Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Both genetic and environmental factors determine the onset of pubertal change in young girls. Puberty may be delayed or may occur earlier, depending on nutrition-related factors and physical activity. Obesity causes earlier onset of puberty, and excessive exercise causes delay. Psychological disorders and chronic isolation may also affect the normal onset of puberty.
The Frisch hypothesis states that an invariant mean weight (48 kg/106 lb) is essential for the initiation of the first menses (menarche). Leptin (a peptide hormone) secreted by adipose tissue may provide the “triggering link” for the initiation of menarche.
The female fetus has the highest lifetime number of oocytes by mid-gestation. Brief follicular maturation and negative feedback on gonadotropin release due to follicular estradiol production also occurs in utero. Peak serum levels of gonadotropins are seen by 3 months after birth and then slowly decline, reaching their nadir at age 4 years. Between the ages of 4 and about 10 years, the “gonadostat” is said to regulate the hypothalamic–pituitary–ovarian axis. A combination of high sensitivity to low levels of estradiol resulting in negative feedback on gonadotropin release, and an intrinsic central nervous system inhibition of gonadotropin-releasing hormone secretion, keep gonadotropins at low levels. By age 11 years (the usual onset of pubertal development), there is a gradual loss of the negative feedback to low levels of sex steroids, and pubertal development begins.
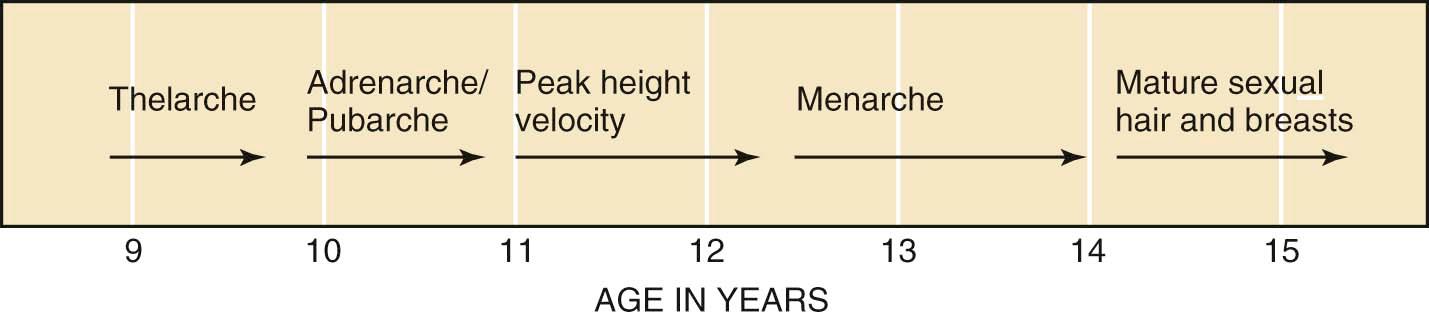
The usual sequence of physical signs of puberty in girls is (1) thelarche (breast budding), (2) adrenarche and/or pubarche (axillary and pubic hair growth), (3) peak height velocity, (4) menarche (first menses), and (5) mature sexual hair and breast growth.
Disorders of puberty include precocious development and delayed puberty. Precocious puberty refers to the development of any sign of secondary sexual maturation at an age earlier than 8 years in girls. Failure to undergo thelarche by the age of 14 years constitutes significant delay of pubertal development and requires evaluation.
Puberty encompasses the development of secondary sexual characteristics and the acquisition of reproductive capability. During this transition, usually between 10 and 16 years of age, a variety of physical, endocrinologic, and psychological changes accompany the increasing levels of circulating sex steroids.
The onset of pubertal changes is determined primarily by genetic factors, including race, and is also influenced by geographic location (girls in metropolitan areas, at altitudes near sea level, or at latitudes close to the equator tend to begin puberty at an earlier age) and nutritional status (obese children have an earlier onset of puberty, and those who are malnourished or have chronic illnesses associated with weight loss have a later onset of menses). Excessive exercise relative to the caloric intake can also delay the onset of puberty. It has been proposed that an invariant mean weight of 48 kg (106 lb) is essential for the initiation of menarche in healthy girls. Leptin, a peptide secreted by adipose tissue, may be the link between weight and the initiation of menarche. Psychological factors, severe neurotic or psychotic disorders, and chronic isolation may interfere with the normal onset of puberty through a mechanism similar to adult hypothalamic amenorrhea.
