Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radiotherapy is the medical use of ionizing radiation, generally as part of cancer treatment to control or kill malignant cells. Ionizing radiation is composed of particles that individually carry enough kinetic energy to liberate an electron from an atom or molecule. Directly ionizing radiation consists of alpha and beta particles. Indirectly ionizing radiation includes photon radiation which may be gamma radiation if produced by radioactive decay or X-rays if produced outside the nucleus.
An alpha particle is identical to a helium nucleus, containing 2 protons and 2 neutrons. Alpha particles are generally produced by a process of alpha decay. If ingested, inhaled or absorbed into the bloodstream, they can cause severe damage. Radium-226, radon-222, and polonium-210 are alpha emitters. Most alpha emitters occur naturally in the environment and are found in various amounts in almost all rocks, soils, and water. This form of radiation is rarely used for the treatment of cancer.
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei. Cobalt-60, strontium-90 and iodine-131 are examples of some of the beta emitters. Iodine-131 is used in the treatment of benign and malignant thyroid disease. Strontium-90 is used in the treatment of metastatic bone cancer and has also been used for treating ocular melanoma. Beta particles are also a source of positrons used in positron emission tomography (PET).
Gamma rays are produced by nuclear gamma decay and are emitted from a radioactive source, often following the emission of a beta particle. Gamma rays are less ionizing but more penetrating than alpha and beta rays and can be stopped by a few centimeters of lead. The commonly used gamma emitters are caesium-137, cobalt-60, and technetium-99m. Technetium is widely used for diagnostic studies, whereas caesium and cobalt are used in the treatment of cancer.
X-rays, like gamma rays, are part of the electromagnetic spectrum. They are emitted by electrons. X-rays of varying energies have many uses but are best known for their medical application. Low-energy X-rays (∼10 keV) are used in diagnostic radiography to take images of part of a patient’s body (e.g., chest or abdomen). The differential absorption of the X-rays by bone, air, and soft tissue generates a clear radiograph. High-energy X-rays (photons) are able to ionize atoms and disrupt molecular bonds, thereby causing potential damage to the tissues they pass through. The ionizing capability of X-rays is utilized in radiotherapy, which uses photons to kill malignant cells in the treatment of cancer. In radiotherapy the photons are generated by a machine called a linear accelerator (linac). The linac generates electrons and then speeds them up to almost the speed of light using electrical fields. As the electron is accelerated its kinetic energy is increased until such time as it collides with the target and the energy is released as a photon (Bremsstrahlung radiation).
When radiation interacts with cells, ionization of DNA molecules takes place causing tumor cell death. The biological effect of ionizations is controlled by a number of factors, such as the number of ionizations that can cause DNA damage, free radical scavenging processes, and the degree of cellular repair. There are two mechanisms by which radiation causes cell damage: direct and indirect.
Approximately 60% of the human body is made of water; therefore the majority of ionizations generated by irradiation arise in the water molecules. When radiation interacts with water it breaks the bonds that hold the water molecule together, producing a positively charged hydrogen ion (H ++ ) and an uncharged hydroxyl radical (OH − ). The OH radical is often called a free radical as it has an unpaired electron and is highly reactive, causing cell damage. These free radicals may undergo further reactions to produce hydrogen peroxide, which also contributes to the destruction of the cells. Oxygen plays a key role in this process of radiolysis. Approximately two-thirds of the biological damage caused by X-rays or electrons is by indirect effect.
If radiation causes ionization of the atoms that are part of the DNA molecule, or some other cellular component essential for the survival of the cell, then the cell may be destroyed directly by interference with its life-sustaining structure. This is referred to as the direct effect of radiation.
