Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Respiratory diseases are among the most common reasons for pediatric patients to seek medical attention. Signs and symptoms can be subtle, and a careful history and physical examination are useful in assessing pediatric patients with respiratory complaints. Diseases of the chest can be divided into two major categories: acquired and congenital. Congenital chest diseases are often symptomatic rather than episodical. The spectrum of diseases involving the pediatric respiratory system is primarily dependent on the age of the patient; therefore, age must be a primary consideration when elaborating the differential diagnosis.
Each pediatric history include the should perinatal history. A history of respiratory distress or intubation at birth, however brief, is important. Prematurity with prolonged need for supplemental oxygen may suggest bronchopulmonary dysplasia with associated structural lung abnormalities. Noisy breathing starting early in life suggests congenital airway obstruction. Regardless of the cause, failure to thrive is a worrisome finding, whereas excellent weight gain in a child with noisy breathing is reassuring.
Distinguishing between constant and intermittent symptoms can be one of the most important clues to diagnosis. A good “cough history” ( Table 17.1 ) and “wheeze history” are important and have similar elements. The clinician should inquire about the chronicity of the symptoms, association with feeding, upper respiratory infections, exposures (pets, dust, and especially cigarette smoking are important), and fevers. The effect—or lack thereof—of medications may give important diagnostic information (but may also be confounded by improper administration technique). The nature of the cough is important: wet or dry, paroxysmal or continuous, and staccato (as seen in neonatal chlamydial pneumonia). The cough that awakens the child at night or keeps the child up much of the night is another worrisome historical finding. Conversely, a persistent cough that disappears in sleep suggests the diagnosis of habit (psychogenic) cough. Causes of cough by age and possible diagnostic workup are shown ( Boxes 17.1 and 17.2 ). In pursuing a history of wheeze, it is important to ask the parents or historians what they mean by the term; it may mean “noisy breathing,” and it may even be applied to stridor.
| Characteristic | Associated Condition |
|---|---|
| Loose, productive | Cystic fibrosis, bronchiectasis, ciliary dyskinesia |
| Croupy | Laryngotracheobronchitis |
| Paroxysmal | Cystic fibrosis, pertussis syndrome, foreign body inhalation, Mycoplasma, Chlamydia |
| Brassy | Tracheitis, upper airway drainage, psychogenic cough |
| After feedings | Pharyngeal incoordination, pharyngeal mass, tracheoesophageal fistula, GER |
| Nocturnal | Upper respiratory tract disease, sinusitis, asthma, cystic fibrosis, GER |
| Most severe in morning | Cystic fibrosis, bronchiectasis |
| With exercise | Asthma (including exercise-induced), cystic fibrosis, bronchiectasis |
| Loud, honking, or bizarre | Psychogenic cough |
| Disappears with sleep | Psychogenic cough |
Chlamydia
Viral (e.g., RSV, CMV, rubella)
Bacterial (e.g., pertussis)
Pneumocystis jiroveci
Tracheoesophageal fistula
Vascular ring
Airway malformations (e.g., laryngeal cleft)
Pulmonary sequestration
Cystic fibrosis
Asthma
Aspiration
Recurrent viral bronchiolitis/bronchitis
GER
Interstitial pneumonitides
Lymphoid interstitial pneumonitis
Diffuse interstitial pneumonitis
Inhaled foreign body
Asthma
Suppurative lung disease
Cystic fibrosis
Bronchiectasis
Right middle lobe syndrome
Ciliary dyskinesia syndromes
Upper respiratory tract disease
Recurrent viral infection/bronchitis
Passive smoke inhalation
GER
Interstitial pneumonitides
Pulmonary hemosiderosis
Asthma
Cystic fibrosis
Mycoplasma pneumoniae infection
Psychogenic or habit cough
Cigarette smoking
Pulmonary hemosiderosis
Interstitial pneumonitides
Ciliary dyskinesia syndromes
Recurrent viral illness
Asthma
Cystic fibrosis
Granulomatous lung disease
Foreign body aspiration
Pertussis infection
Complete history and physical examination
Chest and sinus radiographs
CBC with differential
Pulmonary function tests (including bronchoprovocation tests)
Sweat test (pilocarpine iontophoresis method)
Trial of bronchodilators
Sputum for Gram stain, AFB, bacterial, viral, and fungal cultures
Quantitative immunoglobulins
Tuberculin skin test/anergy panel
Serologic tests or PCR for Mycoplasma pneumoniae
Bronchoscopy with bronchoalveolar lavage
Barium swallow
In evaluating the infant with frequent episodes of cough and/or wheeze, the clinician should inquire about symptoms and signs of gastroesophageal reflux (GER) or choking with feeds. As reflux is worse when the patient is supine, symptoms tend to be more prominent at night and during naps.
