Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The function of the pulmonary circulation is both to support the lung's metabolic activities and to engage in gas exchange. The pulmonary circulation should not be considered in isolation but, both anatomically and physiologically, as part of a functional unit which is intimately related to cardiac function and optimised to match ventilation and perfusion. There are many examples of this unique interdependence, including the pulmonary vascular response to hypoxia being arterial constriction as opposed to arterial dilatation in the systemic circulation; and venous congestion due to left heart failure requiring higher pulmonary arterial pressure (PAP) to maintain vascular flow potentially leading to pulmonary hypertension, right ventricular (RV) dysfunction and even failure. The pulmonary circulation consists of both pulmonary and bronchial circuits, of which the bronchial arteries normally contribute only around 1%.
Appreciation of pulmonary anatomy and normal physiology enables a better understanding of abnormal conditions (and their relevant radiographic features).
Acute pulmonary embolism (PE) is common, can be life threatening, and has a high mortality if undiagnosed; chronic PE may lead to pulmonary hypertension and right heart failure. As clinical symptoms are frequently non-specific, imaging plays a major role in diagnosing acute and chronic PE. The second half of this chapter is therefore devoted to demography, pathophysiology and imaging features of pulmonary thromboembolism.
The pulmonary trunk originates from the right ventricle above the pulmonary valve. In adults, the main pulmonary artery (MPA) measures approximately 5 cm in length and is entirely enveloped within the pericardium. The pulmonary valve consists of three cusps preventing retrograde blood flow during diastole. At the base of the pulmonary trunk there is a mild dilation forming the pulmonary sinuses, which lie between the valve cusps and prevent the valve leaflets from adhering to the wall when open.
At about the fifth thoracic vertebral level, the pulmonary trunk divides into a longer right and shorter left pulmonary artery.
The left pulmonary artery runs superiorly over the left main bronchus to enter the left hilum. Within the hilum, it may either continue directly into the left interlobar artery, from which the segmental branches to the upper and lower lobe arise directly, or it may bifurcate into an ascending and descending branch. The ascending branch then divides almost immediately into the apicoposterior and anterior segmental branches which supply the left upper lobe. The descending branch gives a branch to the lingula, which, in turn, divides into two segmental arteries (the superior and inferior lingular segmental artery). The next branch from the descending branch is the superior segmental artery, which supplies the superior segment of the left lower lobe (segment 6). Subsequent branches supply the remaining four segments of the left lower lobe.
The right pulmonary artery runs under the aortic arch, posterior to the superior vena cava and anterior to the right main bronchus; just before entering the hilum, it divides into the ascending (truncus anterior) and the descending (interlobar) branches. The ascending branch divides into apical, anterior and posterior segmental branches, while the posterior segmental branch may, however, also originate at the bifurcation of the right MPA or the right descending trunk. The interlobar artery gives rise to the middle lobe artery (which further divides into the lateral and medial segmental branches) and the right lower lobe artery, which immediately gives off the artery to the superior segment of the right lower lobe. As on the left side, subsequent branches supply the remaining four segments of the right lower lobe.
The arterial branching follows and runs parallel to the divisions of the bronchial tree (and using the same nomenclature), supplying each bronchopulmonary segment. The branching pattern of the lobar and, especially, the segmental arteries shows a high variation, whereas for the more proximal arteries, it is fairly constant. In addition, supernumerary (accessory) branches exist that are not accompanied by bronchial branches and give additional arterial supply to the lung parenchyma. These arteries are usually located in the periphery of the lung.
Normal sizes of the pulmonary arteries have been assessed with computed tomography (CT). There are some contradicting data on the correlation between pulmonary artery diameter and height, weight, Body Surface Index (BSI) and age; in general, diameters tend to be slightly larger in men. According to the literature, in adults, the upper limit of normal for the pulmonary trunk diameter is 29 to 33 mm (for women 27 mm is suggested), and 23 and 22 mm for the right and left pulmonary arteries, respectively.
The pulmonary artery-to-aorta (PA-to-Ao) ratio is used for the screening and evaluation of pulmonary hypertension: A PA-to-Ao ratio of greater than 1 or 1.1 has been proposed as being suggestive of pulmonary hypertension. The PA-to-Ao ratio decreases with age because the ascending aortic diameter increases with age and body size, whereas the PA increases with body size only. In addition, the PA may enlarge in some diseases (e.g. pulmonary fibrosis) without a correlating increase in PA pressure. The pulmonary artery-to-bronchus ratio, which can be assessed on both plain chest radiography and CT, is especially helpful in the assessment of congestive heart failure and volume overload. It has been also suggested to be used for the assessment of pulmonary hypertension.