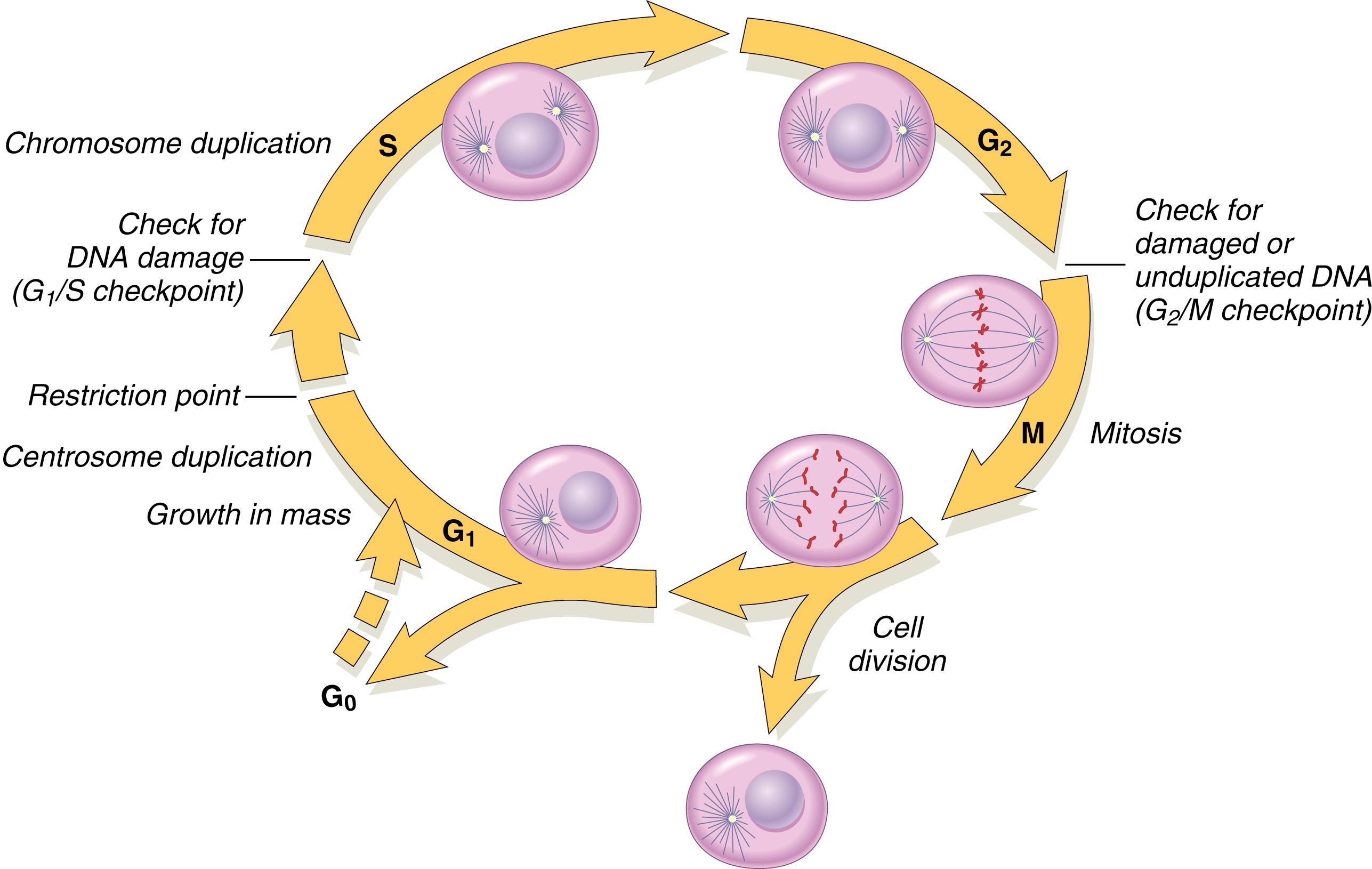
Cell cycle progression is vital for the growth and survival of a cell or organism ( Fig. 12.1 ). The normal cell proliferation cycle goes through two main phases: mitosis (M phase) and DNA synthesis (S phase), which are separated by the G 1 (before cell division) and G 2 (after cell division) phases. G 0 is the resting phase before cell division begins. The average length of a cell cycle is 16 h; this is shorter for cancer cells. Cell cycle arrest normally occurs in response to events that threaten the integrity of the DNA. Cells are most sensitive to radiation in the M and G 2 phases and most resistant in the S phase of the cell cycle. After radiotherapy is delivered, cells may be in different phases of the cell cycle. Fractionating radiotherapy may allow resistant cells time to enter the sensitive phase of the cycle and be more amenable to cell kill. Following radiotherapy, DNA damage can also cause a progressive delay in G 1 , S, and G 2 phases of the cell cycle. This delay gives the cells time to repair the damage.
Radiation kills actively dividing cancer cells efficiently; damage to cancer cells may occur immediately but may take months to take full effect. Along with the cancer cells, some rapidly dividing normal cells (skin, intestines, hair, and bone marrow) are also damaged in the process, leading to unwanted acute effects. Slowly dividing normal cells (nerves, brain, bone, vessels) exhibit radiation-induced damage later.
Radiation-related injury to the cells is divided into three main categories:
Lethal damage is a complex, irreparable and irreversible mechanism leading to cell death. The cell is unable to repair the damage and gives rise to a linear component of cell killing.
Sublethal damage following ionizing radiation can be repaired within hours of the injury taking place and would not lead to cell death, unless further radiation exposure was inflicted on the cells. Cellular repair mechanisms can protect them from further injury, which is important for normal tissues, as they are able to repair themselves between fractions of radiotherapy. The approximate time needed for half the sublethal damage to be repaired (half-life) is different for different tissues. The recovery time is approximately 1 h and is thought to be complete by about 4 h in mammalian cells.
Potentially lethal damage will inevitably kill the cells unless manipulated by repair-facilitated cell cycle arrest. Normal cells can readily be repaired in the S phase but not in the M phase. Tumor cells have defective cell cycle controls and therefore do not undergo cell cycle arrest required for repair, in contrast to the normal cells. A minimum of 6 h are required between fractions of radiotherapy to provide sufficient time for these repair mechanisms to work.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here