A family history of asthma or atopy including eczema and environmental allergies should be investigated. Frequent infections in parents or siblings, particularly those requiring hospitalization, could suggest possible immunodeficiency in the family. Other pulmonary diseases with a strong genetic component include cystic fibrosis (CF), primary ciliary dyskinesia (PCD) and neuromuscular syndromes.
The immunization history is essential, including history of pertussis and seasonal influenza vaccination. Parental report of vaccination status may be confirmed with vaccination records from the primary care physician.
Exercise intolerance is a common manifestation of respiratory disease. The neonate’s main output of energy is in feeding, and thus difficulties with feedings should be monitored; toddlers are expected to keep up with peers and/or siblings in play; the school-age child’s gym performance should be scrutinized. Wheezing or coughing fits following vigorous exercise can occur in asthma. Syncope with exercise is quite worrisome and raises the possibility of pulmonary hypertension.
Examination of the chest in any uncooperative patient is notoriously difficult, but it can be easily accomplished with patience and a few tricks. The infant or toddler is best examined with his or her shirt off while being held upright in the arms of a parent. The patient should face the parent; this maximizes contact with the parent and allows the patient to feel safe. The room should be at a comfortable temperature and the stethoscope head should be warmed before use. The classic four steps in the physical examination—inspection, palpation, percussion, and auscultation—are well applied when examining the pediatric chest.
Decreased subcutaneous adipose tissue typical of CF should be noted. The pattern of breathing should always be evaluated with the child disrobed. Any use of the expiratory muscles is abnormal. Suprasternal and intercostal retractions reflect excessive negative pleural pressure and can be seen in normal children with thin chest walls after vigorous exercise. Subcostal retractions are the result of hyperinflated lungs and a flattened diaphragm pulling inward on the chest wall. In advanced lung disease, the use of accessory muscles of inspiration can be noted; the sternocleidomastoid muscle, for example, helps lift the chest (in a “bucket handle” fashion) and increase its anteroposterior diameter, thereby increasing intrathoracic volume. In respiratory muscle fatigue, a pattern of breathing can be observed in which the diaphragm alternates with the intercostal muscles to inflate the lungs. This is known as respiratory alternans, or paradoxical chest wall motion, and is seen as alternating abdominal and chest expansion instead of the usual pattern of simultaneous chest and abdominal expansion. Chest wall deformities such as pectus excavatum or pectus carinatum (see Chapter 18 ) should be noted.
Chest palpation is performed by placing the hands on either side of the chest as the patient takes a deep breath. The chest should expand symmetrically; asymmetry can be seen in unilateral pulmonary hypoplasia, mainstem bronchial obstruction, and diaphragmatic paresis. Placing fingertips on the upper abdomen just over the insertion of the rectus muscles into the lower rib cage can reveal subtle use of expiratory muscles in children with peripheral (lower) airway obstruction. Similarly, the anterior lower ribs should be assessed with the fingertips. In infants with obstructive lung disease, the lower ribs can be felt pulling inwards on inspiration. This is the palpable aspect of a subcostal retraction. With the patient’s head in the midline position, the trachea should be palpated at the sternal notch to evaluate for tracheal deviation, as is seen with mediastinal shift. Vocal fremitus should be assessed in patients with suspected pleural fluid accumulation; the vibrations transmitted from the larynx as the child says “99” are diminished when there is an accumulation of air or fluid in the pleural space. Infants and children with tracheomalacia and bronchomalacia often have a palpable vibration in the back. Palpable vibrations in only one hemithorax suggest a partial obstruction of the mainstem bronchus in that hemithorax as seen in bronchomalacia.
