral spine segments (seven cervical, 12 rib-bearing thoracic, and five lumbar type vertebral bodies) is not uniformly present; deviation can cause confusion in establishing the origin of pain syndromes, or wrong segment minimally invasive or surgical interventions. It can be reasonably assumed in the human species that there are seven cervical vertebrae. Considerable variation in the number and distribution of thoracic and lumbar segments may be difficult to appreciate on MRI alone; radiographs can establish this enumeration and serve as the foundation for subsequent advanced imaging description.
In a study by Carrino and colleagues using complete spine radiographs, 91.8% of subjects had 24 presacral vertebral segments: 4.8% had 23, and 3.4% had 25. Using full spine sagittal MRI localizer images, Akbar and colleagues identified 23 presacral segments in 3.3% of subjects and 25 in 3.3%; these anomalies of spine enumeration were not mentioned in the radiology report in nearly 70% of cases. In Carrino’s study, if one considers both anomalous number and distribution (e.g. 13 rib-bearing vertebrae + 4 lumbar type vertebrae) of thoracolumbar spine segments, 10.9% of subjects had nonclassical anatomy. For the spine interventionalist, this implies that these situations are encountered in the procedure room regularly; failure of recognition virtually guarantees wrong segment procedures.
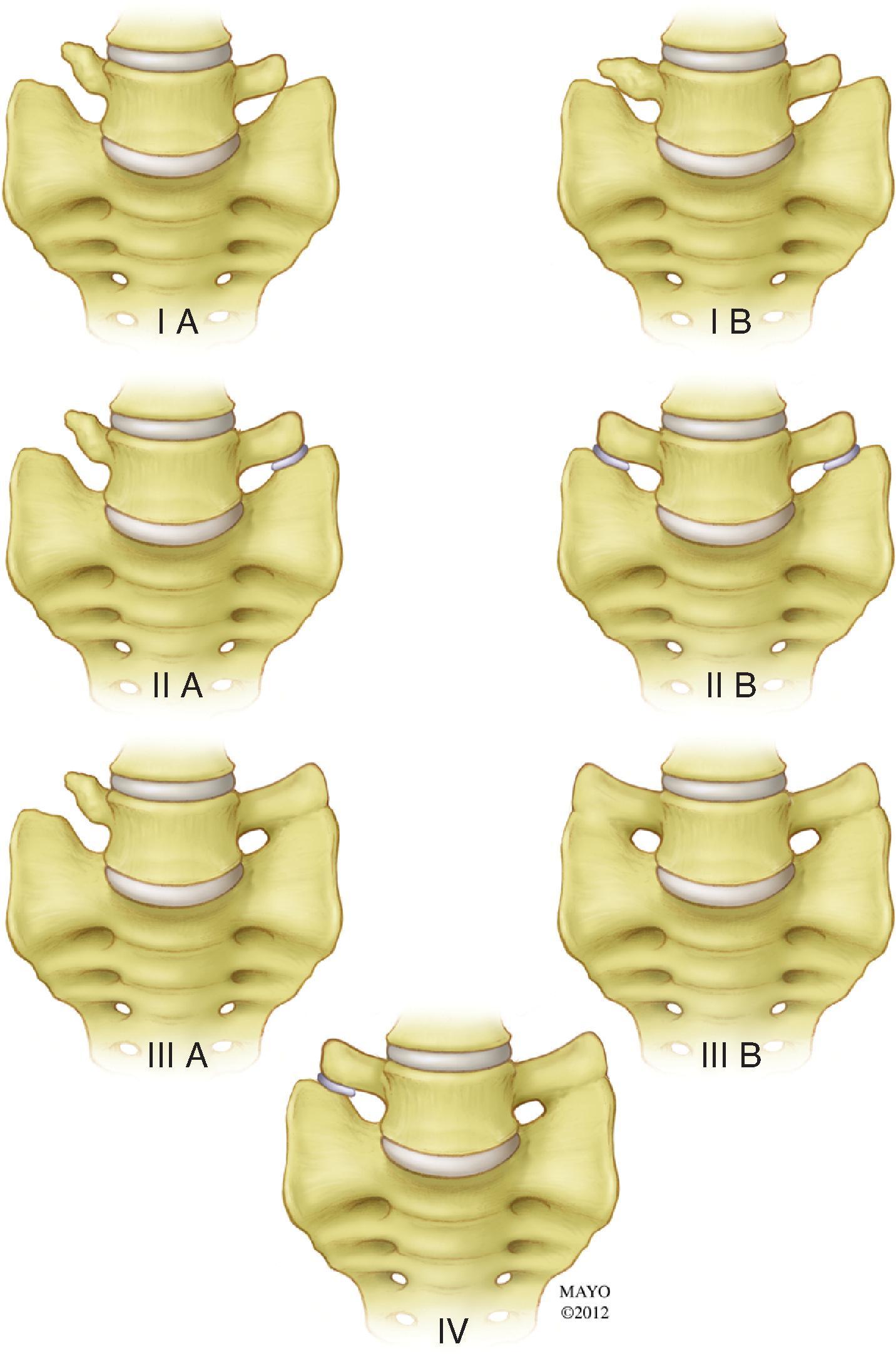
Anomalous segmentation is predicted by the presence of transitional thoracolumbar or lumbosacral vertebral bodies, which may be a source of confusion. Thoracolumbar transitional segments have hypoplastic ribs at the lowest rib-bearing segment; they were present in 4.1% of subjects in Carrino’s study. Lumbosacral transitional segments have characteristics of both the L5 lumbar body and the superior sacral segment; their described prevalence ranges from 4%–30%. The Castellvi classification ( Fig. 20.2 ) describes the morphologic types, ranging from an expanded (height >19 mm) dysplastic transverse process (type I), through a pseudoarticulation between the transverse process and sacral ala (type II), to osseous fusion between the transverse process and the sacrum (type III). Type IV denotes the presence of a type II transition on one side and a type III on the other. Unilateral (a) and bilateral (b) subtypes are also described. On lateral radiographs or sagittal MRI images, a transitional segment can be suggested in the presence of a perceived narrow S1–S2 disc, which extends for the entire anterior-posterior (AP) width of the sacral body with parallel endplates and a squared upper sacral segment ( Fig. 20.3 ). The presence of a transitional lumbosacral segment increased by seven-fold the likelihood of an anomalous number of presacral segments. In patients with radicular pain syndromes and lumbosacral transitional anatomy, the pain practitioner must also know the possibility of an extraforaminal nerve entrapment between the enlarged transverse process and sacral ala.
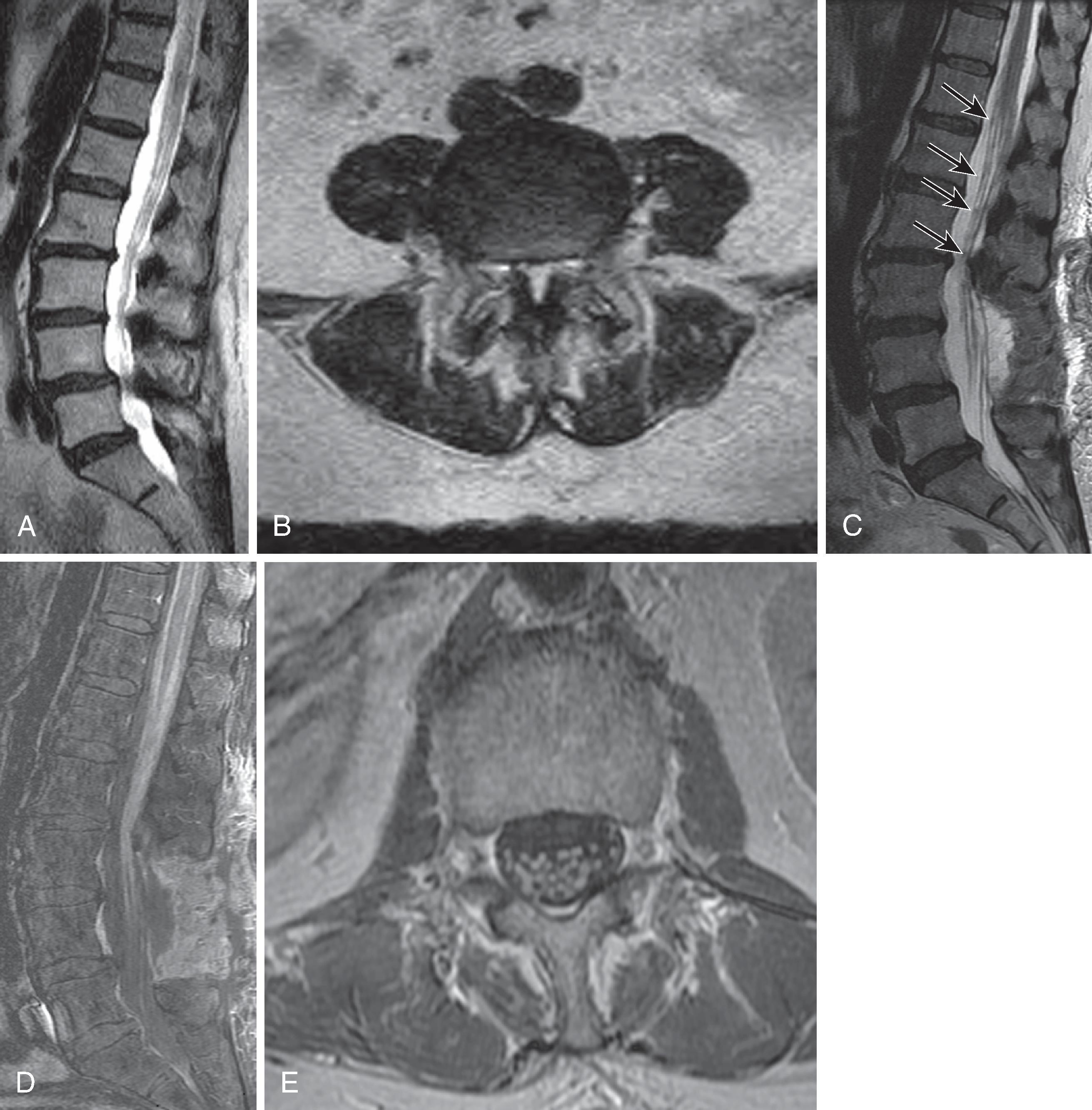
![Figure 20.3, Transitional segments. Lateral radiograph (A) and sagittal T2 magnetic resonance image (B) demonstrate typical findings of a transitional segment interspace (arrows): a narrow disc space with parallel endplates and normal T2 signal intensity. Castellvi IIa transitional segment is demonstrated in frontal (C) and lateral (D) radiographs. Note right pseudoarticulation. In another patient, frontal radiograph (E) shows a left-sided pseudoarticulation, also seen on the axial computed tomography image ( arrows in [F] ). Narrow, parallel endplates are visible in the transitional interspace on the lateral radiograph (G) . This patient had axial pain attributed to the pseudoarticulation; this was injected (H) with relief of the index pain. Figure 20.3, Transitional segments. Lateral radiograph (A) and sagittal T2 magnetic resonance image (B) demonstrate typical findings of a transitional segment interspace (arrows): a narrow disc space with parallel endplates and normal T2 signal intensity. Castellvi IIa transitional segment is demonstrated in frontal (C) and lateral (D) radiographs. Note right pseudoarticulation. In another patient, frontal radiograph (E) shows a left-sided pseudoarticulation, also seen on the axial computed tomography image ( arrows in [F] ). Narrow, parallel endplates are visible in the transitional interspace on the lateral radiograph (G) . This patient had axial pain attributed to the pseudoarticulation; this was injected (H) with relief of the index pain.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/RadiologicAssessmentofPatientWithSpinePain/2_3s20B9780323711012000208.jpg)
Correlation of pain syndromes with imaging findings can be confounded in patients with transitional anatomy or anomalous segmentation. It is best to consider that radicular innervation patterns remain relatively constant when counted caudally from the skull base, but the skeletal anatomy may change about them. For example, the 26th nerve (eight cervical, 12 thoracic, five lumbar, first sacral nerves) from the skull base most commonly innervates the medial head of the gastrocnemius and the soleus muscles, the basis of the S1 radicular pain pattern. A patient with 25 presacral vertebral segments may exit under the pedicle of the lowest lumbar type vertebral body, creating confusion for the unwary practitioner ( Fig. 20.4 ). There is also variation in typical innervation patterns in the presence of transitional anatomy, which may introduce further localization challenges. Only meticulous attention to spine enumeration, best provided by plain radiographs, sometimes supplemented by total spine MRI localizer images, will protect the spine pain patient and interventionalist from wrong segment invasive procedures ( Box 20.2 ). Sometimes, available chest or spinal radiographs or CT examinations of the chest/abdomen/pelvis or thoracic/lumbar spine can be similarly useful in establishing enumeration.