The pulmonary veins, classically two on each side, transport the oxygenated blood from the lung back to the left atrium of the heart. The veins run independently from the pulmonary arteries and bronchi towards the heart. The superior pulmonary veins drain the blood from the upper lobes, including the middle lobe on the right side; the inferior pulmonary veins drain the lower lobes. In addition, the veins from the visceral pleura drain into the pulmonary veins, whereas the veins of the parietal pleura drain into the systemic circulation via the veins of the thoracic wall. There is great interest in pulmonary venous (PV) anatomy variations with regards to ablation procedures for atrial fibrillation,
Bronchial arteries supply various structures in the intrathoracic region: they are responsible for the majority of oxygen supply to the bronchial tree from the central main bronchi to the respiratory bronchioles and lung parenchyma; the upper oesophagus; part of the pericardium; and the visceral pleura. The smallest, most peripheral branches anastomose with branches from the pulmonary arteries in the walls of the bronchioles and the visceral pleura. Systemic branches supplying the thoracic wall also supply the parietal pleura.
The origin as well as the number of the bronchial arteries is subject to considerable variation. In more than 70% of people, the bronchial arteries arise from the descending thoracic aorta, most commonly between the levels of T 5 and T 6 . In most individuals, there are two to four bronchial arteries present, arising either independently or from a common trunk.
The right bronchial artery usually (78% of people) arises within a common stem, with the first aortic intercostal (intercostobronchial artery) from the posteromedial aspect of the descending aorta. On the left side, there is generally a superior and an inferior branch, both arising from the anterior aspect of the descending thoracic aorta. The bronchial arteries run into the hilum, where they branch in a parallel manner and close to the bronchus to the peripheral airways. The diameters of these arteries are small, usually 1–1.5 mm at its origin within the mediastinum.
Anomalous bronchial arteries, defined as bronchial arteries that originate outside the levels of T 5 and T 6 , are found in up to 21% of patients with haemoptysis. These anomalous arteries arise, in the majority of cases, from the aortic arch, and less frequently, from the lower part of the descending aorta, from major aortic branches such as the subclavian arteries, the thyrocervical trunk, the brachiocephalic artery, or the internal mammary artery. Bronchial arteries course into the pulmonary parenchyma parallel to the bronchi, in contrast to the non-bronchial systemic collateral arteries. In the periphery of the lung, bronchial arteries form anastomoses with the pulmonary arteries.
Venous return can occur via the bronchial veins into the azygos vein (right side), accessory hemiazygos vein, left superior intercostal vein (left side), or via the bronchopulmonary arterial anastomoses into the pulmonary veins.
Unlike the systemic circulation, the pulmonary circulation is a low-pressure system with only a relatively small pressure difference between the pulmonary arteries (mean pressure 12 to 20 mm Hg) and the left atrium (7–12 mm Hg). The pressure in the capillaries and the veins approximates the pressure in the left atrium. That is the reason why the elevated pressure in the left ventricle/left atrium (e.g. mitral valve disease) leads via the capillary bed to an increased pulmonary artery pressure.
As with the airways, the combined cross-sectional area of the pulmonary vasculature increases at each generation of branching towards the periphery of the lung. The resistance in the capillaries contributes considerably to the whole vascular resistance. At rest, only one-third of the capillaries are perfused, but, with increasing cardiac output (CO) under stress, the remaining capillaries will be recruited by increasing pressure in order to contribute to gas exchange.
The hydrostatic pressure within the pulmonary capillaries drives fluid into the interstitium. This is partly counteracted by the plasma oncotic (colloid osmotic) pressure, which draws fluid back into the capillaries. An imbalance in these pressures can lead to abnormal fluid shift and thus transudation of fluid into the pulmonary interstitium and alveoli. Pulmonary lymphatic channels drain excess intersitital fluid and their capacity can increase by a factor of 10 if needed (e.g. chronic cardiac insufficiency). However, if the rate of fluid accumulation exceeds the lymphatic clearance capacity, fluid will begin to accumulate within the interstitium. If this process continues, it leads to alveolar fluid accumulation, which may compromise gas exchange (which is not usually the case with isolated interstitial oedema). Accumulation of interstitial fluid accounts for the bronchial wall thickening (cuffing) and subpleural Kerley B lines seen in left heart failure.