Percussion of the chest can reveal much more than hyperresonance and dullness over an area of consolidation. Air trapping is the hallmark of small airway disease and results in a depressed position of the diaphragm. Ordinarily the diaphragm can be found just at or slightly below the tip of the scapula when the patient’s arm is at his or her side in children 5 years old and younger. In the patient with hyperinflation, the diaphragm is found several fingerbreadths below the scapular tips. An area of consolidation or pleural effusion results in dullness to percussion. Another disorder causing asymmetry of percussion of the two hemithoraces is diaphragmatic eventration, a congenital lesion in which the diaphragm is replaced with a thin fibrous membrane without contractile properties. Postoperative diaphragmatic paralysis (which may occur following cardiac surgery) can be diagnosed by percussion of the cooperative patient while holding his or her breath at maximal inspiration and at end-expiration.
Auscultation of the pediatric chest requires patience. One often must wait a minute or two for a deep breath in order to appreciate abnormal breath sounds that are not apparent on shallow breathing. Augmenting the expiratory phase with a gentle squeeze of the thorax while listening with the stethoscope may improve detection of expiratory wheezes.
Abnormal (“adventitial”) breath sounds include crackles and wheezes. Wheezes are continuous sounds, whereas crackles (formerly referred to as rales ) are discontinuous . Wheezes and crackles can be inspiratory or expiratory, although crackles are more commonly heard on inspiration and wheezes are more commonly heard on expiration.
Wheezes probably arise from the vibration within the walls of narrowed large and medium-sized airways. In a patient experiencing an acute exacerbation of asthma, the lungs have wheezes in a range of pitches (described as polyphonic, or heterophonous ) with substantial regional differences in auscultation. Patients with central airway obstruction such as tracheomalacia, on the other hand, have a single pitch of wheeze that sounds the same in all lung fields (monophonic, or homophonous) and is heard loudest over the central airway that is obstructed. Foreign bodies can cause a monophonic wheeze that can vary in pitch depending on the degree of obstruction.