![Figure 20.4, The importance of segmental enumeration. This 36-year-old male who has failed conservative care presents with a left-sided L5 radicular pain pattern involving the lateral thigh, lateral calf, and dorsum of the foot. His magnetic resonance image (MRI) (sagittal T2 [A] and axial T1 [B] at level of dotted line ) shows a subtle disc extrusion in the left lateral recess ( arrow in [B] ) at the second to last interspace. The lowest-most disc appears transitional. MRI scout (C) image demonstrates 24 presacral segments. Frontal and lateral radiographs (D and E) may suggest five lumbar vertebrae on cursory examination. Careful counting at fluoroscopy noted 11 rib-bearing vertebrae. T12 has no ribs, and L5 is transitional, Castellvi type 4. The transforaminal epidural steroid injection (F and G) was performed under the pedicle bearing the dysplastic left T5 transverse process and relieved the patient’s pain.Figure 20.4,cont’d Note (G) the typical appearance of transitional disc space. Careful enumeration of every case is the only way to avoid wrong segment interventions. Figure 20.4, The importance of segmental enumeration. This 36-year-old male who has failed conservative care presents with a left-sided L5 radicular pain pattern involving the lateral thigh, lateral calf, and dorsum of the foot. His magnetic resonance image (MRI) (sagittal T2 [A] and axial T1 [B] at level of dotted line ) shows a subtle disc extrusion in the left lateral recess ( arrow in [B] ) at the second to last interspace. The lowest-most disc appears transitional. MRI scout (C) image demonstrates 24 presacral segments. Frontal and lateral radiographs (D and E) may suggest five lumbar vertebrae on cursory examination. Careful counting at fluoroscopy noted 11 rib-bearing vertebrae. T12 has no ribs, and L5 is transitional, Castellvi type 4. The transforaminal epidural steroid injection (F and G) was performed under the pedicle bearing the dysplastic left T5 transverse process and relieved the patient’s pain.Figure 20.4,cont’d Note (G) the typical appearance of transitional disc space. Careful enumeration of every case is the only way to avoid wrong segment interventions.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/RadiologicAssessmentofPatientWithSpinePain/3_3s20B9780323711012000208.jpg)
Approximately 11% of subjects will have anomalies of number or distribution of thoracolumbar vertebral bodies.
Anomalous segmentation is predicted by the presence of transitional thoracolumbar or lumbosacral vertebral bodies.
Ideally, vertebral numbering should occur from the skull base caudally.
Practically, the human cervical spine is homologous and can be assumed to have seven segments.
T1 is marked by the first upward inclined transverse process.
Meticulous enumeration on every case will prevent wrong segment procedures.
When radiographs are obtained, they should be upright, weight bearing images. Only in a physiologic posture can one assess sagittal and coronal balance ( Fig. 20.5 ). Upright radiographs demonstrate more thoracic kyphosis and lumbar lordosis than do recumbent images. Weight bearing images may also demonstrate instability, most commonly L4–L5 degenerative spondylolisthesis, which would be occult on recumbent films. Radiographs can be complementary to and provide further information than MRI. For instance, spondylolisthesis is often more pronounced on an upright radiograph than MRI, with the latter potentially underestimating the degree of listhesis and associated central spinal canal narrowing. In spinal deformity patients, the exacerbation of spinal curves with weight bearing can be dramatic. Flexion-extension radiographs in the lumbar or cervical spine are not indicated as part of an initial imaging investigation. There is no role for oblique radiographs of the spine. In the lumbar region, they double the gonadal dose and do not provide useful information that will affect clinical decision making. Cervical spine oblique views similarly serve only to irradiate sensitive tissue (thyroid, lens of the eye) without clinical benefit.
When patients who are deemed to be interventional candidates have low back pain lasting over six weeks despite conservative management, advanced imaging (CT, MRI, nuclear medicine) may be obtained. MRI is considered the first line in those with radiculopathic symptoms and in patients with severe or progressive neurologic deficits on presentation and red flag features raising suspicion for a serious underlying condition. CT has undergone a revolution since the early 2000s with the advancement of multidetector technology. A data set for the lumbar spine can now be obtained in a few seconds, eliminating motion artifacts and dramatically improving patient tolerance. This data set can then be reconstructed in any plane without losing spatial resolution or additional radiation exposure. CT provides superior imaging of cortical and trabecular bone when compared with MRI. CT may be necessary to characterize primary bone tumors of the spine. This also helps explain why CT is typically the first line modality used in the acute trauma setting for fracture identification, especially in high energy trauma or other settings meeting the high-risk criteria for potential vertebral injury. CT is also excellent at depicting osseous structural problems such as spondylolysis, pseudarthrosis, fracture, scoliosis, and stenosis, and in the postoperative evaluation of bone graft integrity, surgical fusion, and instrumentation. In addition, CT provides reasonable contrast resolution and can identify root compressive lesions, such as disc herniations or characterize central spinal canal, lateral recess, and foraminal compromise, in most cases. CT cannot typically identify intrathecal pathology and is less sensitive than MRI in the detection of early inflammatory or infectious processes, neoplasm, or paraspinal soft tissue lesions. Radiation dose must always be considered when employing CT, particularly in young patients or in serial studies. One by-product of the rapid technologic advance of CT is that the literature contains no comparative studies between MRI and the latest generation of multidetector CT scanners in the detection and characterization of disc herniations. When advanced cross-sectional imaging of the spine is indicated, but the patient is not an MRI candidate, CT is an excellent alternative.
MRI has been the dominant spine imaging modality since the 1990s, despite modest technologic advancement in the realm of spine imaging in that time span. MRI has superior contrast resolution and thus the ability to distinguish between soft tissue types, allowing it to detect intrathecal pathology and identify subtle root compressive lesions. Therefore it is the first line modality for those suspected of having spinal cord compression, spinal cord injury, or cauda equina syndrome. MRI also has superior sensitivity in the detection of neoplasm and infection.