An important difference between the pulmonary and systemic vasculature is the response to hypoxia. In the pulmonary system, hypoxia results in local vasoconstriction, causing diversion of blood to regions of better ventilation. Although contrary to the vascular response in the rest of the body, this mechanism serves to protect the alveolar–arteriolar p O 2 balance and thus to minimise ventilation–perfusion (VQ) differences in cases of diffuse and regional disease; i.e. it supplies blood to regions of the lung that will most efficiently oxygenate it. This homeostatic mechanism (Euler–Liljestrand reflex, hypoxic pulmonary vasoconstriction) is responsible for ‘matched defects’ seen in cases of pneumonic consolidation on VQ imaging. It is also responsible for different vascular calibres in patients with lobular air trapping.
The bronchial arteries primarily perfuse airways, pulmonary vessel walls, interstitium and pleura, while the more centrally localised bronchial veins drain into the right atrium; the peripherally located smaller bronchial veins drain into the left atrium.
The distribution of perfusion is influenced by gravity and body position. In the upright position, perfusion is greatest in the basal part of the lung, as illustrated by increased lung parenchyma density and larger vessel calibres. In the apical part of the lung (zone I), the intra-alveolar pressure is larger than the intravenous and intra-arterial pressure independent of ventilation and blood volume. In the basal part of the lung (zone III), intravenous and intra-arterial pressure exceed the intra-alveolar pressure. In the middle part (zone II), the intra-arterial pressure is higher than the intra-alveolar pressure followed by the intravenous pressure. In a lying position, zone I is ventrally localised and zone III dorsally accompanied by an apicobasal gradient. In case of acute volume overload or left cardiac failure, in particular, the vessels in zone III are affected.
PV hypertension is caused by increased resistance or flow in the pulmonary veins and is defined by an elevation of the mean pressure > 12 mm Hg. An increased venous pressure automatically leads to an increased capillary pressure. An increase of the mean PV pressure to 12–20 mm Hg results in a redistribution of blood volume (grade 1), a PV pressure of 20–25 mm Hg leads to interstitial oedema (grade 2) and a PVH of > 25–30 mm Hg to alveolar oedema (grade 3).
The most common cause of PV hypertension, by far, is left-sided heart disease ( Table 16.1 ) due to left ventricular failure, mitral valve disease or aortic valve disease. The severity of mitral valve stenosis can be non-invasively gauged by assessing the PV pressure. In cases of aortic valve disease, however, the degree of PV hypertension is more indicative of myocardial failure than severity of stenosis. It has to be noted that an increased left ventricular pressure load does not immediately result in PV hypertension. Only an elevated end-diastolic left ventricular pressure leads to elevation of the left atrial pressure and subsequently to PV hypertension. The PV pressure can be estimated from the pulmonary artery wedge pressure (PAWP) using a Swan–Ganz catheter and is usually < 12 mm Hg.
|
The radiological findings can be thought of as a progressive series of changes that occur in response to the underlying changes in physiology.
Three grades of severity of pulmonary congestion are differentiated ( Table 16.2 ).
| Vascular Redistribution Grade 1 (mm Hg) |
Interstitial Oedema Grade 2 (mm Hg) |
Alveolar Oedema Grade 3 (mm Hg) |
|
|---|---|---|---|
| Acute | 12–19 | 20–25 | > 25 |
| Chronic | 15–25 | 25–50 | > 30 |
As PV pressure rises, the upper lobe veins distend. They initially reach the size of, and eventually become larger than, the lower lobe vessels (thus reversing the normal ‘gravity-dependent’ pattern). This is described as ‘upper lobe venous diversion’ and is often the first recognised radiological sign of PV hypertension ( Fig. 16.1 ). Similar calibres of upper and lower lobe veins do not indicate increased PV pressure if seen in a bedside supine radiograph.
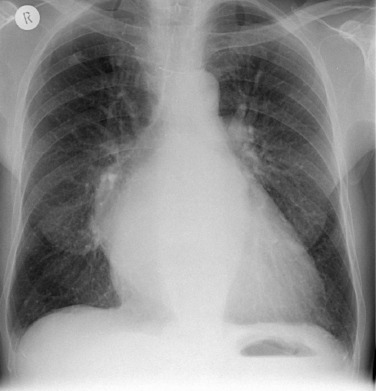
Patients suffering from their first episode of acute PV pressure elevation tend to immediately develop an interstitial or alveolar oedema. Only recurrent periods or chronically increased PV pressure result in distended veins.
If the PV pressure continues to rise and exceeds the plasma oncotic pressure, fluid will begin to accumulate in the lung interstitium. This is known as interstitial pulmonary oedema . Typical radiological signs of interstitial oedema are interstitial (Kerley) lines ( Fig. 16.2 ) caused by thickening of the interlobular septa as a result of fluid accumulation.