Crackles are believed to arise from the popping of fluid menisci within airways. The fine crackles heard in the lungs of patients with interstitial lung disease (ILD) or pulmonary edema may arise from popping open of small airways. Coarse crackles are often audible at the mouth and can be found in CF patients with advanced bronchiectasis. Coarse crackles can also be made by pooled secretions in the central airways.
Other sounds that can be heard include friction rubs, which are creaking sounds heard during both phases of respiration as inflamed pleural surfaces rub over one another. One of the most important abnormal findings in children is the absence of breath sounds over an area of collapse or consolidation.
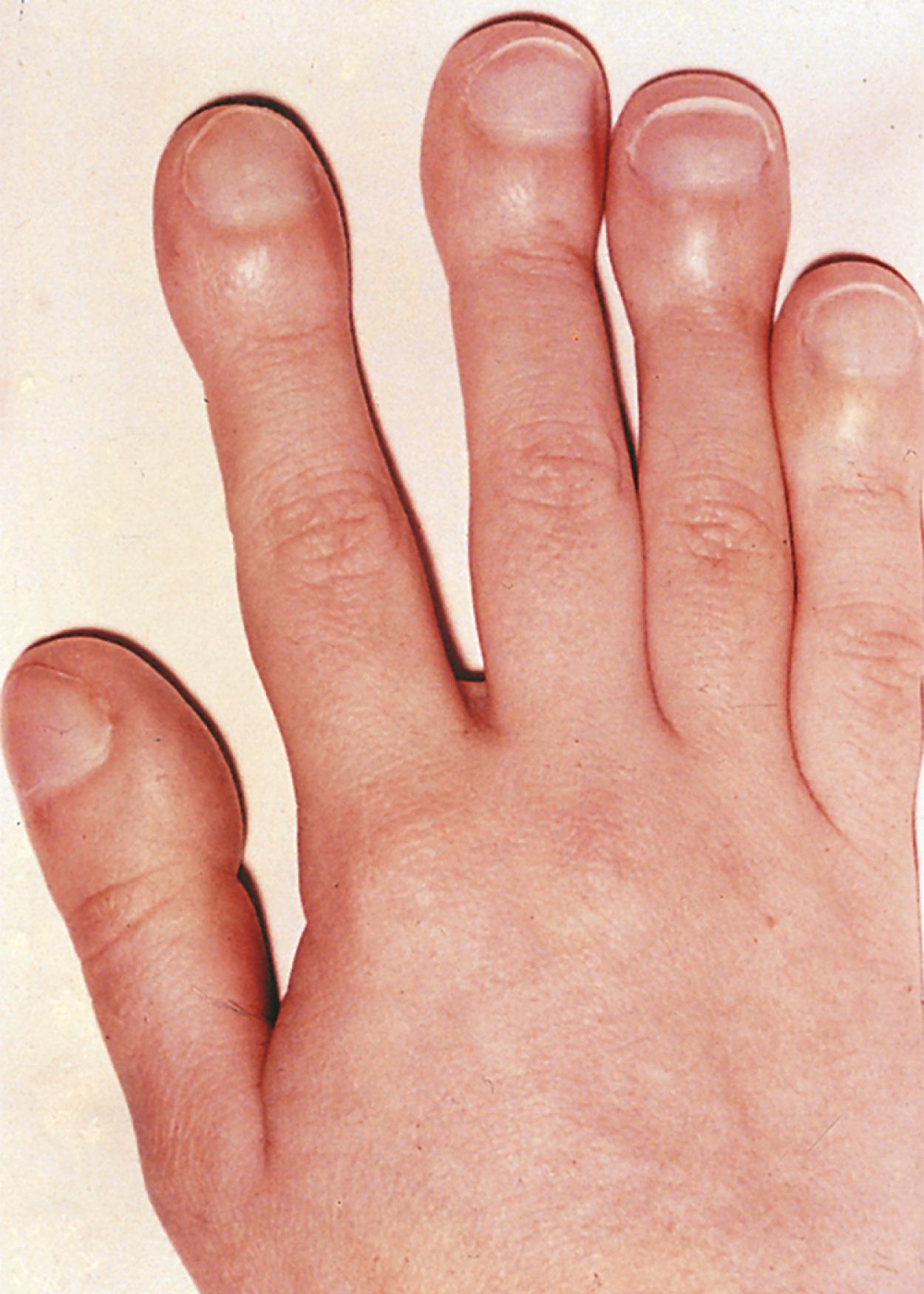
Digital clubbing may suggest lung disease ( Fig. 17.1 ), although extrapulmonary causes include cardiac, inflammatory, gastrointestinal, hepatic, familial causes, as well as clubbing observed with thyrotoxicosis ( Box 17.3 ). Bronchiectasis from CF or from other chronic infectious causes is the major cause of clubbing among all pulmonary diseases. Digital clubbing in any child with a chronic cough or wheezing warrants a thorough evaluation and investigation to determine the underlying disorder.
Pulmonary
Cystic fibrosis
Other bronchiectasis
Pulmonary abscess
Empyema
Neoplasms
Interstitial fibrosis
Pulmonary alveolar proteinosis
Interstitial pneumonitis
Chronic pneumonia
Cardiac
Cyanotic congenital heart disease
Subacute bacterial endocarditis
Gastrointestinal or hepatic
Ulcerative colitis
Crohn disease
Polyposis
Biliary cirrhosis/atresia
Familial
Thyrotoxicosis
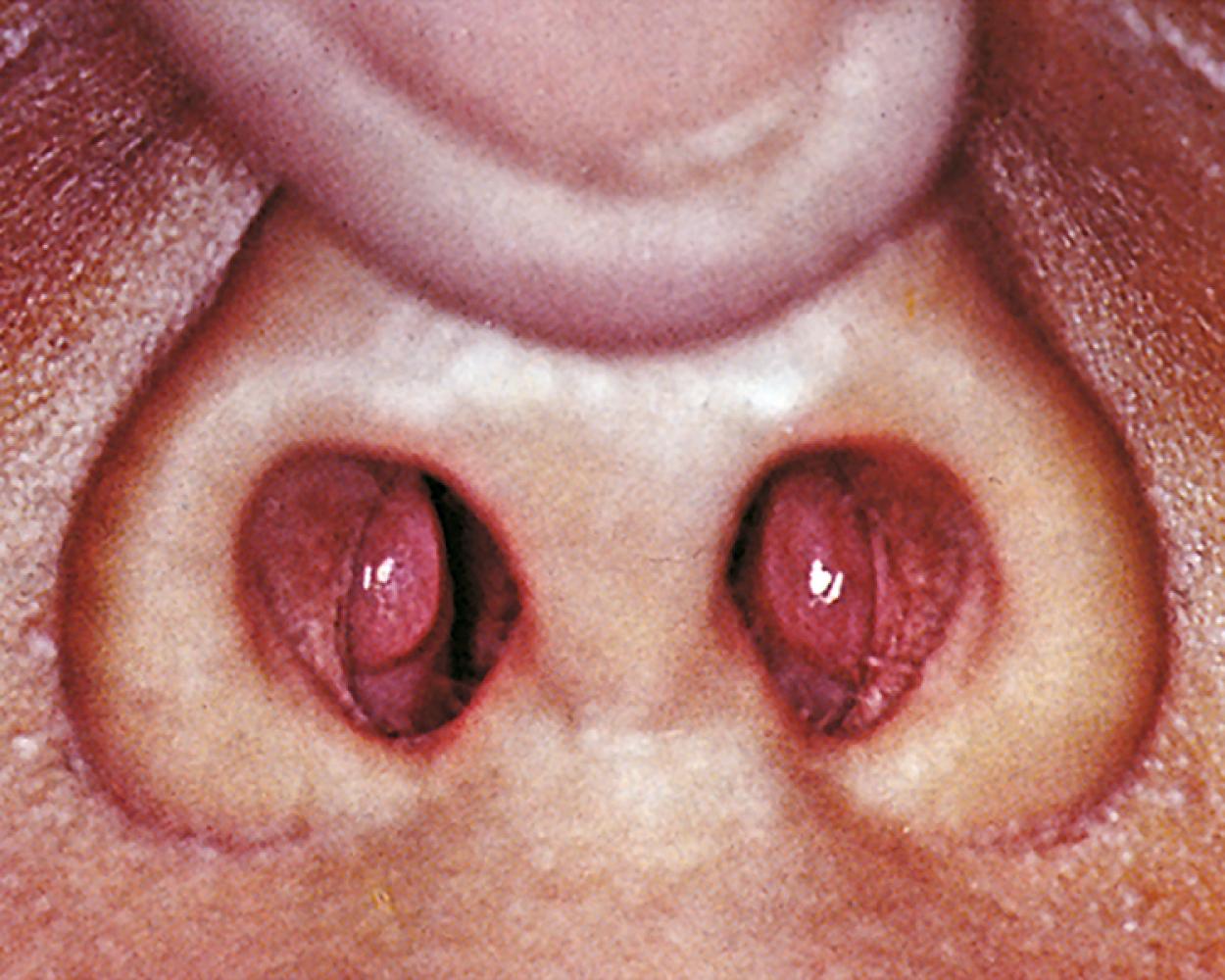
The astute pulmonologist will carefully examine the remainder of the patient. The exam should also include evaluation for nasal polyps (see Fig. 17.2 ), which can be associated with CF, triad asthma, or significant atopy. Tonsillar hypertrophy can be associated with obstructive sleep apnea (OSA). An increased second heart sound, or absence of a split-second heart sound, could suggest pulmonary hypertension.
The adult lung is a complex organ that provides an efficient system of oxygenation and ventilation, with an area of gas exchange of approximately 100 m 2 . Lung morphogenesis is comprised of five distinct developmental periods. Congenital abnormalities with their respective period of development are listed in Table 17.2 . Lung development begins with a ventral outpouching of the foregut endoderm at approximately 3 to 4 weeks post conception. During the