Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The prevalence of mental illness and substance use disorders in the United States is about 30%, so these conditions are often present in patients undergoing anesthesia and surgery. Effects of and potential drug interactions with psychotropic medications are important perioperative considerations, as are potential behavioral issues. In addition, substance use and suicide represent significant occupational hazards for anesthesiologists.
Mood is defined as a temporary state of mind, temper, or feeling. Thus moods are transient. Mood disorders are characterized by disturbances in the regulation of mood, behavior, and affect that are longer lasting or even lifelong. They are typically divided into three classes: (1) depressive disorders, (2) bipolar disorders, and (3) depression associated with medical illness or substance use.
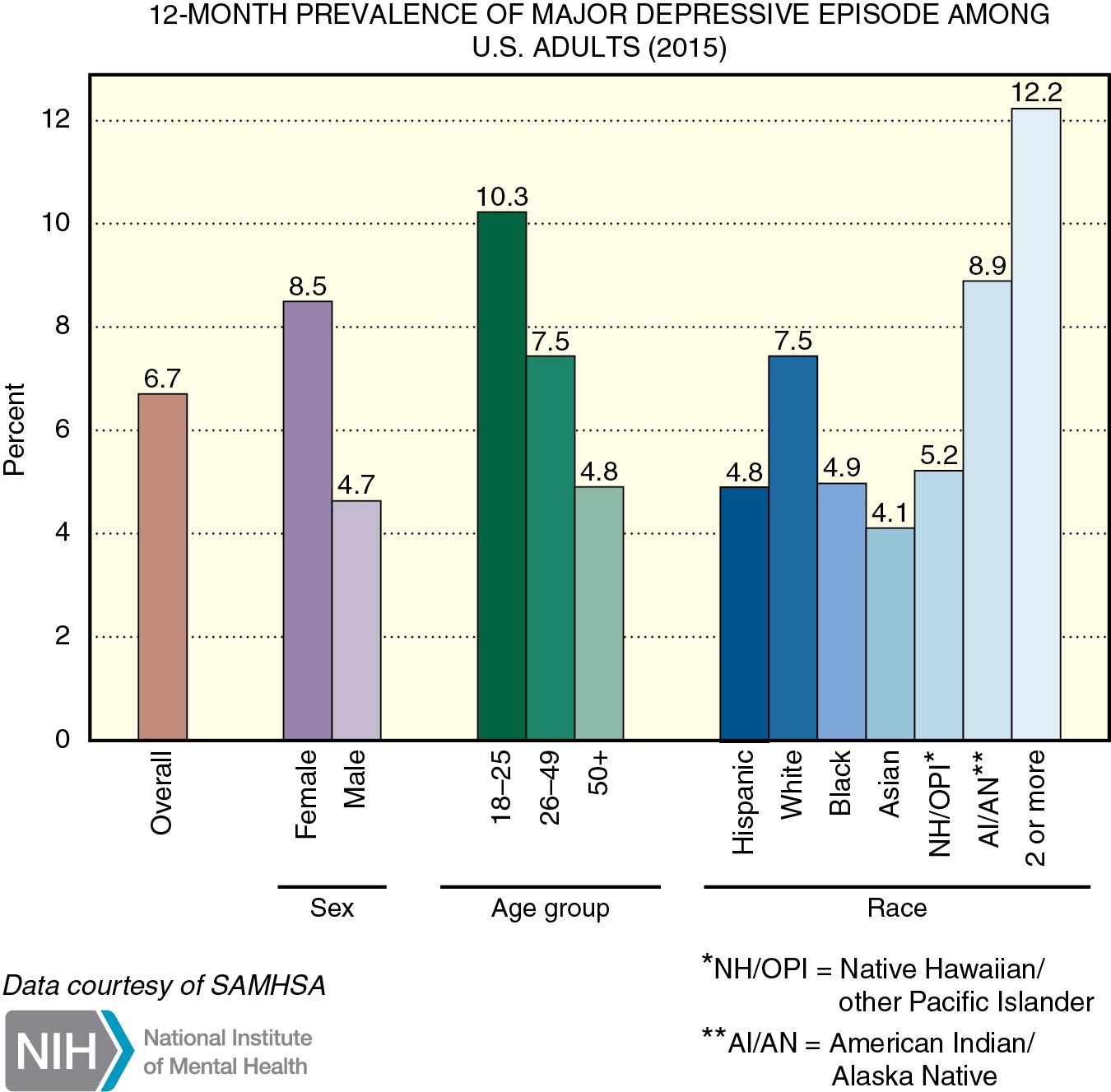
Depression is a common psychiatric disorder, affecting 6% to 7% of the population ( Fig. 29.1 ). It is distinguished from normal sadness and grief by the severity and duration of the mood disturbance. There is a familial pattern to major depression, and females are affected more often than males. A significant number of patients with major depression attempt suicide, and about 15% are successful. Pathophysiologic causes of major depression are unknown, although abnormalities of amine neurotransmitter pathways are the most likely etiologic factors.
