Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The use of electric current for pain management has a long history. As early as the second half of the 19th century, brain lesions were made in animals with the application of direct current, and empirical rules for quantifying lesion size based on current and time were developed. , One of the first uses in humans was for the management of trigeminal neuralgia in 1931 when a direct current was delivered through a needle with a 10 mm uninsulated tip placed in the Gasserian ganglion. This technique produced lesions of unpredictable size. The use of high frequency electric current was later found to produce lesions with a more predictable size. Because frequencies of 300–500 kHz were also used in radio transmitters, the current was called radiofrequency (RF) current. Later, temperature monitoring was suggested to be the most important parameter to obtain a standardized lesion size.
In pain management, RF was first used for percutaneous lateral cordotomy to relieve unilateral pain in cancer patients. A few years later, RF treatment of trigeminal neuralgia was described. The first use of RF current for spinal pain was reported by Shealy, who performed RF lesioning of the medial branch for lumbar zygapophyseal joint pain. Another application for spinal pain was introduced by Uematsu, who described RF lesioning of the dorsal root ganglion (DRG).
At the end of the 1970s, percutaneous cordotomy and RF treatment of the Gasserian ganglion were the only widely accepted RF procedures. A turning point came in 1980, when small-diameter electrodes, known as the Sluijter Mehta Kit (SMK) system, were introduced for the treatment of spinal pain. The system consists of a 22-gauge disposable cannula with a fine thermocouple probe inside for measurement of temperature. The smaller electrode size resulted in diminished discomfort during the procedure. Because there was less risk for mechanical injury to major nerve trunks, targets in the anterior spinal compartment were no longer off-limits, and procedures such as RF lesions adjacent to the DRG (RF-DRG) and lesions of the communicating ramus, , and sympathetic chain became part of the treatment armamentarium.
Over the years, the concept that the clinical effect of RF current was caused by the formation of heat had not been challenged. A selective effect of heat on thin nerve fibers was thought to interfere with the conduction of nociceptive stimuli.
The role of heat was finally questioned for several reasons. First, the classic concept presupposes a strict configuration: the RF lesion must be made in between the nociceptive focus and the central nervous system (CNS). However, RF lesions were also successful when performed distal to the nociceptive focus and the CNS. For example, in the treatment of acute radicular pain from a herniated disk, the electrode is placed distal to the nociceptive focus. Second, RF-DRG induces only transient sensory loss, which is possibly heat related, whereas the pain relief may be of much longer duration. Finally, no differences in outcome were noted when two different tip temperatures (i.e. 40°C and 67°C) were applied. It is against this background that pulsed radiofrequency (PRF) treatment was developed. PRF delivers strong, fluctuating electric fields while the temperature effects are kept to a minimum. PRF was conceived as a novel, potentially safer mode of administration of RF energy. It can be specifically useful for treatments in which RF lesioning is not indicated, such as peripheral neuropathies, atherogenic pain, painful trigger points, and application to the DRG in patients with neuropathy or radicular pain.
A modern RF generator has the following components:
Continuous online measurement of impedance
Nerve stimulation
RF delivery mode
Pulsed current delivery mode
Monitoring of voltage, current, and wattage during the RF procedure
Temperature monitoring
Electrical impedance is measured to confirm the continuity of the electrical circuit. After placement of the needle under fluoroscopic control, nerve stimulation is performed to confirm the proper position of the electrode. Stimulation is carried out at 50 Hz to ensure proximity of the electrode to sensory fibers; 2 Hz stimulation is performed to detect muscle contractions, which indicates that the position of the needle is too close to motor fibers. If an electrode is actually resting on a nerve, the minimum stimulation level required to produce a discharge is 0.25 V. At a distance of 1 cm from the nerve, 2 V would be required. Thus the stimulation threshold is an indicator of the distance of the electrode from the nerve. Temperature is measured with a thermocouple electrode. A thermocouple electrode consists of a junction of two dissimilar metal elements and produces a voltage proportional to temperature.