embryonic period (3 to 7 weeks post conception), the esophagus and trachea become separate structures and lung buds form from the primitive foregut endoderm with subsequent branching into lobar and segmental bronchi. The respiratory epithelium is lined by columnar epithelium at this stage of morphogenesis, with mucous, secretory and neuroendocrine cells. The lung appears gland-like during the pseudoglandular period (6 to 17 weeks post conception) and the airways are lined by a high columnar epithelium. All conducting airways are formed by the end of the pseudoglandular period, with adjacent smooth muscle and cartilage. The vascular system develops near the airways at approximately 9 to 12 weeks post conception. Further subdivision of the terminal bronchioles occurs during the canalicular period (16 to 26 weeks post conception) with the formation of primitive acini. The capillary bed additionally expands, and bronchial cell types become more differentiated, with cilia and secretory functions. The epithelial lining of the distal tubules consists of cuboidal cells which express increasing amounts of surfactant phospholipids and surfactant proteins A, B and C. Differentiation of type I and type II airway epithelial cells (AECs) also occurs during the canalicular period. Formation of the primitive alveolar-capillary membrane also occurs, with capillaries beginning to surround the distal acinar tubules in close proximity to the epithelium. Gas exchange can occur in infants postnatally at the end of the canalicular period (∼26 weeks post conception), particularly if aided by maternal administration of glucocorticoids, and surfactant administered to the neonate following birth. The saccular period (26 to 36 weeks post conception) is marked by continued development and expansion of the pulmonary acini with formation of true alveoli by septation. The distal respiratory epithelium becomes populated with type I AECs that are now in closer proximity to interstitial capillaries. Fusion of the basal lamina of the endothelium and epithelium forms the thin alveolar-capillary membrane. Surfactant phospholipid and protein production ramps up during this period as well. Alveolarization consists of continued lung maturation and growth that occurs primarily by septation starting at approximately 36 weeks post conception and continuing into adolescence. Alveolar walls become progressively thinner and the conducting airways enlarge.