In the United States and Western Europe, a decrease in the age of menarche (i.e., age at first menses) was noted between 1840 and 1970, from an estimated mean age of 17 years in 1840 down to a reported mean age of 13 years in 1970. This trend has plateaued since then, and currently the mean age of menarche is approximately 12.4 years in the United States.
The fetal hypothalamic–pituitary–gonadal axis is capable of producing adult levels of gonadotropins and sex steroids. By 20 weeks' gestation, levels of gonadotropins —follicle-stimulating hormone (FSH) and luteinizing hormone (LH)— rise dramatically in both male and female fetuses ( Figure 32-1 ). Late in gestation, a surge in levels of glucocorticoids in the fetal circulation occurs. This is essential for normal maturation of fetal lungs and critical for the development of the fetal thyroid, kidney, brain, and pituitary. Recently, it has been suggested that excessive exposure of the developing fetus to glucocorticoids or exposure at the wrong time may lead to lifelong alterations in the function of the hypothalamic–pituitary–adrenal axis.
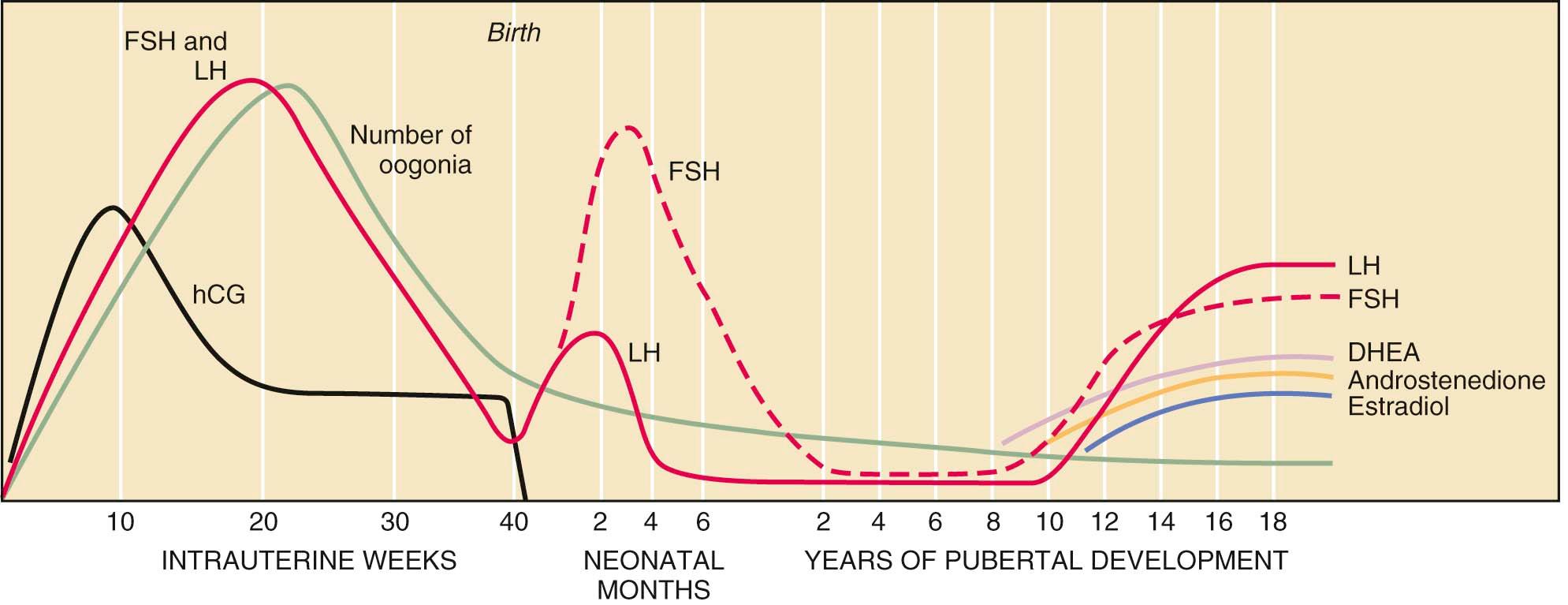
The female fetus acquires the lifetime peak number of oocytes (in utero) by mid-gestation and also has a brief period of follicular maturation and sex steroid production in response to elevated gonadotropin levels in utero. This transient increase in serum estradiol (a sex steroid) acts on the fetal hypothalamic-pituitary unit, resulting in a reduction of gonadotropin secretion (a negative feedback effect), which in turn reduces estradiol production. This indicates that the inhibitory effect of sex steroids on gonadotropin release is operative before birth.