The diagnosis of major depression is based on the persistent presence of at least five of the symptoms noted in Table 29.1 for a period of at least 2 weeks that is a change from previous functioning and not attributed to another medical condition. There is a profound loss of pleasure in previously enjoyable activities (anhedonia). Organic causes of irritability or mood changes and a normal reaction to a major loss (e.g., death of a loved one, loss of a job) must be excluded. Depressive symptoms often are present in patients with cardiac disease, cancer, neurologic diseases, diabetes mellitus, hypothyroidism, and human immunodeficiency virus (HIV) infection. This depression can be a situational depression caused by patients’ reaction to the health condition with which they are now confronted, which may compromise both the quality and quantity of their life. It could also be directly related to the medical illness itself or be a side effect of medications used to treat the medical illness.
| Depressed mood * |
| Markedly diminished interest or pleasure in almost all activities * |
| Fluctuations in body weight and appetite |
| Insomnia or hypersomnia |
| Psychomotor agitation or retardation |
| Fatigue |
| Feelings of worthlessness or guilt |
| Decreased ability to concentrate |
| Suicidal ideation |
* These must be present. Exclude symptoms attributed to another medical condition.
All patients with depression should be evaluated for suicide risk. Suicide is the 10th leading cause of death among Americans, with about 45,000 deaths per year due to this cause. Interestingly, physicians have moderately higher to much higher suicide rates than the general population. Most individuals who commit suicide have been under the care of a physician (not necessarily a psychiatrist) within the month before their death, which emphasizes the need for physicians in all specialties to recognize patients at risk. Hopelessness is the most important aspect of depression associated with suicide.
Depression can be treated with antidepressant medications, psychotherapy, electroconvulsive therapy (ECT), and other nonpharmacologic measures. An estimated 70% to 80% of patients respond to pharmacologic therapy, and most who do not respond to antidepressants do respond favorably to ECT or one of the alternative measures. ECT is typically reserved for patients with depression resistant to antidepressant drugs or those with medical contraindications to treatment with these drugs. Patients with depression plus psychotic symptoms (delusions, hallucinations, catatonia) require both antidepressant and antipsychotic drugs.
Approximately 50 years ago, neurochemical hypotheses regarding depression postulated that decreased availability of norepinephrine and serotonin at specific synapses in the brain is associated with depression and, conversely, that an increased concentration of these neurotransmitters is associated with mania. Subsequent studies have generally supported this hypothesis that norepinephrine and serotonin metabolism are important in mood states, although the exact mechanisms remain to be elucidated. Almost all drugs with antidepressant properties affect the availability of catecholamines and/or serotonin in the central nervous system (CNS) ( Table 29.2 ). These include selective serotonin reuptake inhibitors (SSRIs), selective serotonin-norepinephrine reuptake inhibitors (SNRIs), norepinephrine-dopamine reuptake inhibitors (NDRIs), atypical antidepressants, monoamine oxidase inhibitors (MAOIs), and tricyclic antidepressants.
| Drug Class | Generic Name | Trade Name |
| SSRIs | Fluoxetine Paroxetine Sertraline Citalopram Escitalopram |
Prozac Paxil Zoloft Celexa Lexapro |
| SNRIs | Duloxetine Venlafaxine Desvenlafaxine |
Cymbalta Effexor Pristiq |
| NDRI | Bupropion | Wellbutrin |
| MAOIs | Phenelzine Tranylcypromine Selegiline |
Nardil Parnate Emsam |
| Atypical | Trazodone Vortioxetine Mirtazapine |
Desyrel Trintellix Remeron |
SSRIs block reuptake of serotonin at presynaptic membranes but have relatively little effect on adrenergic, cholinergic, histaminergic, or other neurochemical systems. As a result, they are associated with few side effects.