| Period | WPC a | Developmental Anomalies |
|---|---|---|
| Embryonic | 3–6 weeks | Pulmonary agenesis Laryngeal, esophageal, tracheal and bronchial atresia Tracheoesophageal and bronchoesophageal fistulae Tracheal and bronchial stenosis Bronchogenic cysts Ectopic lobes Extrapulmonary sequestration Congenital diaphragmatic hernia |
| Pseudoglandular | 6–17 weeks | Tracheomalacia and bronchomalacia Intralobar bronchopulmonary sequestration Congenital pulmonary airway malformations (CPAM; previously known as congenital cystic adenomatoid malformation [CCAM]) Acinar aplasia or dysplasia Alveolar capillary dysplasia ± misalignment of the pulmonary veins Pulmonary vascular malformations Congenital pulmonary lymphangiectasia |
| Canalicular | 16–26 weeks | Congenital alveolar capillary dysplasia Pulmonary hypoplasia (related to diaphragmatic hernia, compression from abdominal or thoracic masses, oligohydramnios or renal agenesis) |
| Saccular | 26–36 weeks | Respiratory distress syndrome Bronchopulmonary dysplasia |
| Alveolarization | 36 weeks to adolescence | Alveolar simplification |
Stridor is characteristically a harsh inspiratory noise created by obstruction of the larynx or the extrathoracic trachea. A number of clinical entities can produce persistent or recurrent stridor ( Box 17.4 ), and some of these may also be associated with a chronic cough, as described earlier. With a mild degree of airway narrowing, breath sounds may be normal when the infant or child is at rest, but with any activity that increases tidal breathing (e.g., crying, feeding, agitation), inspiratory stridor may become noticeable.
Croup
Infectious
Allergic/angioneurotic edema, GER
Laryngomalacia
Tracheomalacia
Subglottic stenosis
Extrinsic airway compression
Vascular ring
Mediastinal mass
Lobar emphysema
Bronchogenic cyst
Foreign body in esophagus
Thyromegaly
Pharyngeal or laryngeal masses
Papilloma
Hemangioma
Laryngocele
Web
Foreign body
Tracheoesophageal fistula
Vocal cord paralysis
Psychogenic
In contrast, wheezing is a continuous sound that results from obstruction of airflow in intrathoracic airways. This obstruction can be at the lower trachea “downstream” to the small bronchi and bronchioles. Many diseases that produce chronic wheezing in pediatric patients overlap with those causing coughing or stridor ( Box 17.5 ). Wheezes can be heard on expiration or, less commonly, during both phases of respiration. The pitch of the wheeze, the variation in its pitch throughout the lung fields, and an association with hyperinflation as defined by percussion (described earlier) can help differentiate wheezing resulting from obstruction in the small airways (polyphonic) from that in the large airways (monophonic). Response to bronchodilator and/or steroids is a useful way of differentiating true asthma (which should improve with these treatments) from wheezing resulting from tracheomalacia or bronchomalacia (which does not improve and may even worsen with bronchodilators). Asthma is the most common cause of wheezing in pediatric patients. Asthma may take many different forms, including atopic asthma, exercise-induced asthma, and transient wheezing of infancy.