In both male and female fetuses, serum estradiol is primarily of maternal and placental origin. With birth and the acute loss of maternal and placental sex steroids, the negative feedback action on the hypothalamic-pituitary axis is lost, and gonadotropins are once again released from the pituitary gland, reaching adult or near-adult concentrations in the early neonatal period. In the female infant, peak serum levels of gonadotropins are generally seen by 3 months of age, then they slowly decline until a nadir is reached by the age of 4 years. In contrast to gonadotropin levels, sex steroid concentrations decrease rapidly to prepubertal values within 1 week of birth and remain low until the onset of puberty.
During fetal development, the adrenal glands are large in proportion to their size in adult life (similar to the fetal kidneys). Early in gestation, the fetal adrenal gland produces abundant dehydroepiandrosterone sulfate (DHEA-S), which serves as a precursor for estrogen production by the placenta and is also able to convert placental progesterone into cortisol. It is not until about 23 weeks' gestation that the fetal adrenal cortex expresses the enzyme to directly synthesize cortisol from cholesterol or pregnenolone. In the first few months of postnatal life, the innermost part of the adrenal cortex (the fetal zone) largely regresses, and there is a rapid decrease in the production of DHEA-S.
The hypothalamic–pituitary–gonadal axis in the young child is suppressed between the ages of 4 and 10 years. The hypothalamic–pituitary system regulating gonadotropin release has been termed the gonadostat . Low levels of gonadotropins and sex steroids during this prepubertal period are a function of two mechanisms: (1) maximal sensitivity of the gonadostat to the negative feedback effect of the low circulating levels of estradiol present in prepubertal children, and (2) intrinsic central nervous system inhibition of hypothalamic gonadotropin-releasing hormone (GnRH) secretion. These mechanisms occur independently of the presence of functional gonadal tissue. This is clearly demonstrated in children with gonadal dysgenesis. Agonadal children display elevated gonadotropin concentrations during the first 2 to 4 years of life, followed by a decline in circulating FSH and LH levels by 6 to 8 years of age. By 10 to 12 years of age, gonadotropin concentrations spontaneously rise once again, eventually achieving castration levels. This pattern of gonadotropin secretion in early childhood is similar to that of children with normal gonadal function. These data suggest that an intrinsic central nervous system regulator of GnRH release is the principal inhibitor of gonadotropin secretion from 4 years of age until the peripubertal period. Furthermore, after regression of the fetal zone of the adrenal gland (a few months after birth), very low concentrations of adrenal androgen precursors are available, resulting in decreased adrenal androgen production in early childhood.
In general, androgen production and differentiation by the zona reticularis of the adrenal cortex are the initial endocrine changes associated with puberty. Serum concentrations of DHEA, DHEA-S, and androstenedione rise between the ages of 8 and 11 years. This rise in adrenal androgens induces the growth of both axillary and pubic hair and is known as adrenarche or pubarche. This increase in adrenal androgen production occurs independently of gonadotropin secretion or gonadal steroid levels, and the mechanism of its initiation is not understood at this time. Some studies have suggested that the morphologic and functional changes in the zona reticularis are induced by increasing cortisol levels. In cellular studies, human fetal adrenal cells exposed to cortisol in high concentrations produce DHEA, whereas in human studies, infants treated with high-dose adrenocorticotropic hormone for infantile spasms have been noted to have adrenal androgen production.