Venlafaxine, desvenlafaxine, and duloxetine (SNRIs) are methylamine antidepressants that selectively inhibit reuptake of norepinephrine and serotonin without affecting other neurochemical systems. Bupropion inhibits reuptake of serotonin and dopamine. Other atypical antidepressants have a diverse range of activity ranging from antagonism of specific serotonin receptors, dopamine receptor blockade, presynaptic α 2 blockade resulting in increases in norepinephrine and serotonin release, and histamine receptor blockade
MAOIs are inhibitors of either or both the A and B forms of brain MAO and change the concentration of neurotransmitters by preventing breakdown of catecholamines and serotonin. They are not considered first-line drugs in the treatment of depression because of their adverse effect profile, which includes the risk of hypertensive crises from consumption of tyramine-containing foods and the risk of serotonin syndrome if they are used concomitantly with SSRIs.
Before the availability of SSRIs, tricyclic antidepressants were the most commonly prescribed drugs for treatment of depression. They were thought to affect depression by inhibiting synaptic reuptake of norepinephrine and serotonin. However, they also affect other neurochemical systems, including histaminergic and cholinergic systems. They are now rarely used as first-line therapy for depression but are used more commonly as adjuvant therapy for patients with chronic pain syndromes. Their principal advantage is the existence of well-defined correlations between dosage, plasma concentration, and therapeutic response for nortriptyline, imipramine, and desipramine. Adverse effects include sedation, anticholinergic effects, and cardiovascular abnormalities, including orthostatic hypotension and cardiac dysrhythmias.
There has been a resurgence in the use of amphetamine and its congeners in treating depression. Typically these drugs are used in small dosages in combination with SSRIs. The effects on mood can be remarkable. However, because of their status as class II controlled substances, they are not widely used.
Serotonin is produced by hydroxylation and decarboxylation of l-tryptophan in presynaptic neurons, then stored in vesicles that are released and bound to postsynaptic receptors when needed for neurotransmission. A reuptake mechanism allows for return of serotonin to the presynaptic vesicles. Metabolism is by MAO type A. Serotonin-specific reuptake inhibitors, as their name implies, inhibit reuptake of serotonin from the neuronal synapse without having significant effects on reuptake of norepinephrine and/or dopamine.
SSRIs comprise the most widely prescribed class of antidepressants and are the drugs of choice to treat mild to moderate depression. These drugs are also effective for treating panic disorders, posttraumatic stress disorder, bulimia, dysthymia, obsessive-compulsive disorder, and irritable bowel syndrome. Common side effects include insomnia, agitation, headache, nausea, diarrhea, dry mouth, and sexual dysfunction. Appetite suppression is associated with fluoxetine therapy, though this effect is usually transient. Abrupt cessation of SSRI use, especially use of paroxetine and fluvoxamine, which have short half-lives and no active metabolites, can result in a discontinuation syndrome that can mimic serious illness and can be distressing and uncomfortable. Discontinuation symptoms typically begin 1 to 3 days after abrupt cessation of SSRI use and may include dizziness, irritability, mood swings, headache, nausea and vomiting, dystonia, tremor, lethargy, myalgias, and fatigue. Symptoms are relieved within 24 hours of restarting SSRI therapy.
Among SSRIs, fluoxetine is a potent inhibitor of certain hepatic cytochrome P450 enzymes. As a result, this drug may increase plasma concentrations of drugs that depend on hepatic metabolism for clearance. For example, addition of fluoxetine to treatment with tricyclic antidepressant drugs may result in twofold to fivefold increases in plasma concentrations of tricyclic drugs. Some cardiac antidysrhythmic drugs and some β-adrenergic antagonists are also metabolized by this enzyme system, and fluoxetine inhibition of enzyme activity may result in potentiation of their effects.
Serotonin syndrome is a potentially life-threatening adverse drug reaction that may occur with therapeutic drug use, overdose, or interactions between serotoninergic drugs. A large number of drugs have been associated with serotonin syndrome. These include SSRIs, atypical and cyclic antidepressants, MAOIs, opiates, cough medicine, antibiotics, antiemetic drugs, antimigraine drugs, drugs of abuse (especially Ecstasy), and herbal products ( Table 29.3 ).