Asthma
Exercise-induced asthma
GER
Hypersensitivity reactions (e.g., ABPA)
Cystic fibrosis
Aspiration
Tracheoesophageal fistula
Foreign body
GER
Laryngeal cleft
Pharyngeal dysmotility
Extrinsic masses
Vascular ring
Cystic adenomatoid malformation
Lymph nodes
Tumors
Ciliary dyskinesia syndromes
Tracheomalacia and/or bronchomalacia
Congestive heart failure
Bronchopulmonary hemosiderosis or Heiner syndrome
Endobronchial lesions including localized stenosis
Interstitial pneumonitides
Bronchiolitis obliterans
The inspiratory stridor associated with congenital laryngomalacia generally begins within the first week of life, varies with activity, and is more noticeable in the supine position. The obstruction can be caused by prolapse of the arytenoid cartilages or collapse of the epiglottis due to foreshortened aryepiglottic folds. Clinical symptoms may suggest the diagnosis; but if severe, bronchoscopic visualization of airway dynamics by flexible bronchoscopy is a safe and reliable method of excluding other causes of stridor. Parents can be reassured that this entity is self-limited, becomes less marked after 6 to 10 months of age usually with maturity of innervation or of the larynx, and rarely causes serious problems. In a small percentage of patients, obstruction of the larynx can interfere with feeding and cause dysphagia or failure to thrive, and in some infants it can cause cyanosis with feeding. In these cases, surgical treatment (“supraglottoplasty”) may be considered.
The most common cause of inspiratory stridor in children is infectious croup (acute laryngotracheobronchitis). This disease is most commonly caused by a respiratory virus (parainfluenza, respiratory syncytial, influenza, or rhinovirus), and the patient typically has coryza for 24 to 48 hours before the appearance of croupy cough, hoarseness, and stridor. Occasionally the inflammatory process may spread to the smaller airways and produce wheezing in addition to these symptoms. The “steeple sign” is a characteristic radiographic sign on anteroposterior projections that may be accompanied by marked dilation of supraglottic structures, particularly on lateral films. In most patients, serious airway obstruction does not occur and the disease is self-limited. Acute angioneurotic edema is a less common cause of stridor. In most cases, it results from an allergic reaction and is potentially fatal. Some children with anatomically normal airways suffer recurrent bouts of stridor, usually in the middle of the night, in the absence of signs of viral infection. Treatment for GER is helpful in some patients, suggesting that occult GER can contribute to bouts of recurrent airway obstruction.
Narrowing of the subglottic region can be congenital or acquired, such as in subglottic stenosis associated with endotracheal intubation. Congenital subglottic stenosis improves as the child grows older, but narrowing associated with tracheal intubation may require a tracheostomy, particularly if the infant remains dependent on ventilatory support. The degree of subglottic stenosis can be determined by “sizing” of the airway with various sized endotracheal tubes and assessing for leak. Low grade stenosis may be monitored expectantly or amenable to balloon dilation, while higher grade stenosis may require laryngotracheal reconstruction.
Vocal cord paralysis, either unilateral or bilateral, may be present in the neonatal period, although in the case of unilateral paralysis several weeks may pass before the diagnosis is suspected. A weak or absent cry, hoarseness, inspiratory stridor with or without respiratory distress, and feeding difficulties are usual signs of vocal cord paralysis. Bilateral vocal cord paralysis may be seen with hydrocephalus, myelomeningocele, Arnold-Chiari malformation, or other malformations of the brain. Unilateral and bilateral cord paralyses are observed in patients with abnormalities of the cardiovascular system that are accompanied by cardiomegaly (e.g., ventricular septal defect, tetralogy of Fallot) or that cause abnormalities of the great vessels (e.g., vascular ring, transposition, patent ductus arteriosus). The diagnosis is best made by flexible laryngoscopy under minimal sedation so that vocal cord movement can be examined adequately. Because of a strong association of dysfunctional swallow with vocal cord paralysis, a barium swallow should be performed in suspected cases to assess for aspiration.