The generator establishes a voltage gradient between the (active) electrode and the (dispersive) ground plate. RF current flowing through the tissue results in an alternating electric field. This electric field creates an electric force on ions (electrolytes) in the tissue that causes the ions to move back and forth at a high rate. Frictional dissipation of the ionic movement within the fluid medium results in tissue heating. The lesion size depends on the temperature of the tip of the electrode, and tip temperature depends on the amount of power delivered. Other factors influence lesion size, for instance, heat and the type of tissue. Heat is removed from the lesion area by conductive heat loss and blood circulation (heat “washout”). The larger the heat washout, the smaller the lesion will be for a given tip temperature. The type of tissue factors influences heat washout. For example, bone is an effective heat insulator, and therefore RF lesions close to the bone will have less washout. Similarly, segmental blood vessels, which lie in close relationship to the DRG, may cause more heat washout and thereby reduce the size of the lesion.
The treatment effect of PRF is based on the dual effect of exposure of the tissue to RF fields ( Fig. 66.1 ). Besides the ionic friction that causes the production of heat, there is an independent electric field effect. The mechanism of this electric current effect is thought to be an alteration in synaptic transmission in a neuromodulatory type of effect. , , Alterations have been found in peripheral sensory neurons, the DRG, , neurotransmitters and microglia in the dorsal horn, descending inhibitory pain pathways, and the CSF. ,
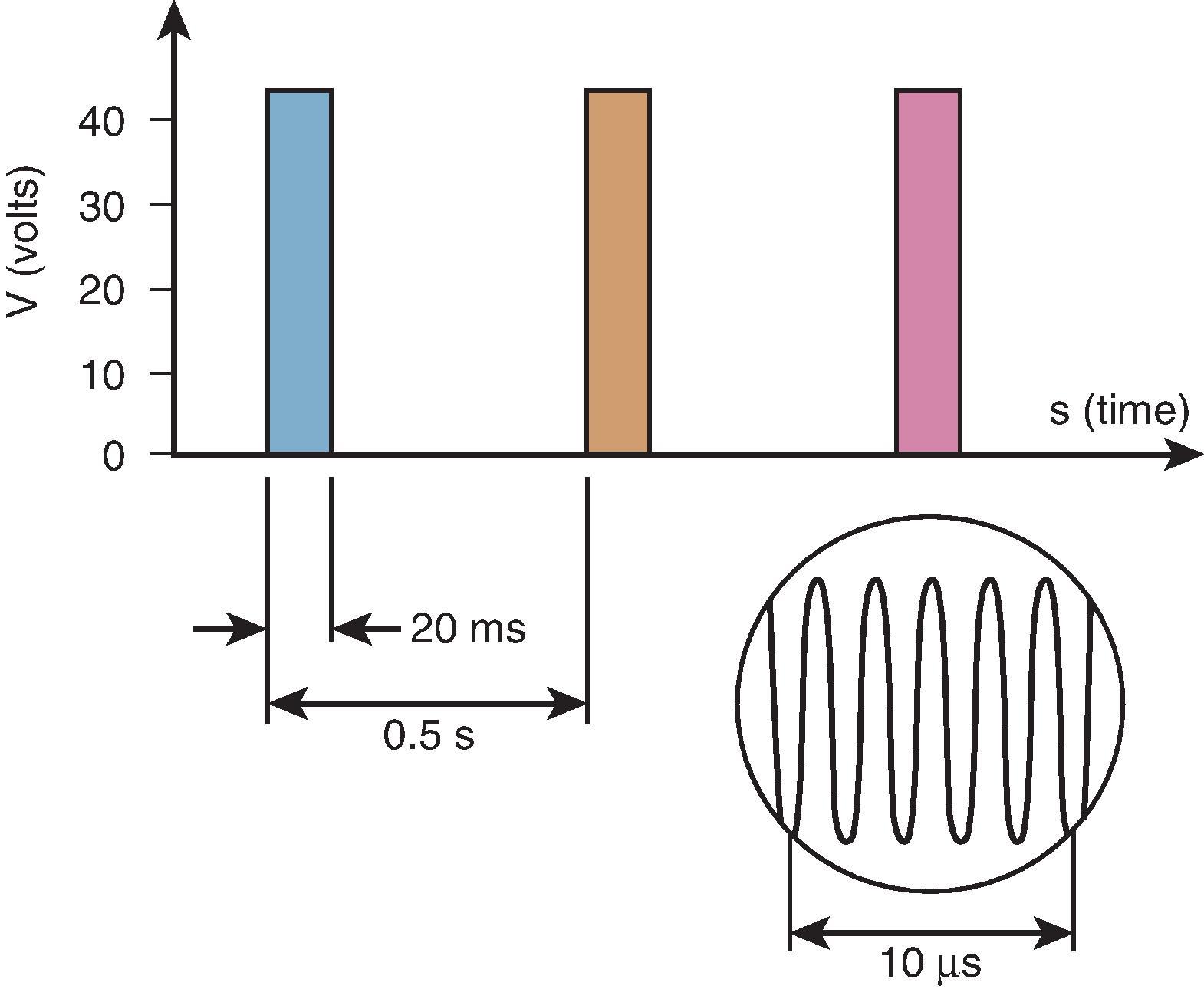
Although no clinical complications have been reported, this technique is considered minimal neurodestructive. Therefore it may be wise to avoid ultralow sensory thresholds (<0.05 V) because such values may reflect intraneural electrode placement. In a small proportion of procedures, the mean tip temperature exceeds 42°C at some point during the PRF procedure. In this case, power output should be decreased as a precaution. This can be done by lowering the voltage or by decreasing either the duration of the active cycle (typically 20–30 ms) or the frequency of the cycle (typically 2 Hz).