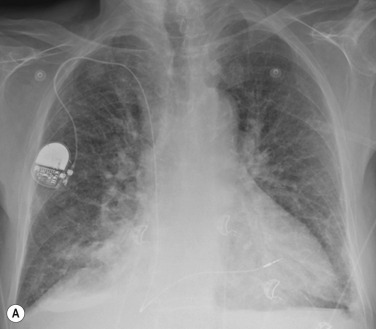
Kerley B lines are the most obvious ones and are short (1 cm or less) interlobular septal lines, found predominantly in the lower zones peripherally, and parallel to each other but perpendicular to the pleural surface. Kerley A lines are deep septal lines (lymphatic channels), radiating from the periphery (not reaching the pleura) into the central portions of the lung and approximately 4 cm long. Their presence normally indicates a more acute or severe degree of oedema.
Septal lines can be differentiated from blood vessels, as the latter are not visible in the outer 1 cm of the lung. In addition, deep septal lines do not branch and are seen with a greater clarity than a blood vessel of similar calibre, as they represent a sheet of tissue ( Fig. 16.3A ).
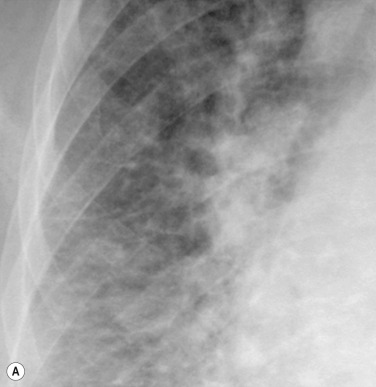
Under normal circumstances septal lines caused by interstitial fluid overload would be expected to resolve after suitable reduction in PV pressure. Exceptionally, however, they may persist, e.g. in long-standing PV hypertension, where haemosiderin deposition or fibrosis has occurred. Alternative causes of persistent septal lines include idiopathic interstitial fibrosis, lymphangitis carcinomatosa and pneumoconiosis.
Other signs of interstitial fluid overload include perihilar haze (loss of visible clarity of the lower lobe and hilar vessels), peribronchial cuffing (apparent thickening of proximal bronchial walls as a result of interstitial fluid accumulating around their walls) and thickening of the interlobar fissure due to thickened subpleural interstitium (to differentiate from interlobar pleural effusion).
As the PV pressure continues to increase, fluid begins to accumulate in the alveolar spaces. This is termed alveolar oedema. Kerley B lines, airspace nodules, bilateral symmetric consolidation in the mid and lower lung zones and pleural effusions may be seen.
Depending on the amount of alveolar fluid overload, there are many variations of increased lung density, ranging from subtle haziness to dense consolidation with air bronchograms (see Fig. 16.3B ).
Certain patterns of opacification may suggest particular diagnoses. The often-cited ‘perihilar bat's wing’ pattern of airspace consolidation is seen most commonly in left ventricular and renal failure, whereas alveolar oedema localised to the right upper zone is suggestive of severe mitral regurgitation ( Fig. 16.4 ). The latter is thought to be a result of predominantly regurgitant blood flow in the right upper lobe pulmonary vein, from the superiorly and posteriorly positioned mitral valve. A predominantly upper lobe oedema is seen in patients with a severe head trauma (neurogenic oedema). Alveolar fluid accumulation changes with patient position and gravity: asymmetric consolidations mimicking a pneumonia may be the result of the left- or right-sided position of the patient.
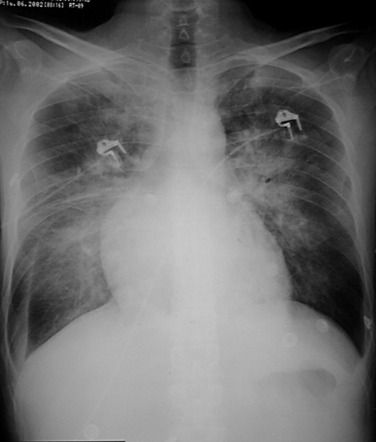
Computed tomography (CT) findings of oedema are similar to the radiographic findings, although very atypical patterns are possible, causing differential diagnostic difficulties.
Interstitial oedema is characterised by smoothly thickened interlobular septa that do not follow the hydrostatic gradient ( Fig. 16.5A ). The peribronchovascular interstitium is thickened, which is best seen in the perihilar area. Commonly, there is also, at least subtly, increased parenchymal density due to alveolar fluid overload.