| Selective serotonin reuptake inhibitors |
| Selective serotonin-norepinephrine reuptake inhibitors |
| Bupropion |
| Atypical antidepressants |
| Monoamine oxidase inhibitors |
| Tricyclic antidepressants |
| Drugs of abuse: ecstasy, lysergic acid diethylamide (LSD), amphetamines |
| Antiemetic drugs: ondansetron, granisetron, metoclopramide, droperidol |
| Analgesics: meperidine, fentanyl, tramadol |
| Lithium |
| Muscle relaxant: cyclobenzaprine |
| Antimigraine drugs: triptans |
| Anticonvulsant drugs: valproate |
| Antibiotics: linezolid, ritonavir |
| Cough medicine: dextromethorphan |
| Dietary supplements: nutmeg, ginseng, St. John wort |
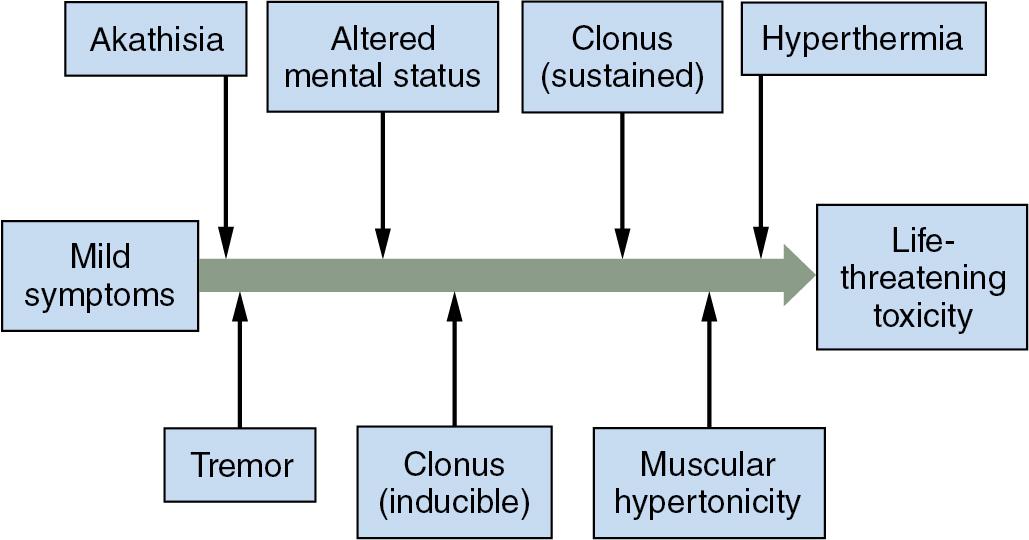
Typical symptoms of serotonin syndrome include agitation, delirium, autonomic hyperactivity, hyperreflexia, clonus, and hyperthermia ( Fig. 29.2 ). Additional syndromes to consider in the differential diagnosis of serotonin syndrome are listed in Table 29.4 . Treatment includes supportive measures and control of autonomic instability, excess muscle activity, and hyperthermia. Cyproheptadine, a 5-hydroxytriptamine (serotonin) type 2A (5-HT 2A ) antagonist, can be used to compete for and bind to serotonin receptors. It is available only for oral use.
| Syndrome | Time to Onset | Causative Drugs | Outstanding Features | Treatment |
| Malignant hyperthermia | Within minutes | Succinylcholine, inhalation anesthetics | Muscle rigidity, severe hypercarbia | Dantrolene, supportive care |
| Neuroleptic malignant syndrome | 24–72 hr | Dopamine antagonist antipsychotic drugs | Muscle rigidity, stupor or coma, bradykinesia | Bromocriptine or dantrolene, supportive care |
| Serotonin syndrome | Up to 12 hr | Serotoninergic drugs | Clonus, hyperreflexia, agitation; possible muscle rigidity | Cyproheptadine, supportive care |
| Sympathomimetic syndrome | Up to 30 min | Cocaine, amphetamines | Agitation, hallucinations, myocardial ischemia, dysrhythmias, no rigidity | Vasodilators, α and β blockers, supportive care |
| Anticholinergic poisoning | Up to 12 hr | Atropine, belladonna | Toxidrome of hot, red, dry skin; dilated pupils; delirium; no rigidity | Physostigmine, supportive care |
| Cyclic antidepressant overdose | Up to 6 hr | Cyclic antidepressants | Hypotension, stupor or coma, polymorphic ventricular tachycardia, no rigidity | Serum alkalinization, magnesium |
Patients whose depression does not respond to other antidepressant drugs may benefit from treatment with MAOIs. MAOIs inhibit norepinephrine and serotonin and tyramine breakdown, so there is more norepinephrine and serotonin available for release. Selegiline is a subtype A MAOI that is reversible and specifically catabolizes serotonin, norepinephrine, and tyramine, the substances most directly linked to MAOI antidepressant activity. It is used in a transdermal preparation that limits enterohepatic MAO inhibition and may help eliminate the need for dietary tyramine restriction.