With the use of heavily T2-weighted imaging sequences (short tau inversion recovery [STIR] or fast spin-echo T2 sequences with fat saturation) or gadolinium-enhanced T1-weighted images with fat saturation, MRI can detect the physiologic parameters of edema, hyperemia, and inflammatory change. It has greater specificity than CT in characterizing the age/acuity of fractures. With gadolinium enhancement, MRI can distinguish between recurrent disc herniation and scarring in the postoperative patient; in this setting, it should be performed without and with intravenous contrast. MRI does not evaluate the cortical bone well. Patient acceptance remains problematic because of prolonged imaging times and up to 10% examination failures caused by claustrophobia; this has decreased as imaging practices have increasingly incorporated both wide bore scanners and/or sedation into a routine offering for MRI (typically with oral benzodiazepines). Open magnets also have improved patient acceptance but at the cost of image quality. Despite advances, including MRI-safe implanted devices becoming more commonplace, a small percentage of patients are MRI incompatible because of some cardiac devices, spinal cord stimulators, or other implanted devices. MRI remains costly. The sensitivity challenges imposed by recumbent MRI imaging were discussed earlier; the hope is that the engineering challenges of high field strength, weight bearing MRI imaging will be met in the near future.
CT myelography retains a problem solving role in the lumbar spine; especially if a routine CT has not answered the clinical question, it will substitute for MRI in the MR-incompatible patient. CT myelography has superior spatial resolution when compared with MRI but lacks its soft tissue contrast resolution. It can provide an exquisite demonstration of root compressive lesions and central spinal canal, lateral recess, and foraminal compromise. In the cervical spine, the superior spatial resolution of CT myelography and its ability to discriminate between bone and soft tissue compressive lesions give it a continuing role. CT myelography is minimally invasive, expensive, operator dependent to a degree, and also requires current CT technology to be maximally useful.
Cone-beam CT, weight bearing myelography holds promise for assessing position dependent pain syndromes, at least in part defeating the sensitivity fault of recumbent advanced imaging. In this technology, arising from rotational angiography roots, the C-arm rapidly rotates about the standing patient who has had contrast introduced into the thecal sac. A flat panel fluoroscopy detector gathers a data set that can be reconstructed in any plane. Soft tissue contrast does not approach that of true CT, and the longitudinal field of view is limited to the length of the flat panel detector. Despite these limitations, the high inherent contrast among intrathecal contrast, the soft tissue structures of the spinal column, and bone allow for a very good depiction of the central spinal canal, lateral recess, and foraminal compromise. This technology will presumably only improve, but it is not in widespread clinical use even at institutions where it is available ( Fig. 20.6 ). A lower-resolution version of this dynamic is multipositional conventional myelography. In a study of 100 patients, Merkle and colleagues showed that compared to supine MRI, 137 additional stenotic segments were diagnosed in 64 patients by standing myelography with extension, suggesting that routine MRI frequently underestimates spinal stenosis.
![Figure 20.6, Ligamentum flavum redundancy (buckling) on upright myelography (cone-beam computed tomography [CT]). A 66-year-old man imaged for neurogenic intermittent claudication. Conventional myelogram in the lateral plane with the patient in the prone position (A) and postmyelogram sagittal (B) and axial CT at L4–5 interspace (C) with the patient in the prone position show minimal redundancy of the ligamentum flavum (black arrows) and mild central spinal canal compromise at the L4–5 level. Cone-beam CT myelography with the patient in an upright position demonstrates a marked increase in ligamentous buckling (white arrow) on the sagittal reconstruction (D) and complete effacement of the thecal sac on the axial reconstruction (E) . (Courtesy of Kent Thielen, MD, Mayo Clinic, Rochester, MN.) Figure 20.6, Ligamentum flavum redundancy (buckling) on upright myelography (cone-beam computed tomography [CT]). A 66-year-old man imaged for neurogenic intermittent claudication. Conventional myelogram in the lateral plane with the patient in the prone position (A) and postmyelogram sagittal (B) and axial CT at L4–5 interspace (C) with the patient in the prone position show minimal redundancy of the ligamentum flavum (black arrows) and mild central spinal canal compromise at the L4–5 level. Cone-beam CT myelography with the patient in an upright position demonstrates a marked increase in ligamentous buckling (white arrow) on the sagittal reconstruction (D) and complete effacement of the thecal sac on the axial reconstruction (E) . (Courtesy of Kent Thielen, MD, Mayo Clinic, Rochester, MN.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/RadiologicAssessmentofPatientWithSpinePain/5_3s20B9780323711012000208.jpg)
Nuclear medicine studies are growing in importance in spine imaging. Technetium pyrophosphate bone scans detect increased blood flow and accelerated bone metabolic activity. With the addition of SPECT and SPECT/CT image fusion, significant additional spatial localization of hyperemia and increased metabolic activity are possible. This imaging is traditionally helpful in assessing the burden of metastatic disease but can also be valuable in assessing non-neoplastic inflammatory states such as spondylolysis. SPECT/CT can potentially identify inflammatory synovitis in the facet and sacroiliac joints, which might guide interventions. However, validation studies of these techniques against accepted reference standards such as comparative blocks in the facet joints or intra-articular sacroiliac blocks have not yet been conducted. When MRI is not technically feasible, technetium bone scanning can be used to characterize the age of vertebral fractures in selecting patients for bone augmentation. The technetium bone scan, combined with gallium scan, offers sensitivity equal to MRI in the detection of spondylodiscitis. However, these techniques provide less anatomic information and a lesser ability to make a specific diagnosis; MRI may ultimately be necessary to characterize the degree of central spinal canal compromise that may influence surgical decision making. PET or PET/CT scans have an increasing role in assessing the burden of metastatic disease and in selecting lesions for percutaneous biopsy.
Axial pain that may have an imaging correlate derives primarily from the stimulation of nociception of the spinal articulations: the intervertebral disc, the zygapophysial or facet joints, and the sacroiliac joint. More broadly, it may include pain originating from the muscular or ligamentous structures in the supporting architecture of the spine. Axial pain is clinically characterized as constant, dull, deep, poorly localized, and aching, located primarily in the paraspinous region with inconstant referral to the proximal extremities. This is in distinction to the neuropathic pain of radicular character, which is typically sharp, electric, lancinating, and experienced in a bandlike distribution into the more distal extremities. DePalma and Maus have well described the prevalence of axial spine pain generators ( Table 20.4 ). , Intervertebral disc disruption (IDD) was the most common axial pain generator in this series, followed by the facet joint, the sacroiliac joint, and insufficiency fractures of the spine or pelvis. This work also emphasized the age dependence of these pain sources. Patients with discogenic pain (IDD) were significantly younger than patients with facet or sacroiliac joint pain. As the age increased, the probability of IDD as a pain source decreased, and the probability of facet or sacroiliac pain increased, up to approximately 70 years of age. A later multivariable analysis also showed a gender relationship, with IDD more prevalent in young men. This echoes the earlier work of Schwarzer, who demonstrated that lumbar facet-mediated pain was uncommon in a population of young workers (∼15%) but more highly prevalent in an aged population (∼32%). ,
| Pain Source | Prevalence (%) | Mean Age (Std Dev) |
| Intervertebral disc disruption (IDD) | 41.8 | 43.7 (10.3) |
| Facet joint | 30.6 | 59.6 (13.1) |
| Sacroiliac joint | 18.2 | 61.4 (17.7) |
| Vertebral insufficiency fracture | 2.9 | 79 (11.8) |
| Pelvic insufficiency fracture | 1.8 | 71.3 (11.7) |
| Baastrup’s disease | 1.8 | 75.3 (4.7) |
| Fusion hardware | 2.9 | 59.6 (19.4) |
The imaging diagnosis of discogenic pain is made challenging by the lack of a pathoanatomic gold standard against which to assess imaging parameters. It is not possible to evaluate a disc, either at surgery or upon histologic examination, and deem it painful. The current most stringent reference standard for the diagnosis of discogenic pain is manometrically controlled provocation discography with normal control levels as documented in the practice guidelines of the Spine Intervention Society (SIS).
However, it is important to observe that examination of the same body of evidence regarding the validity of discography as the reference standard has resulted in diametrically opposed recommendations regarding its use by different physician societies. The SIS, the North American Spine Society, and the American Society of Interventional Pain Physicians accept discography as a useful diagnostic tool in back pain patients and recommend its use. The American Pain Society rejects discography as a diagnostically useful test. A comprehensive review of discography in the journal of the American Society of Regional Anesthesia and Pain Medicine notes that whereas CT discography is the gold standard for the assessment of structural disc alteration, there is no convincing evidence that using discography as a selection tool improves surgical outcomes. Any analysis of imaging findings in discogenic pain patients thus remains based on a reference standard (provocation discography) that is ultimately unproved against a pathoanatomic gold standard. This is further confounded by the evolution of the criteria for a positive discogram since the 1990s.