Recent studies indicate that girls who undergo premature pubarche are more likely than other girls to develop polycystic ovarian syndrome (PCOS) as adults (see Chapter 33 ).
By approximately the 11th year of life, there is a gradual loss of sensitivity by the gonadostat to the negative feedback of sex steroids ( Figure 32-2 ). As a consequence, GnRH pulses (with their mirroring pulses of FSH and LH) increase in amplitude and frequency. The factors that reduce the sensitivity of the gonadostat are incompletely understood. Some studies indicate that a rise in the concentration of leptin, a hormone produced by adipocytes (fat cells) that mediates appetite satiety, precedes and is necessary for this change. This, in turn, supports the association between minimum weight or total body fat and the onset of puberty. The Frisch hypothesis suggests that a critical body weight is necessary for pubertal onset. Further investigations support the concept that fat stores might influence pubertal onset through several mechanisms. First, adipocytes secrete adipokines such as leptin. Leptin appears to serve as a signal to the hypothalamic GnRH pulse generator that there are sufficient energy stores for fertility to commence. Studies have shown that every 1-kg gain in body weight lowers the onset of menarche by 13 days and that every increase of 1 ng/ml in serum leptin lowers the age of menarche by 1 month. Second, aromatase activity in adipocytes is dependent on fat mass, and obesity results in greater peripheral conversion of androstenedione to estrone and of testosterone to estradiol. Last, increasing adipose tissue is related to increasing insulin resistance, which decreases serum levels of sex hormone binding globulin. This leads to an increased level of bioavailable sex hormones.
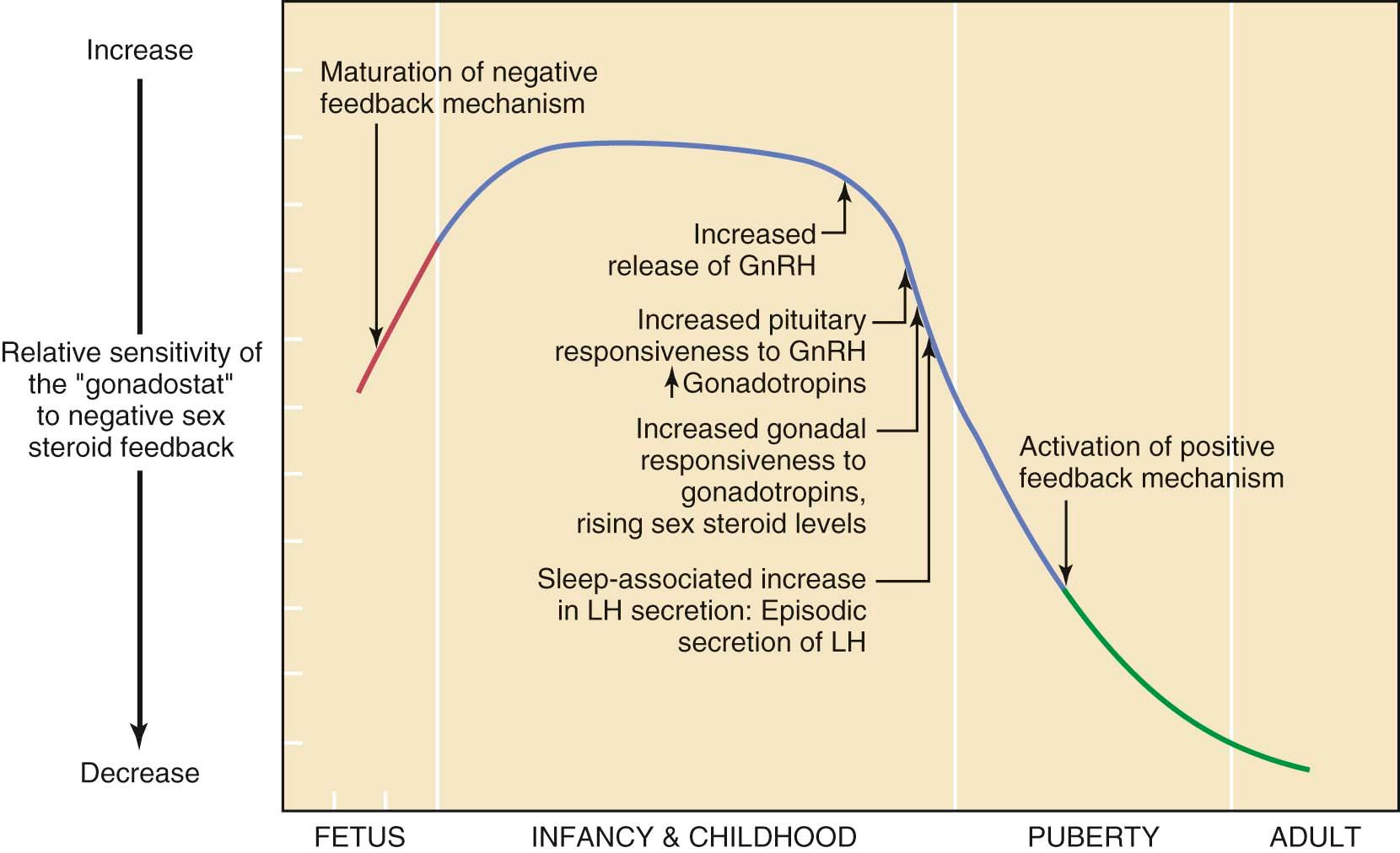
A further decrease in sensitivity of the gonadostat combined with the loss of intrinsic central nervous system inhibition of hypothalamic GnRH release is heralded by sleep-associated increases in GnRH secretion. This nocturnal dominant pattern gradually shifts into an adult-type secretory pattern, with GnRH pulses occurring every 90 to 120 minutes throughout the 24-hour day.
The increase in gonadotropin release promotes ovarian follicular maturation and sex steroid production, which induces the development of secondary sexual characteristics. By middle to late puberty, maturation of the positive feedback mechanism of estradiol on LH release from the anterior pituitary gland is complete, and ovulatory cycles are established.