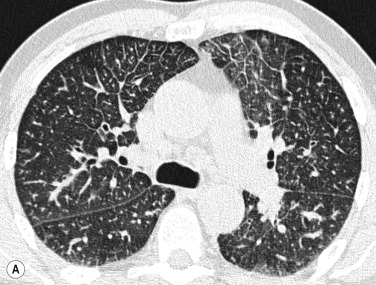
Alveolar oedema may initially be recognised as peribronchovascular airspace nodules progressing to diffuse ground-glass or dense airspace consolidation (see Fig. 16.5B ). Increased density may follow a ventrodorsal gradient but can also be localised predominantly in the perhilar region or in a patchy distribution, the latter causing sometimes quite atypical patterns ( Fig. 16.6 ).
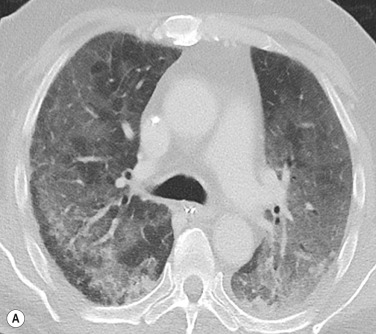
In chronic pulmonary venous hypertension, signs of pulmonary arterial hypertension (PAH) may also develop. In addition, a fine nodular ‘interstitial’ pattern may appear throughout both lungs. These nodules represent haemosiderin deposition. This pattern was previously most commonly seen in patients with long-standing severe mitral stenosis.
Pre-existing underlying lung disease influences the pattern and distribution of oedema, Patients with extensive emphysema do not develop homogeneous consolidation; even though degree of PV pressure or fluid overload would otherwise lead to an alveolar oedema, the fluid remains within the interstitium, leading to thickened septa and a rather ‘interstitial’ fluid distribution, meaning that the radiographic appearance may lead to underestimation of the severity of oedema in these patients ( Figs 16.7 and 16.8 ).
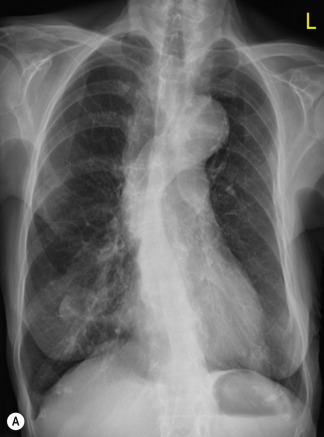
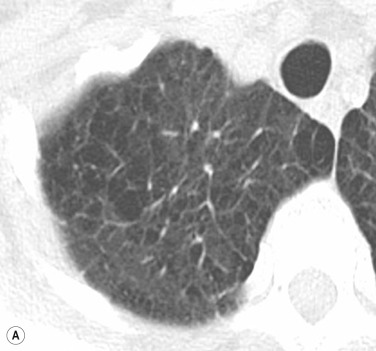
Although most cases of PV hypertension are associated cardiomegaly resulting from valvular and/or myocardial dysfunction, the presence of cardiomegaly is not universal. An important example of this is in the first 24–48 hours post myocardial infarction. This is due to an acute decrease in myocardial compliance, which essentially resolves in the first week after infarction. Other situations, where signs of pulmonary oedema may be seen associated with a normal heart size, are in patients with non-cardiogenic pulmonary oedema, in patients with acute overhydration or drug-induced lung oedema (e.g. heroin, aspirin, nitrofurantoin).
Cardiogenic oedema has to be differentiated from other underlying diseases that result in an imbalance of hydrostatic pressure, colloid osmotic pressure or capillary permeability, all of them resulting in pulmonary oedema ( Table 16.3 ).
|
PAH is defined by a mean pulmonary artery pressure of ≥ 25 mm Hg at rest, as measured invasively at right heart catheterisation. This can result from a broad spectrum of disease processes originating in the lungs, pulmonary vasculature or heart diseases with different pathophysiologies, treatments and prognoses. Irrespective of its underlying cause, PAH is a progressive disease leading to substantial morbidity and mortality.
Because symptoms are non-specific and evaluation of pulmonary artery pressure is relatively inaccessible, there is often considerable delay between the onset of symptoms and the diagnosis of PAH. Imaging, such as high-resolution computed tomography (HRCT), computed tomography pulmonary angiography (CTPA), magnetic resonance imaging (MRI) and echocardiography, plays a crucial role in the diagnostic work-up of patients with known or suspected pulmonary hypertension. Imaging also plays an important role in raising the possibility of PAH, both in those at risk of developing PAH due to co-morbidities and in those presenting with non-specific cardiorespiratory symptoms where a de novo diagnosis may be suggested. Imaging is particularly important for identifying patients with recurrent or chronic pulmonary thromboembolism where it is fundamental in assessing disease extent and distribution and evaluating the technical feasibility of pulmonary thrombendarterectomy.