Although challenging, there is motivation to make the diagnosis of discogenic pain via noninvasive imaging. Discography has historically been considered a minimally invasive and nondestructive test. Recent in vitro and in vivo evidence has raised concern that disc puncture or discography may contribute to disc dysfunction. Korecki and colleagues noted that in a bovine disc model, single punctures with a 25-gauge needle resulted in biomechanical degradation of disc function with cyclic loading. Carragee and coworkers demonstrated using a 10-year follow up that asymptomatic subjects who had undergone investigational discography showed more degenerative phenomena on imaging than did matched control subjects and incurred more spine related health care, including surgery. , Discography in these series allowed intradiscal pressures up to 100 per square inch (PSI) and were not performed on back pain patients. Another study, utilizing back pain patients with intradiscal pressures restricted to <50 PSI per SIS Guidelines, did not show any difference in degenerative imaging findings, including disc herniations, in punctured vs. unpunctured discs. Although the clinical significance of these observations remains uncertain, a noninvasive diagnosis is desirable.
The specificity fault inherent in spine imaging was previously discussed: manifestations of disc “degeneration” including loss of T2 hyperintensity, loss of disc space height, and disc contour abnormalities (disc bulges and protrusions) are ubiquitous, often asymptomatic, and primarily represent normal age change ( Fig. 20.7 ). However, in a population of symptomatic patients with suspected discogenic pain, are there imaging findings that correlate with positive provocation discography?
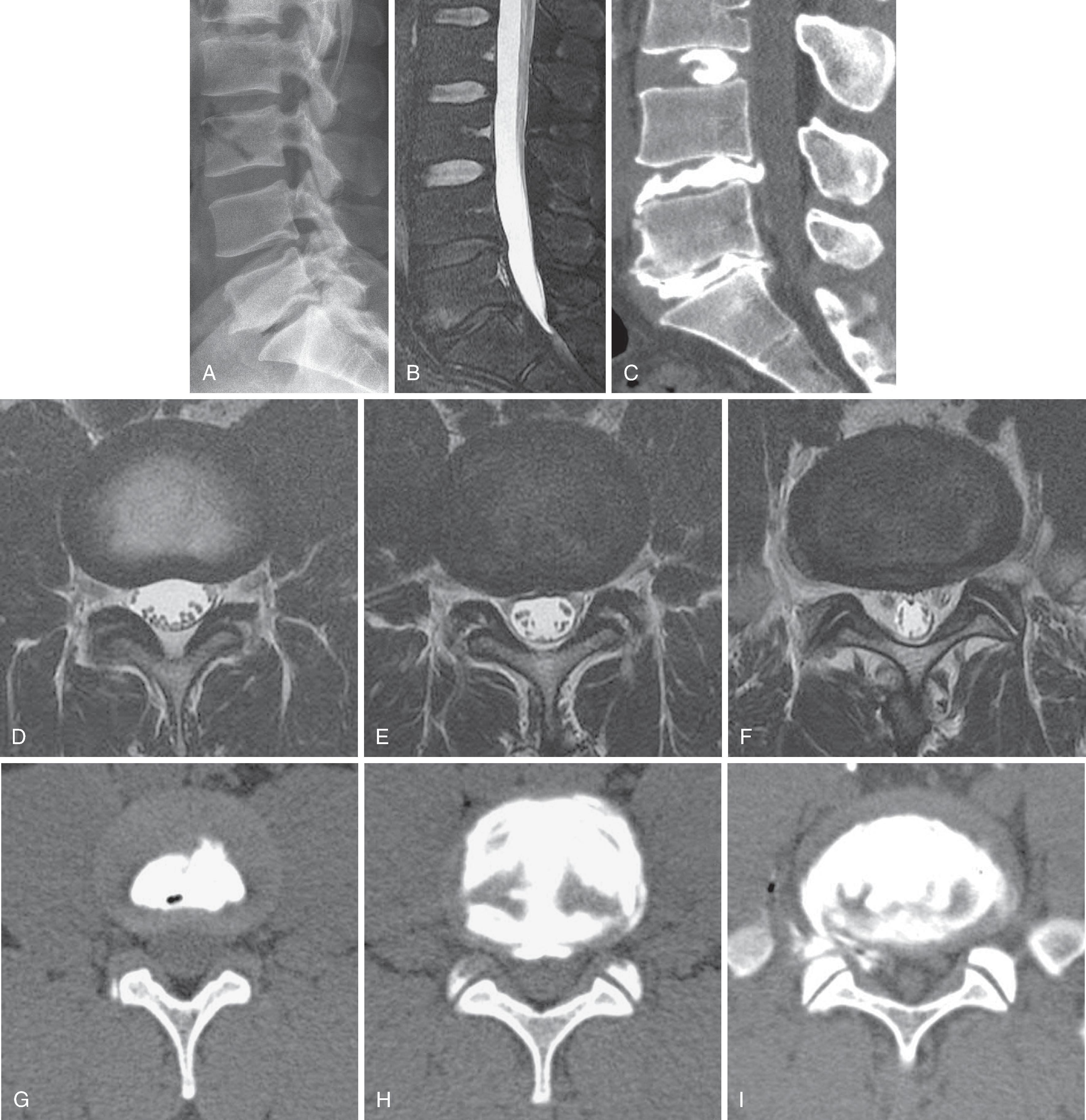
The functional unity of the disc and the cartilaginous endplate is manifested in signal changes within the endplate and adjacent subchondral marrow that accompany nuclear matrix degradation. Endplate marrow changes were originally classified by Modic in 1988 ( Fig. 20.8 ). Type I change represents ingrowth of vascularized granulation tissue into sub-endplate marrow; it exhibits a hypointense T1 and hyperintense T2 signal on MRI and may enhance with gadolinium. Type II change exhibits elevated T1 and T2 signals and reflects fatty infiltration of sub-endplate marrow. Type III change is hypointense on T1 and T2; it correlates with osseous sclerosis. Type I change is thought to represent an active inflammatory state, with type II more quiescent and type III post-inflammatory. Ohtori and colleagues noted elevated levels of protein gene product 9.5-immunoreactive nerve fibers and tumor necrosis factor alpha (TNFα) immunoreactive cells in the cartilaginous endplates of patients with Modic changes. The immunoreactive nerve ingrowth was noted exclusively in patients with discogenic low back pain. TNFα immunoreactive cells were more common in type I endplate changes.
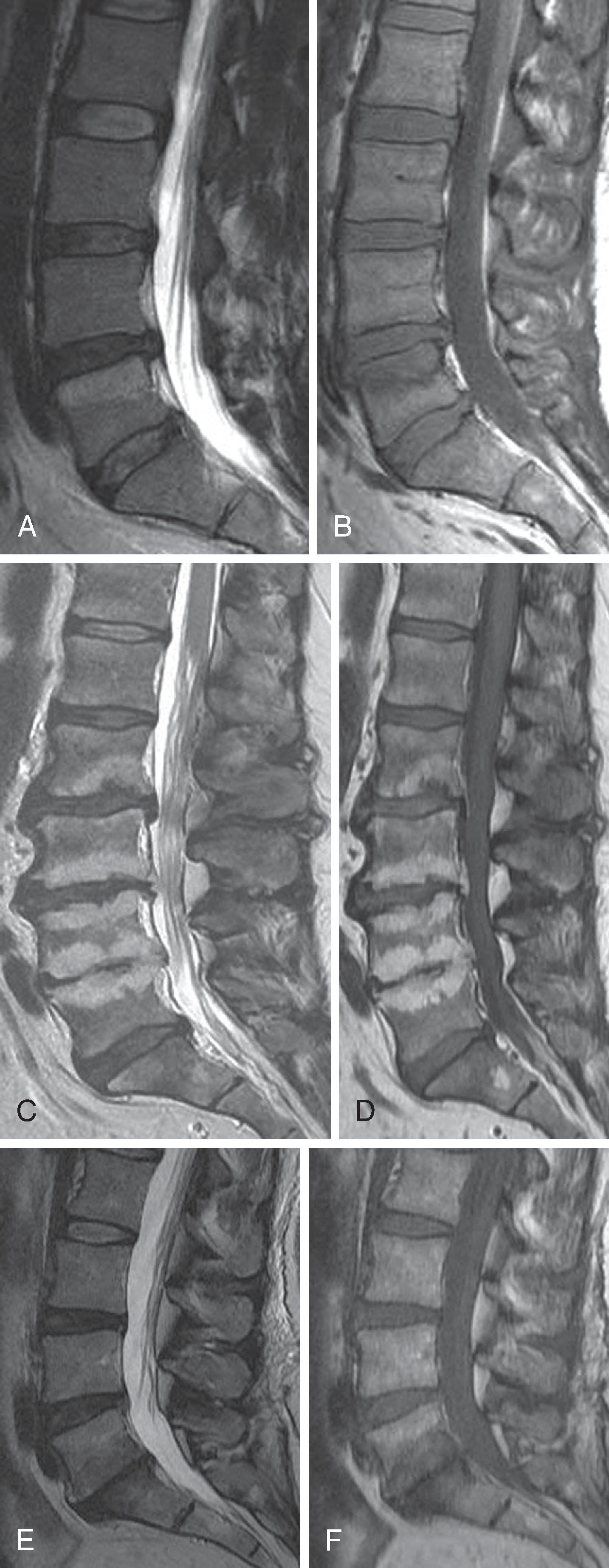
Modic endplate changes do carry an association with low back pain, particularly type I change. Albert and Manniche reported low back pain in 60% of patients with Modic changes and in only 20% of those without Modic changes. Type I change was more strongly associated with low back pain than type II change. Thompson and colleagues examined nearly 2500 discs. Type I change had a positive predictive value (PPV) for positive provocation discography of 0.81, while type II change yielded a PPV of 0.64. Weishaupt and colleagues, with a threshold at 25% of vertebral body height for either type I or type II change, saw no false-positive results (100% specificity). Bogduk compiled the data of six studies examining Modic change of either Type I or II, revealing a specificity of 0.83 (sensitivity = 0.24) and a likelihood ratio of 3.4 as a predictor of positive discography.