The principal clinical problems associated with use of MAOIs, especially the nonselective irreversible forms, include the need for dietary restrictions, the potential for drug interactions, and adverse side effects. Probably the most dreaded occurrence is very significant systemic hypertension if patients ingest foods containing tyramine (cheeses, wines) or receive sympathomimetic drugs. Both tyramine and sympathomimetic drugs are potent stimuli for norepinephrine release. Interestingly, however, orthostatic hypotension is the most common adverse effect observed in patients being treated with MAOIs ( Table 29.5 ). The mechanism for this hypotension is unknown, but it may involve accumulation of false neurotransmitters such as octopamine that are less potent than norepinephrine. This mechanism may also explain the antihypertensive effects observed with long-term use of MAOIs.
| Sedation |
| Blurred vision |
| Orthostatic hypotension |
| Tyramine-induced hypertensive crisis |
| Excessive effects of sympathomimetic drugs |
| Potential for serotonin syndrome |
Adverse interactions between MAOIs and serotoninergic drugs have been observed. In the anesthetic environment the interaction between MAOIs and the opioid meperidine has been the most notable.
Anesthesia can be safely conducted in patients being treated with MAOIs despite earlier recommendations that these drugs be discontinued 14 days before elective surgery to permit time for regeneration of new enzyme. Proceeding with anesthesia and surgery in patients being treated with MAOIs influences selection and doses of drugs to be administered. Benzodiazepines are acceptable for pharmacologic treatment of preoperative anxiety. Induction of anesthesia can be safely accomplished with most intravenous (IV) induction agents, but it should be kept in mind that CNS effects and depression of ventilation may be exaggerated. Ketamine, a sympathetic stimulant, should be avoided. Serum cholinesterase activity may decrease in patients treated with phenelzine, so the dose of succinylcholine may need to be reduced. A volatile anesthetic with or without nitrous oxide is acceptable for maintenance of anesthesia. Anesthetic requirements may be increased because of increased concentrations of norepinephrine in the CNS. Fentanyl has been administered intraoperatively to patients being treated with MAOIs without apparent adverse effects. The choice of nondepolarizing muscle relaxants is not influenced by treatment with MAOIs. Spinal or epidural anesthesia is acceptable, although the potential of these anesthetic techniques to produce hypotension and the consequent need for vasopressors may argue in favor of general anesthesia. Addition of epinephrine to local anesthetic solutions should probably be avoided.
During anesthesia and surgery, it is important to avoid stimulating the sympathetic nervous system as, for example, by light anesthesia, topical application of cocaine spray, or injection of indirect-acting vasopressors to decrease the incidence of systemic hypertension. If hypotension occurs and vasopressors are needed, use of a direct-acting drug such as phenylephrine is recommended. The dose should probably be decreased to minimize the likelihood of an exaggerated hypertensive response.
Provision of analgesia during the postoperative period is influenced by the potential adverse interactions between opioids, especially meperidine and MAOIs, which can result in serotonin syndrome. If opioids are needed for postoperative pain management, morphine is a preferred drug. Alternatives to opioid analgesics such as nonopioid analgesics, nonsteroidal antiinflammatory drugs (NSAIDs), and peripheral nerve blocks should be considered. Neuraxial opioids provide effective analgesia, but experience is too limited to permit recommendations regarding use of this approach in patients being treated with MAOIs.
For patients who do not respond well to antidepressant drug therapy, there are several forms of treatment for severe depression that do not include antidepressant medications but rather rely on various forms of brain stimulation. At the present time these alternatives include transcranial magnetic stimulation and ECT. Magnetic seizure therapy (MST) is in investigational trials.