PAH is a clinical and haemodynamic syndrome that results from increased vascular resistance in the pulmonary circulation. This may be secondary to raised PV pressures (left heart disease), chronic hypoxia resulting either from disease or altitude, or primary disease of either the large vessels (e,g, chronic thromboembolic disease) or the small vessels of the lung. In each of these disease processes exposure of the pulmonary circulation to persistently raised pressures results in ongoing small vessel remodelling and progressive PH. Increase in pulmonary vascular resistance requires high driving pressure to maintain CO, which in turn leads to RV hypertrophy. Ultimately, if untreated, the RV will fail initially during exercise, later at rest, with death from RV failure occurring a medium of 2.8 years after diagnosis.
There are several different causes of PAH. The current PAH classification ( Tables 16.4 and 16.5 ) was developed at the Fifth World Symposium on Pulmonary Hypertension in Nice, France, in 2013 and represents a modification of the previous Dana Point classification. The classification groups together diseases sharing similar pathophysiological mechanisms, clinical presentation and therapeutic options.
|
| Definition | Haemodynamic Characteristics | Clinical Groups (See Table 16.3 ) |
|---|---|---|
| PH | PAPm ≥ 25 mm Hg | All |
| Pre-capillary PH | PAPm ≥ 25 mm Hg PAWP ≤ 15 mm Hg |
1—Pulmonary arterial hypertension 3—PH due to lung diseases 4—Chronic thromboembolic PH 5—PH with unclear and/or multifactorial mechanisms |
| Post-capillary PH Isolated post-capillary PH (Ipc-PH) Combined post-capillary and pre-capillary PH (Cpc-PH) |
PAPm ≥ 25 mm Hg PAWP > 15 mm Hg DPG < 7 mm Hg and/or PVR ≤ 3 WU DPG ≥ 7 mm Hg and/or PVR > 3 WU |
2—PH due to left heart disease 5—PH with unclear and/or multifactorial mechanisms |
Group 1 comprises diseases primarily affecting the pulmonary arterioles with vascular remodelling resulting in progressive luminal obliteration. While collectively described as Group 1 or PAH, this group encompasses idiopathic and heritable PAH, PAH associated with drugs, connective tissue diseases, HIV infection, congenital heart disease, portal hypertension and schistosomiasis ( Fig. 16.9 ). PAH is characterised clinically by the presence of pre-capillary PAH (a pulmonary capillary wedge pressure (PCWP) <15 mm Hg) and the exclusion of other causes of PAH. The rare diseases pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary haemangiomatosis (PCH) are separated into a Group 1’, which reflects the more distal anatomical location of disease and potential adverse response to targeted vasodilator therapy used for Group 1.
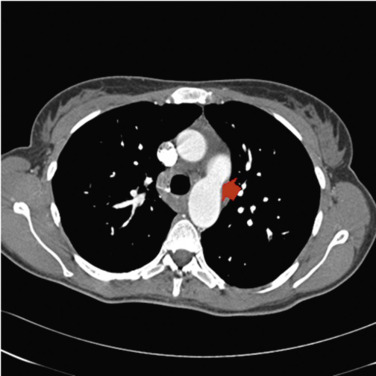
Groups 2 and 3 are by far the most common causes of PAH. Group 2 comprises patients with PAH secondary to left heart disease, causing an increased pulmonary artery pressure and an elevated PCWP greater than 15 mm Hg. Group 3 represents pulmonary hypertension due to lung disease or hypoxia. Group 4 is mainly comprises chronic thromboembolic pulmonary hypertension (CTEPH), which results from pulmonary arterial obstruction by an organised thrombus. In dedicated centres, removal of the obstruction, whether by surgical pulmonary endarterectomy or balloon pulmonary angioplasty, may result in cure of PAH in selected cases.
The diagnostic work-up of patients with suspected PAH includes a medical history, physical examination, chest radiography, echocardiography, right heart catheterisation and advanced imaging such as CT, MRI and scintigraphy.