Modic type I change may also be associated with segmental instability. Persistent type I change after fusion surgery raises concern for pseudarthrosis. Patients with solid fusions are more likely to have either persistent type II change or resolution of all marrow abnormality. One caveat about Modic changes is that their detection and classification are heterogeneous across different studies. Among the multiple reasons are a lack of standardized reporting guidelines and technical factors such as magnetic field strength and pulse sequence parameters. As in other disciplines, artificial intelligence will likely play an increasing role in the imaging evaluation of back pain, including the potential for automated detection/calculation of characteristics such as Modic changes and spinal stenosis grades.
In 1992, Aprill and Bogduk described the high intensity zone (HIZ) as an imaging marker of a painful disc at provocation discography ( Fig. 20.9 ). Their definition of the HIZ is a “ high intensity signal (bright white) located in the substance of the posterior annulus fibrosis, clearly dissociated from the signal of the nucleus pulposis in that it is surrounded superiorly, inferiorly, posteriorly and anteriorly by the low intensity (black) signal of the annulus fibrosis and is appreciably brighter than that of the nucleus pulposis” (Page 362).
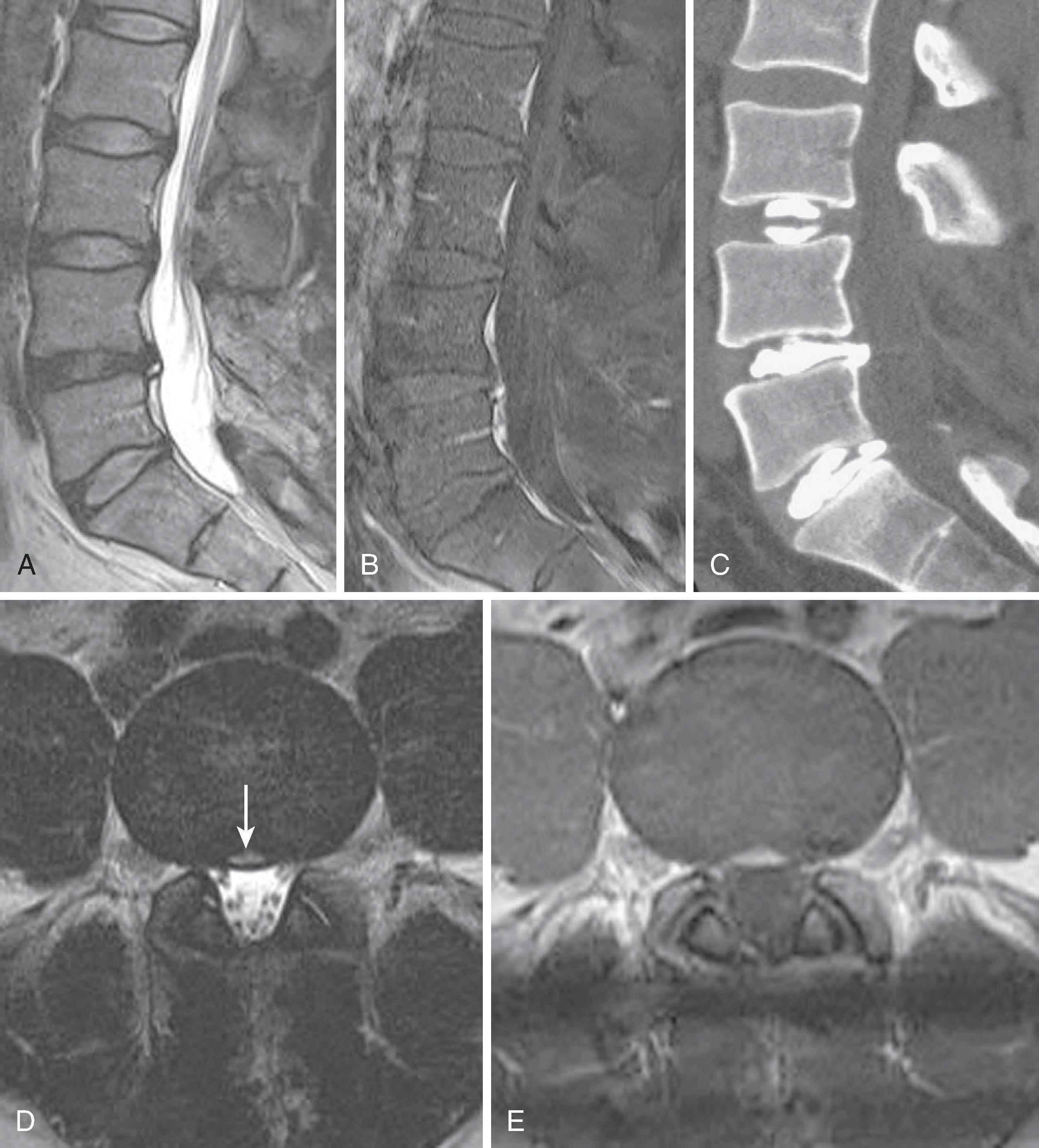
This finding was identified on a midsagittal T2-weighted image; it may occur centrally in an otherwise normal annulus, in a bulging annulus, or be located superiorly or inferiorly behind the edge of the vertebral body in a severely bulging annulus. In a series of 500 consecutive patients, the per-patient prevalence was 29%; the per-disc prevalence of an HIZ was 6%. The majority of HIZs were present at the L4 and L5 disc levels, confirmed in later studies.
The relationship of the HIZ to pain production was evaluated in a subset of 41 patients selected for the presence of an HIZ on prediscography MR imaging. Discography was performed with the requirement of a nonpainful control disc for a diagnosis of discogenic pain. Pain responses were tabulated both as “exact” reproduction of pain as well as “similar” pain. In all, 118 discs were tested in 41 patients; the per-disc prevalence of the HIZ was 34%, reflecting selection bias. In detecting exact pain, the HIZ had a sensitivity of 82% and specificity of 89%, a 70% PPV, and a positive likelihood ratio (+LR) of 7.3. When the discographic criteria were relaxed to exact or similar pain, the specificity rose to 97% with a PPV of 95%; there were only two false-positive cases where a disc bearing an HIZ was nonpainful. The investigators postulated that the HIZ represents a complex grade 4 fissure where the nuclear material has been trapped within the lamellae of the annulus fibrosis and become inflamed, accounting for the T2 hyperintensity, brighter than that of the parent nucleus. They advanced the HIZ finding as pathognomonic of a symptomatic disc. The publication of these findings elicited considerable interest and many subsequent studies , attempting to verify or refute its conclusions. Bogduk compiled the results of 12 studies addressing the HIZ as a predictor of a positive discogram, revealing a pooled specificity of 88% (sensitivity 45%) and a likelihood ratio of 3.8 for this imaging finding. A more recent retrospective analysis of nearly 2500 discs in patients who underwent discography showed that the presence of an HIZ had a PPV of 0.71.
Disc contour abnormalities have been studied as predictors of positive discography, and although associations have been identified, there is considerable disparity among studies without a clear pattern. , ,
Conventional imaging techniques for assessment of the intervertebral disc and spinal pain are qualitative (not quantitative), unable to evaluate disc substructure, and unable to discriminate among early/subtle changes of disc degeneration. Although not in routine clinical use in most radiology practices, emerging advanced MRI techniques attempt to address these limitations. These include quantitating the degree of “degenerative” changes affecting the intervertebral disc, quantifying its biochemical composition, discriminating among early degenerative changes, and identifying subtle changes in key pain generator phenotypes. Such techniques include but are not limited to T2 mapping, T1 rho mapping, MR spectroscopy, and ultra-short time-to-echo (UTE) MRI.
T2 mapping (also known as T2 relaxometry) involves quantification of the content of water, proteoglycan, and collagen sequence breakdown. These different tissues can be objectively quantitated based on different relaxation times (T2 “values” or “times”), which is an intrinsic tissue property that reflects the disc’s molecular environment. These T2 relaxation times tend to decrease with age, and decreased T2 relaxation times correlate well with an increased grade of both disc and cartilaginous endplate degeneration. Objective and continuous grading of disc generation may be possible by histogram analysis. Some studies suggest that disc degeneration as quantified by T2 mapping may correlate with increasing low back pain, allowing differentiation of chronic low back pain patients from controls. Others suggest that quantitative T2 mapping may be able to indicate discs at-risk for future herniation and correlate with patient’s clinical status at longer term follow up. One potential advantage of T2 mapping is the ability to indicate pathologic changes of the disc before it is evident subjectively to human readers. However, to date, normative values of T2 times are lacking, both within individual patients and across patient populations. Thus T2 mapping is not yet able to supplant traditional visual assessment of MR images. Some data suggest that healthy discs in a given patient may provide the best definition of normal when available, to which abnormal levels could be compared. There are many reasons why such quantitative imaging is not a panacea for the assessment of back pain. For instance, T2 calculations may be affected by unique subtle variations in the magnetic environment, such that different values may result from scan to scan, even when imaging equipment and scanning parameters are identical. T2 times can also be affected by confounding variables such as specific lumbar levels among non-degenerated and degenerated discs, time of the day, and axial loading of the spine. Precisely where to measure the quantitative features is an added complexity for quantitative imaging; for example, discs can be analyzed at the level of the anterior outer annulus, posterior outer annulus, or nucleus pulposus/inner annulus. The inherent limitation of not being to precisely delineate these different substructures further complicates matters. In addition, the study methodology varies, including the use of either axial or sagittal imaging. Many publications in the quantitative imaging arena are also limited by relatively small sample sizes.