Physical changes of puberty involve the development of secondary sexual characteristics and the acceleration of linear growth (gain in height). The Marshall and Tanner classification of breast and pubic hair development is employed for descriptive and diagnostic purposes ( Figures 32-3 and 32-4 ). A useful acronym for remembering the usual chronologic order of the stages of female pubertal development is TAPuP ME (standing for thelarche, adrenarche, pubarche, peak growth velocity, and menarche).
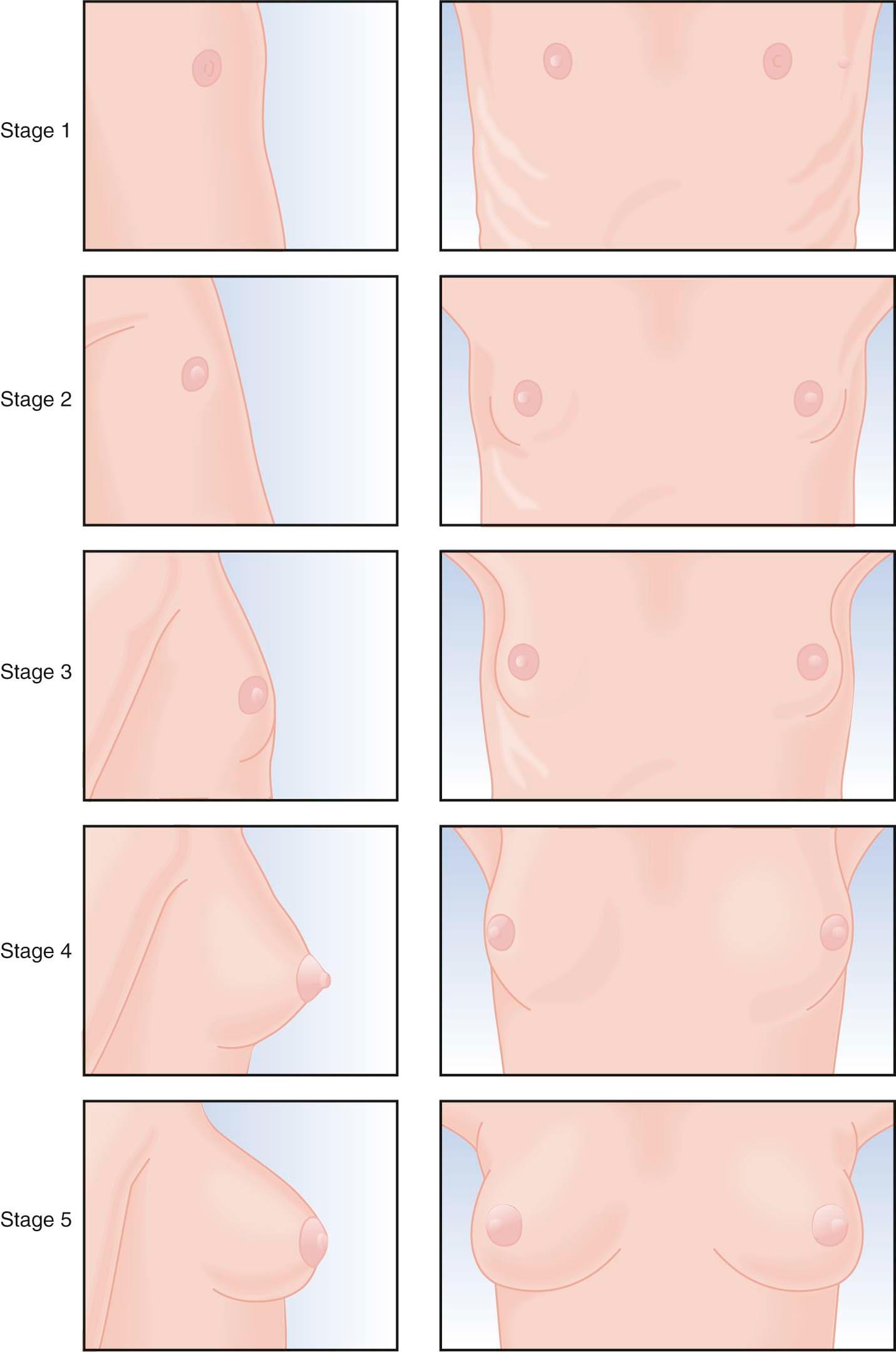
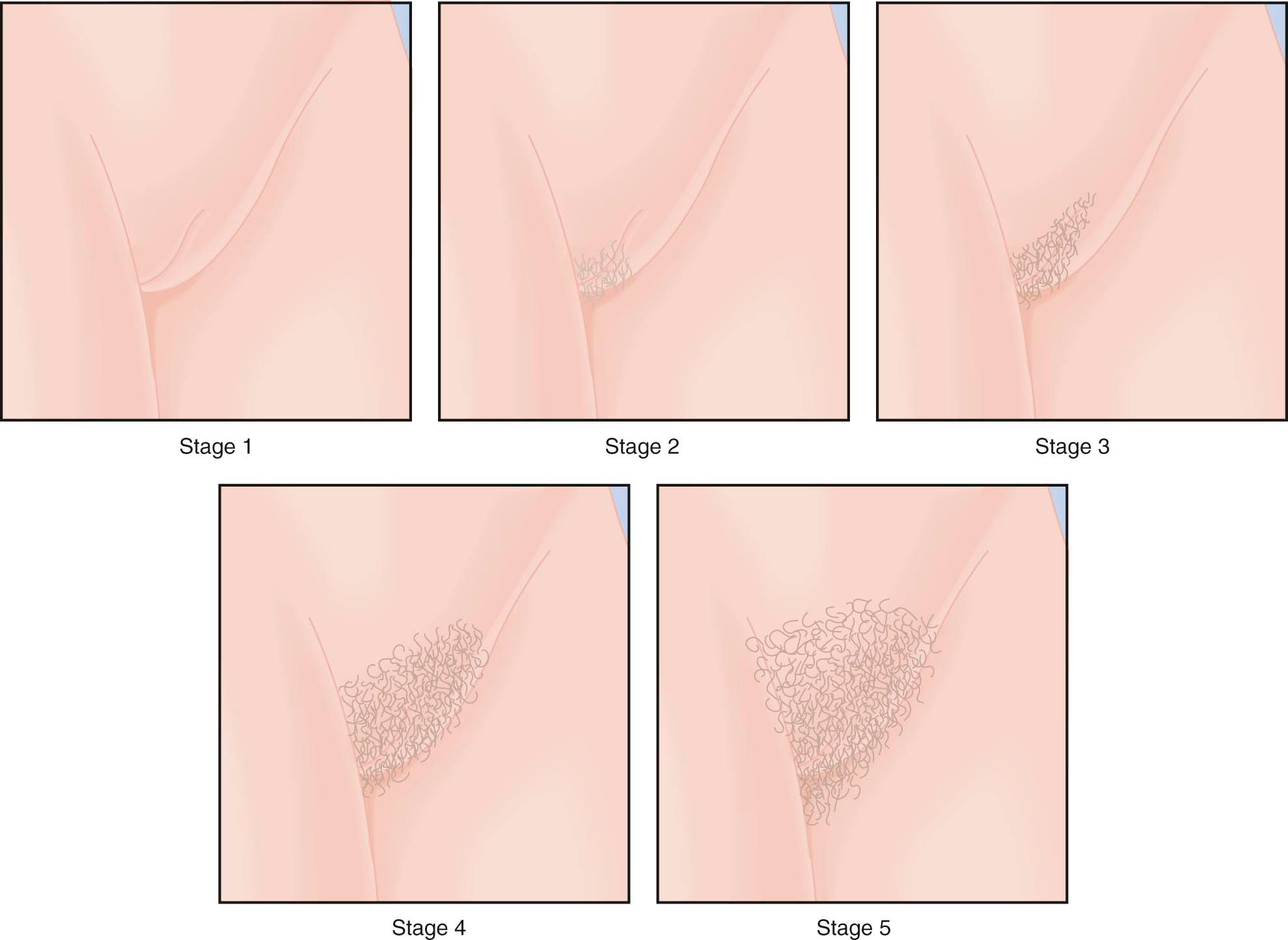
The first physical sign of puberty is usually breast budding (thelarche), followed by the appearance of axillary or pubic hair (adrenarche/pubarche). Unilateral breast development is not uncommon in early puberty and may last up to 6 months before the development of the contralateral breast. Maximal growth or peak height velocity is usually the next stage, followed by menarche (the onset of menstrual periods). The final somatic changes are the appearance of adult pubic hair distribution and adult-type breasts. In approximately 15% of normally developing girls, the development of pubic hair occurs before breast development. The sequence of pubertal changes generally occurs over a period of 4.5 years, with a normal range of 1.5 to 6 years ( Figure 32-5 ).
Race plays a role in determining the age of the onset of puberty. African American girls begin puberty earlier than girls in other racial groups (on average between the ages of 8 and 9 years), followed by Mexican Americans and whites ( Table 32-1 ). In African American girls, thelarche and adrenarche can occur as early as 6 years of age, whereas in whites, they can occur as early 7 years of age.
| Puberty Milestone | Non-Hispanic White (mean age * ) | Black (mean age * ) | Mexican American (mean age * ) |
|---|---|---|---|
| Pubic hair † | 10.5 | 9.5 | 10.3 |
| Breast development † | 10.3 | 9.5 | 9.8 |
| Menarche † | 12.7 | 12.1 | 12.2 |
| Menarche ‡ | 12.7 | 12.3 | 12.5 |
* Estimated with application of weights for the examination sample of the National Health and Nutrition Examination Survey III (NHANES III).
† Estimated using probit model for the status quo data of the puberty measurements.
‡ Estimated using failure time model for the recalled age at menarche.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here