Radiographically , cardiac enlargement (right atrial and RV enlargement), dilatation of the central pulmonary arteries (MPA and its branches down to the segmental level) ( Fig. 16.10 ) and tapering of peripheral arterial branches (vessels beyond segmental level)—termed ‘peripheral pruning’—are seen. In long-standing cases, the central pulmonary arteries may develop calcification due to atheroma, a feature absent in non-hypertensive pulmonary arteries. Central arterial enlargement may superficially mimic enlarged hilar lymph nodes, but the smooth outline of arterial enlargement can usually be distinguished from the lobulated border of lymphadenopathy. Measurement of the transverse diameter of the right descending pulmonary artery at its midpoint may be used to suggest pulmonary hypertension. With a cut-off of greater than 17 mm being described. However, while a relatively specific sign, the sensitivity of chest radiography for the diagnosis of (mild) PAH is low.
Transthoracic ultrasound ( transthoracic echocardiography; TTE ) is often used as a screening tool that is recommended in all patients with suspected PAH. Echocardiography enables assessment of right- and left-sided cardiac chamber size and function, identification of intracardiac shunts and estimation of PA systolic pressure (by measuring the velocity of the tricuspid regurgitant jet). When peak tricuspid regurgitation velocity is difficult to measure (trivial/mild tricuspid regurgitation), the use of contrast-enhanced ultrasound significantly increases the Doppler signal, allowing for more precise measurement of the peak tricuspid regurgitation velocity. However, the literature is not uniform with respect to the diagnostic accuracy of TTE; bedside measurements show considerable user dependency and examinations may suffer from variable quality, especially in obese patients and those with chronic lung disease.
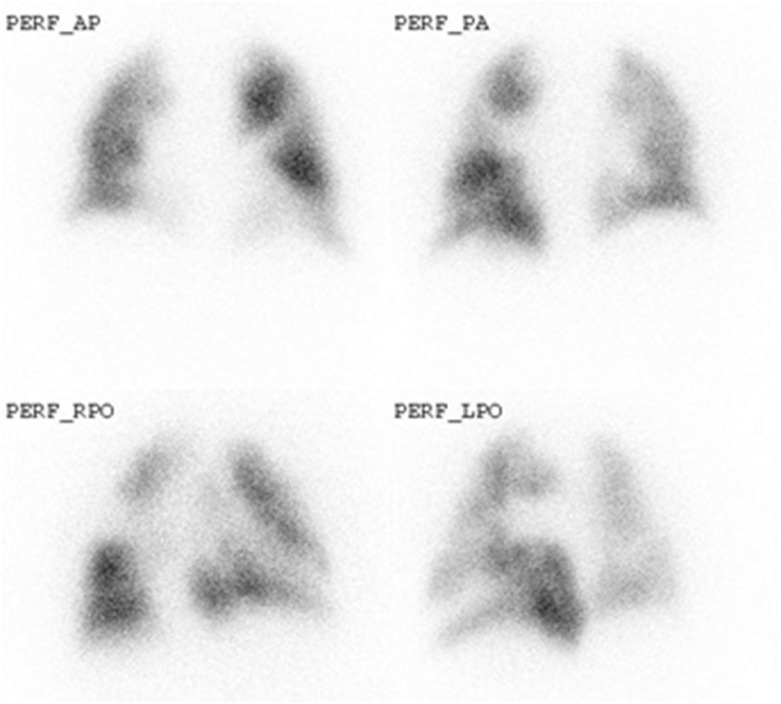
The ventilation–perfusion (VQ) lung scintigram is a nuclear medicine test widely performed in patients with suspected PAH to exclude CTEPH ( Fig. 16.11 ). According to the European Society of Cardiology and the European Respiratory Society guidelines published in 2009, it is recommended in all patients as the screening method of choice to diagnose or rule out CTEPH. A normal or low-probability VQ result effectively excludes CTEPH with a sensitivity of 90%–100% and a specificity of 94%–100%. Unmatched perfusion defects may also be seen in PVOD and sometimes in patients with PAH. In such cases. CTPA may be used as a complementary investigation. As CTPA is more frequently being used as a first-line test in patients with unexplained symptoms, VQ may not be required if CT has already proven the presence of chronic thromboembolic disease.
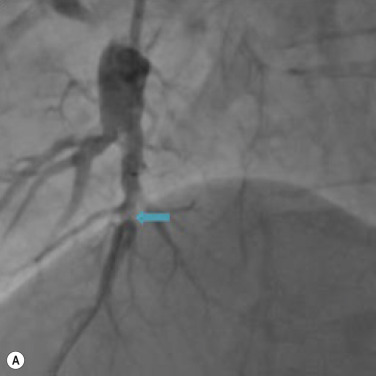
Traditional pulmonary angiography is still considered the reference standard in the anatomical assessment of the pulmonary arteries, though non-invasive imaging techniques are increasingly utilised. In some centres, angiography is considered mandatory for the work-up of CTEPH to identify patients who may benefit from thrombendarterectomy. Non-invasive imaging in many centres is playing an increasing role in the surgical evaluation or CTEPH. However, with increasing recognition of a potential role for balloon pulmonary angioplasty in CTEPH, a greater emphasis is being placed on angiography in inoperable cases ( Fig. 16.12 ). Angiography may also be helpful in the evaluation of possible vasculitis or pulmonary arteriovenous malformations (PAVMs).