Meanwhile, mapping of the T1 rho time constant quantifies the degree of cartilaginous degeneration by assessing water molecule dispersion within the discal cartilage. T1 rho is an MRI relaxation time parameter that is sensitive to low frequency interactions between macromolecules and bulk water. The dynamic range of T1 rho values is greater than that of T2 relaxation times. , Lower T1 rho values are found in degenerated discs and may correlate with discogenic pain. In a high field strength (9.4 T) animal research, data from Paul and colleagues suggest that T1 rho mapping correlates better than T2 mapping with biochemical, histologic, and glycosaminoglycan characteristics of the disc.
MR spectroscopy (MRS) can also evaluate the biochemical structure of tissues. After initial reports of the potential utility of this technique for assessing the intervertebral disc based on cadaveric and ex vivo data, in vivo studies began emerging. A recent study by Gornet and colleagues showed a correlation between discography results and MRS analysis of disc structure (proteoglycan and collagen) and acidity (lactate, alanine, propionate). MRS showed the ability to discriminate discogram positive from negative discs and the potential for this information to affect surgical outcomes. Some investigators have correlated multifidus muscle fatty atrophy with chronic low back pain and disc degeneration, with intramyocellular but not extramyocellular lipid accumulation as detected by MRS showing association. ,
Quantitative imaging has also been applied to the cartilaginous endplate, such as with the use of UTE MRI. On conventional T2-weighted images, the endplate signal is hypointense and its integrity is difficult to assess. UTE MRI results in the endplate as signaling hyperintense and affords the potential of assessing the morphology and biochemical structure of the endplate. Quantitative measurements of endplate thickness on 3-dimensional UTE MRI appear to be associated with adjacent disc degeneration. Some investigators have also used UTE MRI to assess the intervertebral disc. A hypo- or hyperintense band within the disc on UTE imaging may correlate well with disc degeneration, low back pain, and disability.
In summary, quantitative high-resolution imaging techniques such as those described above show promise in evaluating degenerative findings involving the intervertebral disc. They offer the possibility of evaluating both structural and functional characteristics of the disc. The hope is that one or more such techniques could be implemented into routine or problem solving clinical practice to identify clinically meaningful and potentially actionable evidence of subtle/early disc degeneration.
Imaging identification of discogenic pain is challenging for a variety of reasons. (1) There is no pathologic or surgical gold standard. (2) The existing standard of comparison, discography, is ultimately unproven, is subjective in its interpretation, and has evolved over time in its criteria for a positive test. None of the studies reviewed earlier use the most current and restrictive criteria, those of Spine Intervention Society (SIS). (3) The imaging findings likely have threshold effects, where only a significant expression of the finding (intensity of an HIZ, extent of marrow change) is a useful predictor of discogenic pain. Most studies do not account for this factor. (4) Imaging findings are likely technique dependent to an unknown degree, and imaging techniques are evolving.
The best available imaging predictors of discogenic pain, when applied to a select population of patients with axial back pain, in whom other pain generators have been excluded, are Modic endplate changes and the HIZ. Discography remains the reference standard for the diagnosis of discogenic pain. Quantitative imaging techniques have emerged and are evolving, but not in widespread clinical use.
This discussion has purposely focused on the lumbar spine. The lumbar spine segment is most commonly afflicted with discogenic pain, and the literature regarding its pathogenesis and evaluation with imaging and provocation discography, although challenging, is of greatest depth. The cervical intervertebral disc is structurally distinct from the lumbar disc. There is no posterior annulus, and the small nuclear compartment disappears early in life; the residual fibrocartilaginous plate normally develops fissures as mere age-related change. There are no morphologic features at discography that contribute to a diagnosis of discogenic pain. Diagnosis is reliant entirely on the provocation of concordant pain, with the requirement of nonpainful control discs.
As in the lumbar segment, structural age changes (loss of T2 signal, loss of disc space height, contour abnormality) are ubiquitous in cervical MRI studies. The Matsumoto study cited earlier examined 2480 cervical discs in asymptomatic subjects and noted loss of T2 signal in 17% of males and 12% of females between ages 20–30, and 89% of males and 86% of females over 60 years old. A 10-year follow up of this study by Okada and colleagues demonstrated that progression of degenerative findings occurred in most patients (81%). The only factor related to this progression was age. Clinical symptoms developed in a smaller proportion of subjects (34%). Daimon and colleagues then published a 20-year prospective longitudinal follow up of this study in 2018. Progression of degenerative findings was essentially ubiquitous, seen in 95% of patients. The rate of progression of structural findings on MRI increased with age. Only the progression of foraminal stenosis had any association with the development of clinical symptoms (specifically, the onset of upper limb pain). In another study by Okada, 89% of asymptomatic subjects (mean age 49 years) exhibited structural age changes on MRI; another group of patients (mean age 46 years) with symptomatic lumbar disc herniations but asymptomatic of neck pain showed cervical disc age-related change in 98% of the patients. There is a paucity of literature addressing the correlation of imaging findings with cervical discography. An early study by Parfenchuck (1994) showed only a modest ability of MRI findings of T2 signal loss or disc contour abnormality to predict a positive cervical discogram (sensitivity = 73%, specificity = 67%). Schellhas’ (1996) study suggested that MRI cannot reliably predict a positive cervical discogram. A subsequent study by Zheng again demonstrated only a modest predictive value of MRI using parameters of T2 signal loss and disc contour abnormality (sensitivity = 73%, specificity = 49%). The inflammatory disc parameters that proved to have such high specificity in the lumbar region are either unusual or little studied (Modic change) or have no anatomic existence (HIZ) in the cervical region. Occasionally foci of elevated T2 signal are observed in the posterior cervical disc, but in the absence of a posterior annulus, the anatomic correlate is unclear. Imaging identification of discogenic pain in the cervical spine remains elusive. Cervical discography remains the reference standard in the diagnosis of cervical discogenic pain. In patients with neck pain with or without neurologic symptoms, a study of 1059 patients revealed that the number of degenerated disc levels and the degree of cervical disc degeneration increases significantly with age. C5–6 was the most common degenerated level, followed by C4–5 and C6–7. C5–6 and C6–7 were most common in those patients with two-level degeneration, followed by C4–5 and C5–6, and C3–4 and C-5. Degeneration was seen at contiguous levels, with skip level degeneration uncommon.
The imaging investigation of discogenic pain highlights a theme common to this chapter: physiologic parameters have greater significance in pain syndromes than purely structural alteration. To be ultimately valuable in the diagnosis of discogenic pain, imaging must move beyond macroscopic descriptions of morphology to the realm of biochemical imaging, quantifying the change in nuclear constituents over time. Besides characterizing biochemical nuclear matrix degradation, imaging will also need to identify inflammatory mediators in the disc and adjacent cartilaginous endplate more precisely. Perhaps then we will truly be capable of the noninvasive diagnosis of discogenic pain.
Recently, the so-called vertebrogenic model of pain has emerged as a potential alternative to the discogenic model. The vertebrogenic model is based on the hypothesis that nociceptors in the endplates can cause acute or chronic pain. This theory, that low back pain may arise from the endplates rather than the disc, has been increasingly supported by a growing body of literature. ,
At the root of the vertebrogenic model of pain is the concept that endplates are inherently prone to spondylotic changes. This is because of the conflicting functional roles held by endplates. Endplates must be strong enough to withstand the substantial physiologic stress put upon the spine. However, because discs lack a dedicated blood supply, endplates must also be porous enough to allow for passive transport from the vertebral body capillaries. This porosity, in the setting of substantial tensile strains placed upon the vertebral bodies, frequently results in damage. Some endplate regions are particularly susceptible to destructive changes, likely related to normal anatomic variations in endplate composition. For example, an endplate’s center is thinner than its periphery, and superior endplates are thinner than their inferior counterparts.
Once endplates are damaged, pro-inflammatory mediators can communicate between the disc and the vertebral body bone marrow. This is typically followed by a cascade of spondylotic changes, in which cross-talk between the disc and bone marrow leads to progressively increasing inflammatory mediators. Ultimately, such changes result in the degradation of endplates in regions often specific to the spinal level and location within the endplate.
On MRI, this spondylotic process typically appears as Modic changes. Modic changes, as discussed above, are associated with low back pain and are relatively specific imaging markers for predicting the reproduction of symptoms during provocative discography. The association between Modic changes and provocative discography would seem to support the discogenic model. However, the closely related innervation between the annulus and endplates means that pain could be elicited from the endplate or disc. Some authors have opined that the correlation between MR findings and provocative discography presents some of the best evidence for the vertebrogenic model of low back pain. Nevertheless, it remains impossible to distinguish between vertebrogenic and discogenic pain confidentially.