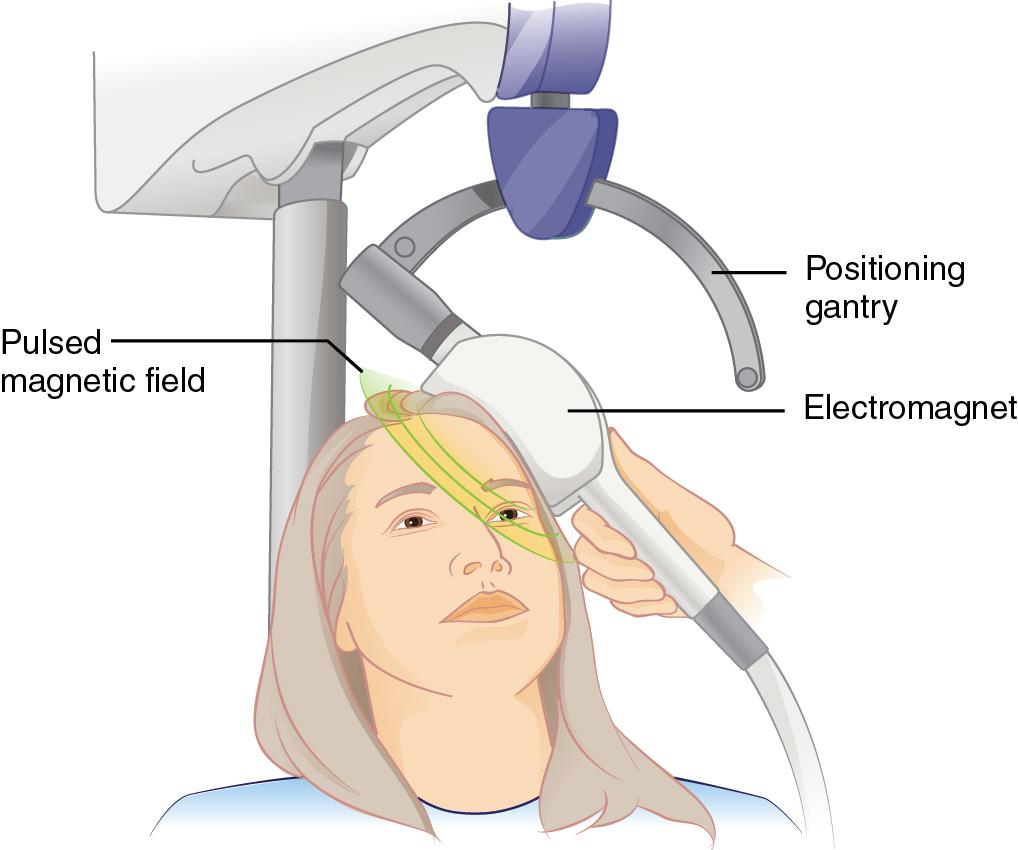
Repetitive transcranial magnetic stimulation (rTMS) uses a magnet instead of electric current to activate the brain. An electromagnetic coil is placed against the forehead near the region of the brain thought to be involved in regulation of mood ( Fig. 29.3 ). Then short electromagnetic impulses are administered through the coil. These cause small electric currents that stimulate cells in the targeted region. The impulses can apparently not travel farther than about 2 inches from the point of origin, so the treatment is localized to the area of interest, which is typically the left or right prefrontal cortex. The impulses have about the same strength as those in use during an magnetic resonance imaging (MRI) exam. A major advantage of this treatment is that anesthesia is not needed. Most complications consist of headaches and scalp discomfort. In 2008, the US Food and Drug Administration (FDA) approved rTMS for treatment of depression in patients who have failed treatment with at least one antidepressant medication.
MST uses elements of both rTMS and ECT. It uses a magnetic pulse instead of electricity to stimulate a target area in the brain, but it uses a higher frequency of electromagnetic stimulation, with the aim of inducing a seizure. Because of this, an anesthetic is required in a manner similar to that needed for ECT. There is some evidence that MST may reduce the incidence and severity of cognitive side effects compared to traditional ECT.
Despite many decades of use of ECT, the exact mechanism for its therapeutic effect remains unknown. Alterations in neurophysiologic, neuroendocrine, and neurochemical systems are thought to be involved but have not been clearly elucidated. What is evident is that electrically induced seizures of at least 25 sec duration are necessary for a therapeutic effect. ECT is indicated for treatment of severe depression in patients who show no response to drug therapy, cannot tolerate the adverse effects of psychotropic drug therapy, or are suicidal. The electric current may be administered to both hemispheres or only to the nondominant hemisphere (which may reduce memory impairment). The electrical stimulus produces a grand mal seizure consisting of a brief tonic phase followed by a more prolonged clonic phase. The electroencephalogram shows changes similar to those present during spontaneous grand mal seizures. Typically patients undergo 6 to 12 induction treatments during hospitalization and then may continue weekly, biweekly, or monthly maintenance therapy. More than two-thirds of patients receiving ECT show significant improvement in their depressive symptoms.