It is not desirable to adjust the voltage during a PRF procedure to reach the mean tip temperature since the mean tip temperature does not affect the outcome of the procedure. Because there is a large variation in heat washout, large and unpredictable variations in voltage will occur. There is no optimal duration of treatment. However, durations of 240 s are often reported. Typical PRF treatment settings are 45 V for 240 s at 2 Hz and 20 ms pulse width.
The use of cooled radiofrequency treatment was first described for the treatment of low back/sacroiliac joint pain. Later, it was introduced for the treatment of chronic knee pain.
Cooled electrodes increase lesion size by removing heat from adjacent tissue and allowing power delivery to be increased without tissue scarring and high impedance. A randomized controlled trial (RCT) study showed a higher success percentage and possibly a longer effect with the use of cooled radiofrequency for the treatment of knee pain. There is an increasing interest in the use of cooled RF.
Patients with trigeminal neuralgia have brief episodes of sharp, shooting pain in one or more of the trigeminal divisions that are typically provoked by touch. This so-called trigger area need not be in the division where the patient experiences pain. In the classic case, the patient is free of pain between episodes. However, residual pain has been reported in 42% of cases. These patients have been described as having a combination of trigeminal neuralgia and atypical facial pain. Trigeminal neuralgia predominantly occurs in older age groups (50+ years), although it may occasionally be seen in very young patients. It is thought to be caused by vascular compression of the trigeminal root. In patients with multiple sclerosis, it occurs frequently and may indeed be the first symptom of the disease. In a study evaluating the clinical characteristics of patients with trigeminal neuralgia, 22 had multiple sclerosis. Six of them had atypical trigeminal neuralgia, and 16 had signs of brainstem involvement. It is not clear whether the pain is caused by plaques in the CNS in these patients, but clinically there was no distinction between patients with and without brainstem involvement. Trigeminal neuralgia may also be caused by a primary brain tumor (acoustic neuroma), which should always be excluded before symptomatic treatment is considered (red flag).
In younger patients, posterior fossa craniotomy with microvascular decompression (MVD) is the treatment of choice. This treatment has a high rate of success and avoids the sensory loss that is one of the consequences of thermocoagulation of the ganglion. This procedure has a low complication rate. Complications are mostly serious neurologic deficits. In patients with multiple sclerosis, the procedure should be combined with partial sectioning of the trigeminal nerve. This could be an indication of a more central mechanism in these patients. Pain relief is substantially longer after microvascular decompression than after thermocoagulation of the ganglion. If the pain recurs, recurrent vascular compression is seldom found during reoperation. In such cases, partial sectioning of the nerve could be performed. However, other forms of treatment such as thermocoagulation are generally recommended because the incidence of complications of MVD is distinctly higher after reoperation. , The outcome of thermocoagulation in operated patients is less favorable. PRF seems a safer alternative that was shown to be effective in case reports. , An RCT comparing RF with PRF found a much shorter duration of efficacy for PRF. More recently, an RCT that compared short RF, long RF, and PRF showed that all groups experienced significant differences in pain than baseline. No differences were found between the groups. A retrospective study and an additional RCT found that high voltage PRF resulted in a longer-lasting pain relieving effect than standard voltage PRF. , The choice between MVD, RF, and PRF treatment of the Gasserian ganglion is a clinical decision in which age, physical condition, and personal preference of the patient have to be taken into account.