Anatomically, vertebrogenic pain is usually attributed to nociceptive innervation by branches of the basivertebral nerve (BVN). The BVN stems from the sinuvertebral nerve and enters the vertebral body via the posterior basivertebral foramen before branching along the endplates. , The BVN has been shown to contain substance P, protein S-100, PGP 9.5, and calcitonin gene-related peptide, confirming its role in nociception. ,
The innervation density of the vertebral body endplates is similar to the annulus and is greatest within the central endplate. In healthy endplates, the innervation is modest in quantity. However, damage to cartilage and subchondral bone induces proliferation of sensory nerve fibers, thought to be a chemotactic response to elements of disc breakdown. , , Spondylotic changes may lead to a greater density of innervation by branches of the BVN, accounting for the localization of pain to the vertebral body endplates.
The vertebrogenic model has led to the recent development of novel therapeutic approaches to treat low pain back. Among these, BVN ablation using radiofrequency (RF) energy has gained the greatest interest. , Both single-arm studies , and randomized control trials , have been completed. The results have overwhelmingly demonstrated the efficacy of BVN ablation. For example, Fischgrund et al. found that patients treated with intraosseous BVN ablation showed superior improvement in Oswestry Disability Index (ODI)—an outcome marker for patients with low back pain—and a greater rate of response compared to control patients treated with sham procedures. Khalil et al. compared the efficacy of BVN RF ablation to standard care in patients with low back pain. The authors reported clear superiority of ODI-based outcome in the treatment group, with early statistical power resulting in termination of study enrollment and offering cross-over to the control group. Laser ablation of the BVN, too, has been used with promising results.
Despite the encouraging results demonstrated in these early trials, additional studies will have to determine the safety and long-term efficacy of BVN ablations. In addition, trials to date have been restricted to the lower spine (L3–S1), and patients with Modic I or II changes on MRI. Further investigation is needed to understand whether procedural success can be translated to other levels in the spinal column. Finally, it will be important to develop a reliable set of clinical, imaging, and/or testing (e.g. provocative discography) criteria by which vertebrogenic pain is diagnosed and whether such criteria can be used to differentiate vertebrogenic from discogenic pain.
The supporting structure of the posterior column of the spine includes the paired facet joints with their associated capsules, the ligamentum flavum, the intraspinous and supraspinous ligaments joining the spinous processes, and the intertransverse ligaments. The inferior articular process of the facet joint faces anteriorly and is convex in configuration. On axial images, the inferior articular process is the more posterior component of the joint. The superior articular process (SAP) has a concave articular surface that faces posteriorly and medially. On axial images, it appears as the anterior component of the joint. In an erect standing position, the lumbar facet joints bear approximately 16% of the compressive load; they bear essentially no load in a flexed sitting position. With the loss of disc space height, the lumbar facet joints will bear proportionally more axial load. The fibrous joint capsule has been demonstrated to be richly innervated by nociceptors and proprioceptive fibers. In a normal state, nociceptors such as those seen in the facet joint capsule have a high threshold and would not be expected to discharge unless loads are supraphysiologic. However, in the presence of pathologic joint inflammation, synovitis, chemical mediators may sensitize these nociceptors, and supraphysiologic levels of stress may no longer be required to stimulate pain. Such inflammatory mediators (substance P, bradykinin, phospholipase A2) have been detected in the facet joint capsule.
There is thus a pathoanatomic basis for facet joint-mediated pain, particularly in the presence of facet synovitis. The imaging challenge in identifying a potentially painful joint lies in the well demonstrated specificity fault, that imaging changes of morphologic facet arthrosis (subchondral sclerosis, erosions, or cyst formation, osteophytes, joint space narrowing, vacuum phenomena) are age-related changes and do not correlate with pain. The reference standard for facet pain is positive comparative dual medial branch blocks, as there are no reliable physical exams or historical features allowing a confident diagnosis of Z-joint pain. Schwarzer and colleagues semi-quantitatively scored CT finding of structural facet arthrosis and found no relationship to Z-joint pain as determined by placebo controlled medial branch blocks. More recently, Cohen and associates showed no association between MRI findings of structural facet arthrosis and pain relief with dual medial branch blocks and subsequent radiofrequency rhizotomy. These structural changes become universal by the sixth or seventh decade of life; they do not represent active inflammatory disease causal of pain.
Instead, imaging must look to identify physiologic, not structural, markers of active facet synovitis. Imaging techniques that may be applicable include the technetium pyrophosphate bone scan (including its technologic evolution SPECT and SPECT/CT) and the MRI physiologic imaging parameters of T2 hyperintensity and gadolinium enhancement. Technetium pyrophosphate bone scans detect hyperemia and accelerated bone turnover, which may be considered manifestations of active inflammation. Dolan and associates compared the response to intra-articular facet joint injection guided by clinical exam versus positive SPECT studies; there was a significantly greater short term (one- to three-month) clinical response in the SPECT-guided injections. A similar study by Holder and colleagues, using response to uncontrolled intra-articular injections as the reference standard, identified a 100% sensitivity and 71% specificity for SPECT scans in the detection of facet joint-mediated pain. Pneumaticos and colleagues prospectively studied patients undergoing intra-articular injections in three groups: injections guided by positive SPECT scans, injections in patients with negative SPECT scan guided by clinical exam, and injections in patients without SPECT studies guided by clinical exam. The patients whose injections were directed to SPECT-positive joints had significantly better clinical outcomes than the other two groups, as well as lower costs as fewer joints were injected. McDonald used SPECT/CT to identify joints for injection in 37 patients with clinical facet joint lumbar pain. The mean visual analog score dropped from 7.2–2.8, with an average duration of benefit of 2.2 months; only 1 in 37 patients did not report benefit. The fused SPECT/CT images were useful in distinguishing the L4–5 and L5–S1 facet joints.
There is no correlation between the degree of morphologic facet arthrosis and the intensity of SPECT activity. Rather, the phase of active synovitis correlating with pain production may occur relatively early in the progressive development of structural changes of facet arthrosis. Kim and colleagues correlated MRI findings in the facet joints with SPECT; they noted that the MRI appearance of T2 hyperintensity in the synovium, intrasynovial fluid, and cartilage disruption best correlated with a positive SPECT, here used as a reference standard for active facet synovitis. More extensive structural manifestations of facet arthrosis did not demonstrate the physiologic finding of SPECT activity. Cervionke and colleagues have demonstrated an indirect association of T2 hyperintensity within and about the facet joint with axial pain. T2 hyperintensity in the adjacent pedicle has also been associated with axial pain of facet origin; this finding can also be seen in pedicle or pars stress fractures. T2 hyperintensity accompanying facet synovitis may also be seen in the surrounding multifidus muscle, occasionally sufficiently extensive enough to raise concern of a sinister process. Gadolinium enhancement in and about the facet joint also suggests facet synovitis. Ultimately, the physiologic parameters of edema, hyperemia, and accelerated metabolic activity as detected by increased SPECT or SPECT/CT activity, and T2 hyperintensity and gadolinium enhancement on MRI must be evaluated as predictors of facet joint pain against the current reference standard, comparative medial branch blocks. Prevalence studies of these parameters in populations asymptomatic of back pain are also needed for researchers to understand the specificity of these imaging observations. Despite the lack of absolute validation, if the patient is going to undergo an MRI for evaluation of axial pain, a fat-saturated or STIR sequence should be performed, as it represents the best chance of demonstrating physiologic findings (T2 hyperintensity), which may identify an axial pain generator ( Fig. 20.10 ).
Various investigations have provided insight into the potential correlation of facetogenic pain with physiologic imaging findings of the facet joints. Lehman and colleagues retrospectively reviewed the utility of 212 SPECT/CT exams of the spine and sacrum over four years. These exams were most commonly used in the diagnosis and management of pain, helping to identity pain generators and frequently resulting in a resultant change in clinical management. However, Lehman and his colleagues also studied 74 patients who underwent subsequent percutaneous facet joint steroid injection or comparative medial blanch blocks after a SPECT/CT scan. Interestingly, the targeted facet joint treatment(s) was frequently discordant with the facet joint(s), demonstrating increased uptake on the nuclear medicine study. In approximately one-third of patients, the activity did not correlate with clinical findings. Similarly, when comparing facet joint activity on SPECT/CT with facet joint signal change on MRI with fat suppression, Lehman and colleagues found these two imaging findings did not always correlate and therefore should not be considered interchangeable.
Besides SPECT/CT, other hybrid imaging modalities that are being used in at least the research setting to evaluate facet joint arthropathy are PET/CT and PET/MRI. PET/MRI is an alternative, emerging imaging modality that offers the possibility of evaluating facet joint inflammation with both radiotracer uptake and MRI findings of edema and/or enhancement. One of the elements for its appeal is the lack of ionizing radiation associated with the cross-sectional (MR) component, unlike the CT aspect of SPECT/CT and PET/CT. In a prospective study of 10 patients with clinically suspected facetogenic low back pain at specific levels, 18F-fluorodeoxyglucose (FDG) activity did show high concordance with MRI findings of considerable facet inflammation (prominent posterior element osseous or adjacent soft tissue edema/enhancement). However, there was low concordance of both MRI findings of inflammation and FDG activity with the clinically implicated joints. Future research is needed to further elucidate the role of such hybrid imaging technologies, including ideally a comparison of imaging findings with the results of medial branch blocks. One such prospective study by Freiermuth and colleagues included 29 patients; the sensitivity and specificity of SPECT/CT for a positive response after a medial branch block were 57% and 77%, respectively. In addition to FDG-PET, some investigators are using sodium fluoride ([ 18 F]-NaF) PET (combined with either CT or MRI) to evaluate facet joint inflammation. This radiotracer can detect bone turnover. In one study of six patients with facetogenic low back pain who underwent [ 18 F]-NaF PET/MRI, a significant positive correlation was observed between maximum facet joint tracer uptake and clinical disability. Such pilot data are promising but require further validation.