Right heart catheterisation is indicated in all patients with suspected PAH to confirm the diagnosis, to evaluate the severity and when PAH-specific drug therapy is considered. Vasoreactivity testing is indicated in patients with idiopathic pulmonary arterial hypertension (IPAH), heritable PAH and other types of PAH; however, it should only be performed in specific centres and under controlled conditions. This testing is not recommended for other PAH groups.
MRI plays an important role in diagnosis of PAH because of its ability to assess RV dysfunction caused by increased afterload. Magnetic resonance angiography (MRA) of the pulmonary vasculature ( Figs 16.13 and 16.14 ) and parenchymal perfusion can be combined with dynamic quantitative assessment of ventricular volumes and function ( Fig. 16.15 ). Cardiac MRI provides direct evaluation of the RV size, morphology and function, and allows non-invasive assessment of parameters including stroke volume, CO and RV mass, as well as evaluation of left ventricular and valvular function. Phase contrast MRI permits evaluation of flow in the PA and aorta and, therefore, quantification of any intracardiac shunt as well as dynamic evaluation of the distensibility of the PA.
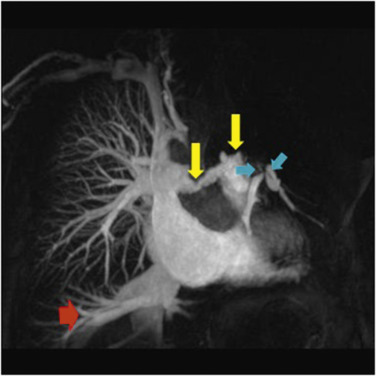
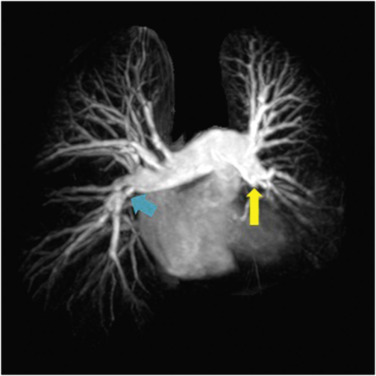
The lack of ionising radiation and objective nature of cardiac MRI assessment of right heart haemodynamics are particularly valuable for follow-up purposes. MRI data can be of prognostic importance—decreased stroke volume, increased RV end-diastolic volume and decreased LV end-diastolic volume being poor prognostic indicators. Using temporally resolved MR perfusion techniques combined with spatially resolved MRA patients with CTEPH can be accurately differentiated from those with PAH. Pulmonary arterial obstruction or stenosis leads to wedge-shaped perfusion defects or perfusion delay, while in PAH, the perfusion is reduced and heterogeneous. Quantitative evaluation of perfusion in patients with PAH has shown a significantly reduced pulmonary blood flow (PBF) and prolonged mean transit time (MTT) in patients when compared with healthy volunteers, but this did not correlate with the severity of pulmonary hypertension.
Computed tomography plays a major role in assessing patients with suspected or known PAH and may suggest the presence of PAH, even when clinically unsuspected. CT is widely available, non-invasive, inexpensive and well tolerated. CTPA permits comprehensive evaluation of the pulmonary vasculature, heart and lung parenchyma. Systematic evaluation of each component is key to full pathophysiological understanding. While CTPA's role in diagnosing acute and chronic PE is indisputable (see below), the comprehensive evaluation that it provides also demonstrates the impact on the right ventricle, identifies any left heart comorbidity and evaluates the lung parenchyma for signs of chronic lung disease and features of vasculopathy or hypoperfusion (mosaic perfusion).
CT may suggest the presence of PAH, independent of its cause when the ‘generic signs’ of PAH are present. The generic signs can include both vascular and cardiac features, which are described below. In the presence of known PAH, or CT signs suggesting its presence, a detailed evaluation of its potential cause should be sought. This will include a systematic evaluation of cardiac causes (left ventricular dilatation, signs of prior infarct, presence of valvular disease or an intracardiac shunt), large vessel obstruction (by chronic thromboembolic disease or rarely tumour or vasculitis) and the lung parenchyma (for primary lung disease, signs of vasculopathy/mosaic perfusion).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here