Following the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system on assessing the quality of evidence, there is a low quality of evidence that RF of the Gasserian ganglion reduces the pain of patients suffering from idiopathic trigeminal neuralgia for at least six months. There is evidence of very low quality that PRF is effective in the treatment of trigeminal neuralgia. However, there is extensive experience with RF treatment of trigeminal neuralgia. A review of 25 years’ experience in 1600 patients undergoing percutaneous RF trigeminal rhizotomy for idiopathic neuralgia indicated acute pain relief in 97.6% and continued complete pain relief at five years’ follow up in 57.7%. Comparisons with other techniques are based mainly on retrospective evaluations.
Comprehensive considerations for the usage of continuous radiofrequency (CRF) or pulsed radiofrequency (PRF) treatment in idiopathic trigeminal neuralgia are outlined by Guo et al.
The technique for placing a radiofrequency needle in the Gasserian ganglion is as follows:
The oval foramen is visualized first by using a tunnel view technique. To attain this, the direction of the x-rays should be reversed from the normal configuration because the image intensifier is too bulky to avoid contact with the patient’s chest ( Fig. 66.2 ). The position of the C-arm should be adjusted until the oval foramen is identified just medial to the mandibular processes and just lateral to the maxilla.
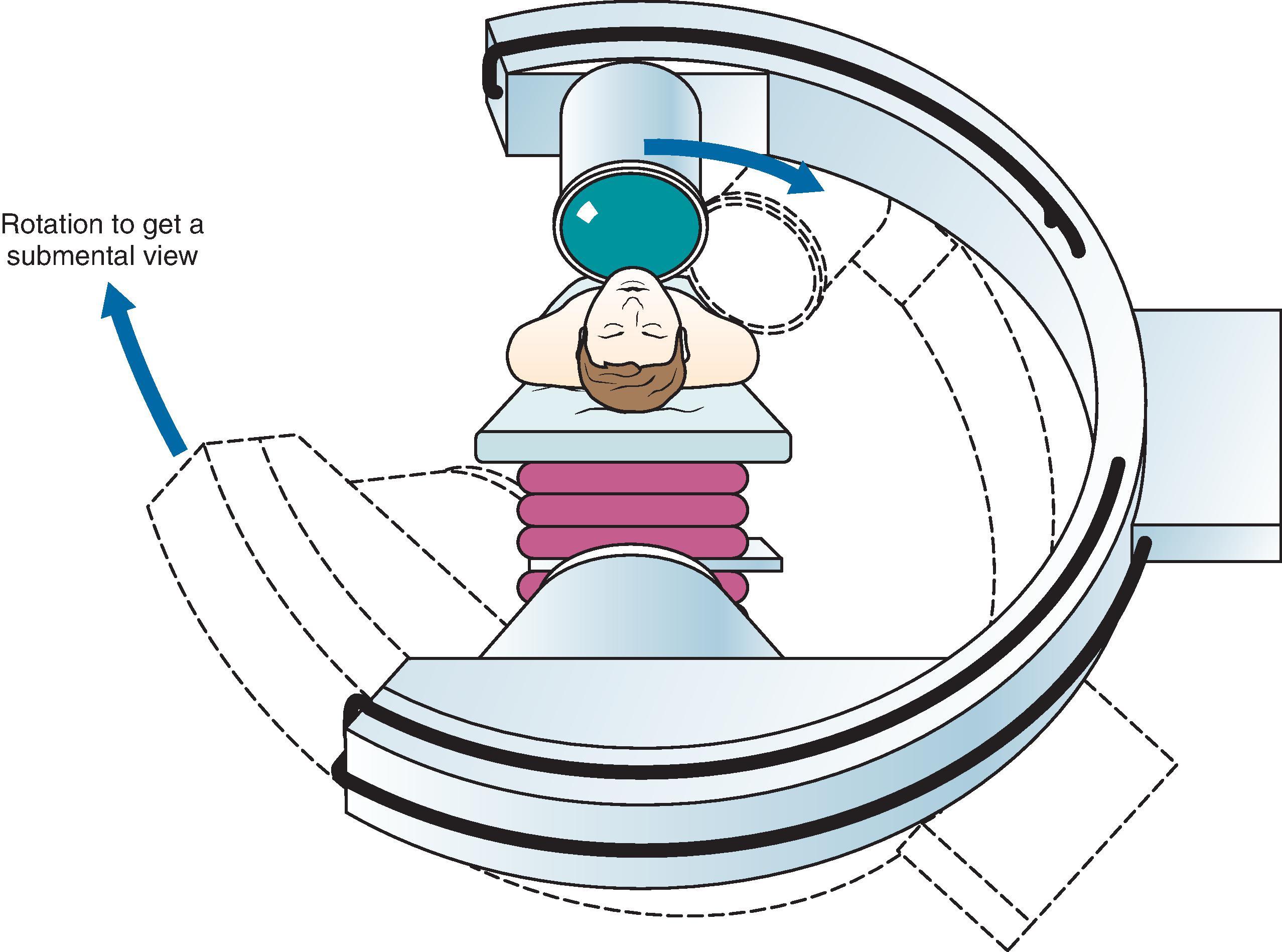