In addition to the seizure and its neuropsychiatric effects, ECT produces significant cardiovascular and CNS effects ( Table 29.6 ). The typical cardiovascular response to the ECT stimulus consists of 10 to 15 sec of parasympathetic stimulation producing bradycardia with a reduction in blood pressure, followed by sympathetic nervous system activation resulting in tachycardia and hypertension lasting several minutes. These changes may be undesirable in patients with ischemic heart disease. Indeed, the most common causes of death associated with ECT are myocardial infarction and cardiac dysrhythmias, although overall mortality rates are extremely low, approximately 1 in 5000 treatments. Transient myocardial ischemia, however, is not an uncommon event. Other cardiovascular changes in response to ECT include decreased venous return caused by the increased intrathoracic pressure that accompanies the seizure and/or positive pressure ventilation and ventricular premature beats that presumably reflect excess sympathetic nervous system activity. Patients with acute coronary syndromes, decompensated congestive heart failure, significant dysrhythmias, and severe valvular heart disease require cardiologic consultation prior to initiation of ECT.
|
|
|
|
|
|
|
|
|
|
|
Cerebrovascular responses to ECT include marked increases in cerebral blood flow (up to sevenfold) and cerebral blood flow velocity (more than double) compared with pretreatment values. Cerebral oxygen consumption increases as well. The rapid increase in systemic blood pressure may transiently overwhelm cerebral autoregulation and result in a dramatic increase in intracranial pressure. Thus the use of ECT is prohibited in patients with known space-occupying lesions or head injury. The cerebral hemodynamic changes are also associated with increased wall stress on cerebral aneurysms, and intracranial aneurysm disease is another contraindication to ECT.
Increased intraocular pressure is an inevitable side effect of electrically induced seizures. Increased intragastric pressure also occurs during seizure activity. Transient apnea, postictal confusion or agitation, nausea and vomiting, and headache may follow the seizure. The most common long-term effect of ECT is memory impairment.
Anesthesia for ECT must be brief, provide the ability to monitor and limit the physiologic effects of the seizure, and minimize any interference with seizure activity or duration. Patients must fast before the procedure. IV administration of glycopyrrolate 1 to 2 min before induction of anesthesia and delivery of the electric current may be useful in decreasing excessive salivation and bradycardia. The magnitude of treatment-induced hypertension can be ameliorated with use of nitroglycerin intravenously, sublingually, or transdermally. Likewise, esmolol 1 mg/kg IV administered just before induction of anesthesia can attenuate the tachycardia and hypertension associated with ECT, and it does so better than labetalol. Many other drugs, including calcium channel blockers, ganglionic blockers, α 2 -agonists and antagonists, and direct-acting vasodilators, have been used to treat the sympathetic overactivity during ECT, but they do not appear to offer any specific advantages over esmolol or nitroglycerin therapy.
Methohexital (0.5–1 mg/kg IV) is the traditional drug used for induction of anesthesia for ECT. It has a rapid onset, short duration of action, minimal anticonvulsant effects, and rapid recovery. Because of shortages of barbiturates in the United States, other induction drugs are now commonly used for ECT. Propofol is an alternative to methohexital and is associated with a lower blood pressure and heart rate response to ECT. Recovery time is similar after administration of methohexital and propofol, but the anticonvulsant effect of propofol can be manifested as a shortened seizure duration. Ketamine and etomidate improve the quality and duration of the electrically induced seizure, but ketamine is associated with a prolonged reorientation time after the procedure, and etomidate is associated with more hypertension after the seizure and the possibility of spontaneous seizures before the electrical stimulus is delivered.
IV injection of succinylcholine promptly after induction is intended to attenuate the potentially dangerous skeletal muscle contractions and bone fractures that can result from seizure activity. Doses of 0.3 to 0.5 mg/kg IV are sufficient to attenuate skeletal muscle contractions and still permit visual confirmation of seizure activity. The most reliable method to confirm electrically induced seizure activity is the electroencephalogram. Alternatively, tonic and clonic movements in an extremity that has been isolated from the circulation by applying a tourniquet before administration of succinylcholine are evidence that a seizure has occurred. Succinylcholine-induced myalgias are remarkably uncommon, occurring in only about 2% of patients undergoing ECT. There is no evidence that succinylcholine-induced release of potassium is increased by ECT. Ventilatory support and oxygen supplementation are continued as necessary until there is complete recovery to pretreatment cardiopulmonary status. Because repeated administration of anesthetics is necessary, it is possible to establish the exact doses of the anesthetic induction drug and succinylcholine that produce the most predictable and desirable effects in each patient.
Occasionally ECT is necessary in a patient with a permanent cardiac pacemaker or cardioverter-defibrillator. Fortunately most of these devices are shielded and not adversely affected by the electric currents necessary to produce seizures, but it is prudent to have a magnet available to ensure the pacemaker can be converted to an asynchronous mode should malfunction occur in response to the delivered electric current or to myopotentials from the succinylcholine or the seizure. Monitoring the electrocardiogram (ECG) and the plethysmographic waveform of the pulse oximeter and palpation of peripheral arterial pulses will document uninterrupted function of a cardiac pacemaker. Implantable cardioverter-defibrillators should be turned off before ECT and reactivated when the treatment is finished.