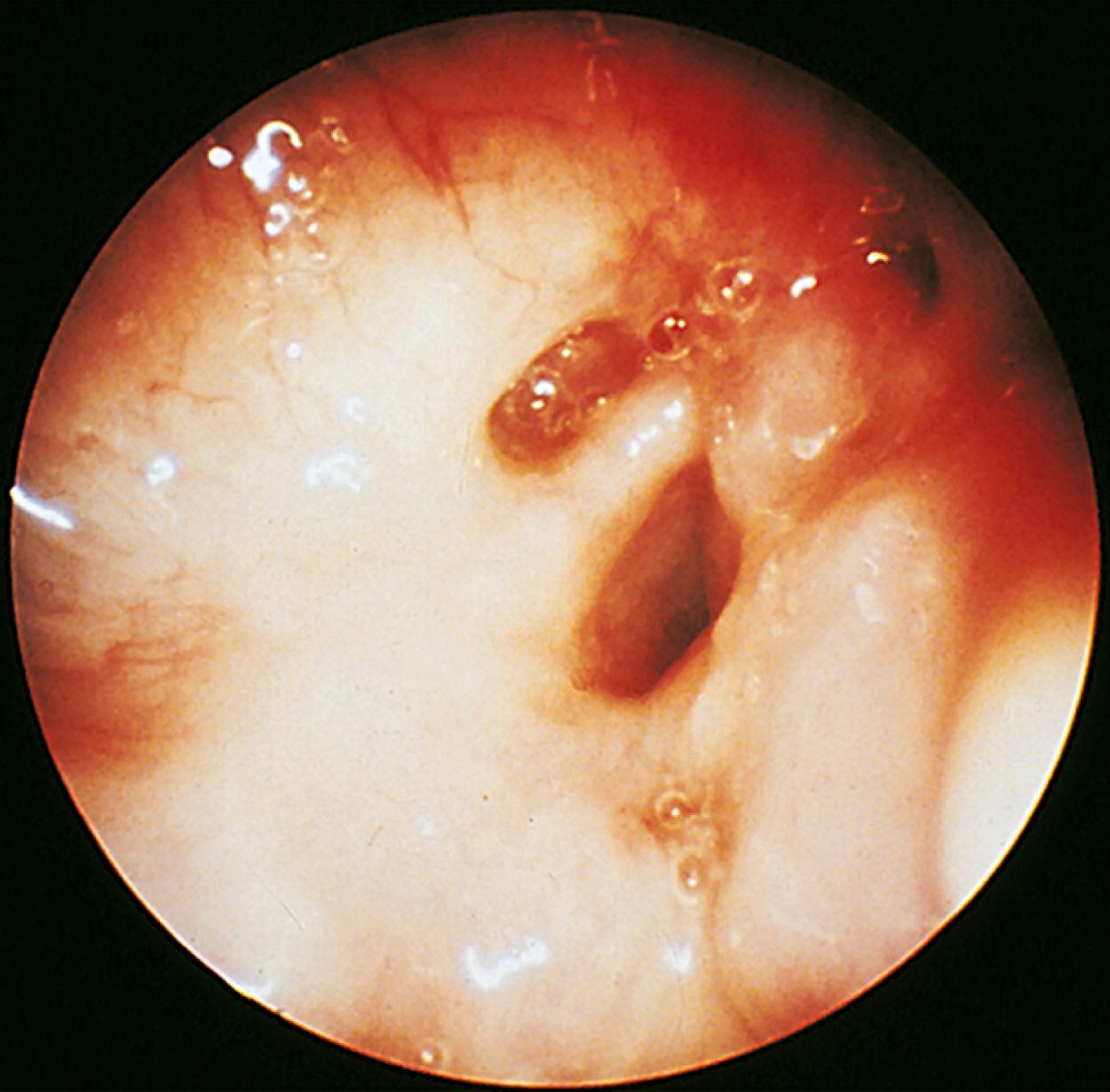
Congenital laryngeal or pharyngeal masses can also produce stridor by obstructing the airflow. Although inspiratory stridor may be observed, hoarseness is a more common presenting feature. Laryngeal webs, papillomas, or hemangiomas may all present with ongoing stridor, as well as brassy or dry cough. Hemangiomas of the larynx or trachea may also produce stridor or a brassy or dry cough. Cutaneous or mucosal hemangiomas noted during the physical examination suggest the possibility of additional organ system involvement. Laryngeal webs ( Fig. 17.3 ), cysts, and laryngoceles are quite uncommon and are accompanied by respiratory distress, stridor, feeding difficulties, and cyanosis. Diagnosis is made by bronchoscopy. A foreign body in the pharynx or larynx may also cause stridor. For additional information on stridor, see Chapter 24 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here