The shape of the foramen varies with the angle of the x-rays in the horizontal plane. A more vertical direction will transform the foramen into a round, almost circular shape. A more horizontal direction will make the foramen flat, like a split. The C-arm should be adjusted so that the foramen optimally has an oval shape. The skin entry point is now marked over the target point and is located lateral to the corner of the mouth. The entry point may be just superior to the mandible, but it also may be much more superior, close to the maxilla ( Fig. 66.3 ).
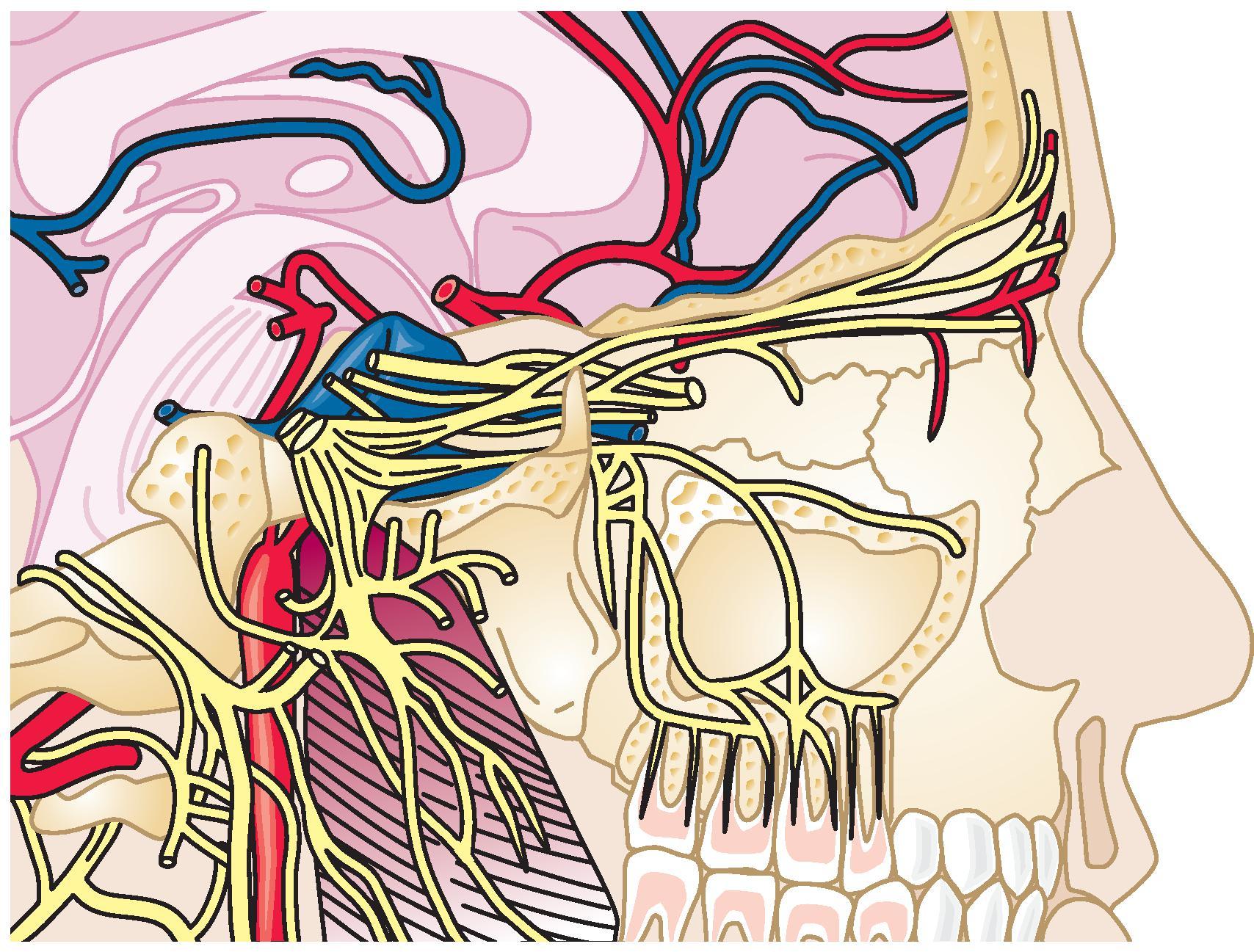
RF treatment of the Gasserian ganglion is associated with very low morbidity and virtually no reported mortality. Reports vary considerably regarding recurrence of pain. This may be because of variations in technique. If a dense sensory loss is produced, there is a low incidence of recurrence. , However, loss of facial sensation and the accompanying paresthesia account for 80% of the side effects of the procedure with an incidence of 18.8%. If RF treatment with a less intense lesion is performed, it might be associated with a lower incidence of paresthesia but potentially earlier recurrence. Other complications include corneal hypesthesia (6.6%), masseter weakness and paralysis (4.1%–6.2%), anesthesia dolorosa (0.6%–1%), keratitis (0.6%–1.2%), transient paralysis of cranial nerves III and IV (0.8%), CSF leak (0.11%), hearing loss (0.13%), and meningitis (0.02%). A much less frequent complication is permanent palsy of the abducens nerve.