A tissue pathway between contralateral same segment facet joints was initially described in the cervical region by Dr. Kikuzo Okada in 1981; 80% of cervical facets demonstrated a communication between the joint capsules via a space situated dorsal to the ligamentum flavum in the axial plane and the interlaminar space in the coronal plane. This communication is frequently observed in intra-articular injections of cervical facet joints that are structurally normal on imaging or exhibit only modest evidence of synovitis or structural arthrosis. In the lumbar region, this communicating pathway is commonly observed only in the face of advanced facet arthrosis; it may also be observed in the presence of defects in the pars interarticularis. This lumbar retroligamentous pathway also commonly communicates with an adventitial bursa within the interspinous ligament (i.e. Baastrup’s disease).
This pathway may serve as a means of transmission of infection or, more commonly, noninfectious inflammatory change between multiple joints and tissue compartments. When the imaging findings of inflammation and/or fluid in the space of Okada are correlated with low back and/or radicular back pain, Lehman and colleagues in their pictorial review termed this clinical setting as the posterior ligamentous complex inflammatory syndrome. In patients with lumbar axial pain and spondylolytic defects, this posterior element inflammatory complex may involve four facet joints, the bilateral pars defects, and the interspinous ligament. It may have a capacity of 4–6 cc of fluid in the author’s experience. It is also a potential confounding space in epidural steroid injections. For example, this space may provide a false loss of resistance superficial to the dorsal epidural space during interlaminar injections; contrast injection may subsequently fill either facet joint at that segment level or the interspinous ligament ( Fig. 20.11 ). Similarly, during cervical and lumber transforaminal epidural steroid injections, in which the needle tip may be at least partially in or near the facet joint capsule, contrast injection will occasionally demonstrate flow from the ipsilateral facet joint to the space of Okada, simulating dorsal epidural flow.
![Figure 20.11, The space of Okada. Frontal fluoroscopic image (A) demonstrates the space of Okada providing communication between the bilateral C6–7 facets via the right-sided injection. In another patient (B), an attempted right L4 transforaminal injection opacified the superior recess of the L4–5 facet, traversed an L5 pars defect (white arrow) to the right L5–S1 facet, and opacified the space of Okada to a left L5 pars defect (black arrow) and the left L4 and L5 facets. Subsequent computed tomography (C, D, E, F) confirms the opacification of all these structures. The space of Okada is marked by the white arrows in (D) . The space of Okada may contain a small amount of fluid, which appears as T2 hyperintensity ( white arrows, G, H, I ). Note continuity with interspinous ligament ( arrow in [H] and [I] ). Figure 20.11,cont’d. In a final patient, an attempted interlaminar injection (needle placement images J, K ) opacified the interspinous ligament ( white arrow in [L] ) and the left L4–5 facet ( black arrows in [L] ) via the space of Okada. This space ( black arrows in [M] ) is posterior to the ligamentum flavum; the needle was advanced ventrally into the epidural space without incident. (From Murthy NS, Maus TP, Aprill C. The retrodural space of Okada. AJR Am J Roentgenol . 2011;196:W784-W789.) Figure 20.11, The space of Okada. Frontal fluoroscopic image (A) demonstrates the space of Okada providing communication between the bilateral C6–7 facets via the right-sided injection. In another patient (B), an attempted right L4 transforaminal injection opacified the superior recess of the L4–5 facet, traversed an L5 pars defect (white arrow) to the right L5–S1 facet, and opacified the space of Okada to a left L5 pars defect (black arrow) and the left L4 and L5 facets. Subsequent computed tomography (C, D, E, F) confirms the opacification of all these structures. The space of Okada is marked by the white arrows in (D) . The space of Okada may contain a small amount of fluid, which appears as T2 hyperintensity ( white arrows, G, H, I ). Note continuity with interspinous ligament ( arrow in [H] and [I] ). Figure 20.11,cont’d. In a final patient, an attempted interlaminar injection (needle placement images J, K ) opacified the interspinous ligament ( white arrow in [L] ) and the left L4–5 facet ( black arrows in [L] ) via the space of Okada. This space ( black arrows in [M] ) is posterior to the ligamentum flavum; the needle was advanced ventrally into the epidural space without incident. (From Murthy NS, Maus TP, Aprill C. The retrodural space of Okada. AJR Am J Roentgenol . 2011;196:W784-W789.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/RadiologicAssessmentofPatientWithSpinePain/10_3s20B9780323711012000208.jpg)
The sacroiliac joint is a large irregular synovial joint lined by thick hyaline cartilage on its sacral surface and thinner fibrocartilage on its iliac surface. The inferior and anterosuperior aspects of the radiographically perceived joint are synovial; its superior and posterior aspect is ligamentous. The posterior surface of the joint is covered by thick interosseous and dorsal sacroiliac ligaments. There is minimal movement of the joint, except under the hormonal influences of pregnancy. However, this modest mobility is vital for appropriate gait. The synovial portion of the joint is uniformly present in young adults, with a cartilage thickness of 2–5 mm; the synovial space may be attenuated in older adults by fibrous adhesions, but fusion across the joint is not a normal change of aging. Potential communications between the synovial space and the dorsal sacral foramina, the L5 epiradicular sheath, and the ventrally situated lumbosacral plexus have been observed. These could explain radicular pain associated with sacroiliac joint dysfunction, as well as occasional lumbosacral temporary motor block after sacroiliac joint intra-articular injections, related to the spread of anesthetic. Although the surface area of the joint is large, its synovial space is of small volume, ranging from 1–2.5 cc. , Innervation of the sacroiliac joint is complex and remains controversial. Consensus suggests a dominant dorsal innervation from the L5 and S1–S4 dorsal rami.
Osteoarthrosis of the sacroiliac joints may be seen in pathologic specimens of young adults, but it is not generally appreciable radiographically until middle age. Changes in cartilage degeneration are more prominent on the iliac side of the joint. Beyond 40 years of age, many subjects have detectable narrowing of the sacroiliac joint, especially in its inferior portion. This may be accompanied by subchondral sclerosis and osteophyte formation, most prominent anteriorly and inferiorly. A vacuum phenomenon may be seen. As in the facet joint, changes of osteoarthrosis are a normal aging phenomenon and are not predictive of pain.
Evaluation of imaging features predictive of sacroiliac joint pain is yet again confounded by the lack of a pathoanatomic gold standard. Medical history and physical exam provocative maneuvers cannot consistently identify painful sacroiliac (SI) joints. The reference standard for sacroiliac joint pain is the relief of index pain with an intra-articular anesthetic block. This should ideally be either placebo controlled or a comparative block paradigm. Potential leakage from the joint capsule may complicate diagnostic specificity. Schwarzer studied patients with chronic low back pain experienced below the lumbosacral junction. Using response to single blocks as the diagnostic criteria for sacroiliac pain yielded a prevalence of 30%. Requiring a positive block and a ventral capsular tear on postarthrographic CT yielded a prevalence of 21%; adding pain provocation with joint distension to the criteria lowered prevalence to 16%. The Maigne study using double blocks suggested a prevalence of 18.5%. Subsequent work by DePalma identified a prevalence of 18%; sacroiliac joint pain increased in prevalence with age up to approximately 70 years.
Structural changes of osteoarthrosis in the sacroiliac joint are poor predictors of pain. Elgafy and colleagues scored CT features of arthrosis in SI joints and noted a sensitivity of 58% and a specificity of 69%. Physiologic imaging parameters suggesting the presence of hypervascularity, edema, or inflammation are more likely to predict pain. In the studies by Maigne and Slipman, technetium bone scans achieved specificities of 89.5% and 100%, although with low sensitivity, for sacroiliac pain as referenced by uncontrolled intra-articular injections. The MRI physiologic parameters of T2 hyperintensity and gadolinium enhancement have been studied primarily in the context of inflammatory spondyloarthropathies. MRI evidence of active inflammation (sacroiliitis) is intrinsic to the diagnoses of spondyloarthropathies and correlates well with clinical disease activity and therapeutic response to disease modifying agents (TNFα inhibitors) ( Box 20.3 ).
Structural arthrosis, as seen by radiographs, computed tomography (CT) or magnetic resonance image does not predict pain.
Physiologic parameters of hyperemia, edema, and increased metabolic activity may predict pain.
These physiologic parameters may be assessed by T2 hyperintensity (STIR or fat-saturated T2 images), gadolinium enhancement, or increased uptake on single-photon emission computed tomography (SPECT) or SPECT/CT.
There are no validation studies of physiologic parameters against accepted reference standards (dual, comparative blocks).
There are no studies addressing specificity—that is, the prevalence of these findings in asymptomatic subjects.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here