No complications have been reported with PRF treatment of the Gasserian ganglion.
The pterygopalatine ganglion is a parasympathetic ganglion located in the pterygopalatine fossa just beneath the maxillary nerve. It is in or close to the foramen that connects the pterygopalatine fossa to the nasal cavity. Preganglionic fibers reach the ganglion from the facial nerve through the greater superficial petrosal nerve and the nerve of the pterygoid canal. There are also connections through the deep petrosal nerve that joins with the greater superficial petrosal nerve to form the Vidian nerve ( Fig. 66.4 ). Many afferent fibers originating from the nasal mucosa, the soft palate, and the pharynx cross the ganglion on their way to the maxillary nerve and eventually to the Gasserian ganglion.
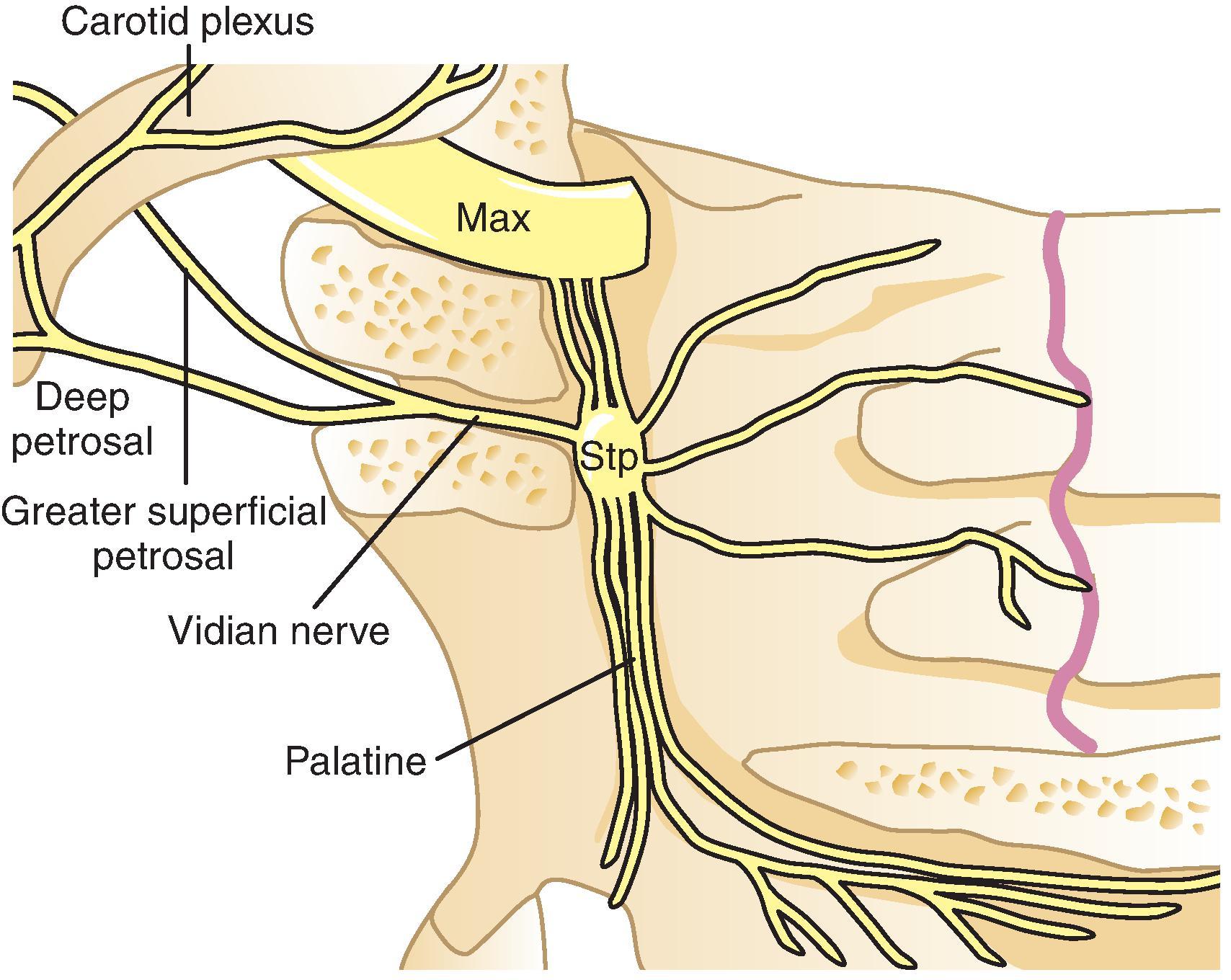
The rationale for RF treatment of cluster headaches is based on parasympathetic symptoms during an attack. Following the GRADE system, there is a very low quality of evidence that RF of the pterygopalatine ganglion reduces intensity and frequency of cluster headache attacks up to 18 months. , A retrospective study of 56 patients with episodic and 10 patients with chronic cluster headache treated by RF treatment of the pterygopalatine ganglion and followed 12–70 months. In the group with episodic cluster headache, 60.7% experienced complete pain relief, while only three out of ten patients with chronic cluster headache had the same result. Another retrospective study of 15 patients reported significant improvement in both mean attack intensity and mean attack frequency for up to 18 months.
Since cluster headache is a very rare but severe clinical condition, the authors recommend that RF of the pterygopalatine ganglion could be used in patients with cluster headache refractory to conservative treatment.
In a retrospective study assessing the effect of RF treatment of the pterygopalatine ganglion on persistent idiopathic facial pain (PIFP), 60% (nine out of 15 patients) showed considerable pain relief. However, repeated RF treatment procedures were needed in most cases. Treatment of atypical facial pain in the second division of the trigeminal nerve using RF of the pterygopalatine ganglion has also been described. A case report on PRF treatment of the pterygopalatine ganglion for posttraumatic headache described 17 months of pain relief. Analysis of PRF treatment of the pterygopalatine ganglion in 30 patients suffering from chronic head and facial pain showed complete relief of pain in 21% and mild to moderate relief in 65%. In a case series ( N = 8) on refractory chronic short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and with cranial autonomic symptoms (SUNA) reported an average improvement in pain of 57% (30%–100%), with an average duration of six (2–10) months.
Evidence for the use of PRF is weak, but given the safe character of this treatment, the authors recommend the use of PRF for these aforementioned conditions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here