Safe and successful use of ECT has been described in patients following cardiac transplantation. In such patients, lack of vagal innervation to the heart eliminates the risk of bradydysrhythmias. However, the sympathetic responses still occur.
Bipolar disorder, previously called manic-depressive disorder, is characterized by marked mood swings from depressive episodes to manic or hypomanic episodes, with normal behavior often seen between these episodes. Between 8% and 10% of patients with bipolar disorder commit suicide. The manic phase of bipolar disorder is manifested clinically by sustained periods of expansive euphoric mood in which the patient expresses grandiose ideas and plans. The mood disturbance may be sufficiently severe to cause impairment in occupational functioning, social activities, and relationships, so there is risk of harm to self and others. Irritability and hyperactivity are also present; in severe cases, psychotic delusions and hallucinations may appear that are indistinguishable from those of schizophrenia ( Table 29.7 ).
| Expansive euphoric mood |
| Inflated self-esteem |
| Decreased need for sleep |
| Flight of ideas |
| Greater talkativeness than usual |
| Distractibility |
| Psychomotor agitation |
Genetic patterns in bipolar disorders suggest autosomal dominance with variable penetrance. Presumably there are abnormalities in neuroendocrine pathways that result in aberrant regulation of one or more amine neurotransmitter systems. Thus the pathophysiology of bipolar disorder—to the extent it is known—is similar to that of major depressive illness. Note that evaluation of mania must exclude the effects of substance abuse drugs, medications, and concomitant medical conditions.
Mania necessitates prompt treatment, usually in a hospital setting to protect patients from potential harmful actions. Lithium remains a mainstay of treatment, but antiepileptic drugs such as carbamazepine and valproate are often used. Olanzapine is another treatment option. When manic symptoms are severe, lithium may be administered in combination with an antipsychotic drug until the acute symptoms abate.
Lithium is an alkali metal, a monovalent cation, and is minimally protein bound. It does not undergo biotransformation and is excreted by the kidneys. Lithium is efficiently absorbed after oral administration. Its therapeutic serum concentration for acute mania and for prophylaxis is approximately 0.8 to 1.2 mEq/L. Because of this narrow therapeutic window, the serum lithium concentration must be monitored to prevent toxicity. The therapeutic effects of lithium are most likely related to actions on second-messenger systems based on phosphatidylinositol turnover. Lithium also affects transmembrane ion pumps and has inhibitory effects on adenylate cyclase.
Common adverse effects of lithium therapy include cognitive dysfunction, weight gain, and tremor. Lithium inhibits release of thyroid hormone and results in hypothyroidism in about 5% of patients. Long-term administration of lithium may also result in polyuria due to a form of vasopressin-resistant diabetes insipidus. Cardiac problems may include sinus bradycardia, sinus node dysfunction, atrioventricular block, T-wave changes, and ventricular irritability. Leukocytosis in the range of 10,000 to 14,000 cells/mm 3 is common.
Toxicity occurs when the serum lithium concentration exceeds 2 mEq/L, with signs of skeletal muscle weakness, ataxia, sedation, and widening of the QRS complex. Atrioventricular heart block, hypotension, and seizures may accompany severe lithium toxicity. Hemodialysis may be necessary in this medical emergency.
Lithium is excreted entirely by the kidneys. Reabsorption of lithium occurs in the proximal tubule in exchange for sodium, so diuretic use can affect the serum lithium concentration. Thiazide diuretics trigger an increase in lithium reabsorption in the proximal tubule, whereas loop diuretics do not promote lithium reabsorption. Administration of sodium-containing solutions or osmotic diuretics enhances renal excretion of lithium and results in lower lithium levels. Concomitant administration of NSAIDs and/or angiotensin-converting enzyme (ACE) inhibitors increases the risk of lithium toxicity.
Evidence of lithium toxicity is an important consideration during the preoperative evaluation. The most recent serum lithium concentration should be reviewed, and inclusion of a lithium level in measurements of the patient’s serum electrolyte concentrations during the perioperative period is very useful. To prevent significant renal reabsorption of lithium, it is reasonable to administer sodium-containing IV solutions during the perioperative period. Stimulation of urine output with thiazide diuretics must be avoided. The ECG should be monitored for evidence of lithium-induced conduction defects or dysrhythmias. The association of sedation with lithium therapy suggests that anesthetic requirements may be decreased in these patients. Monitoring the effects of neuromuscular blockade is indicated because the duration of action of both depolarizing and nondepolarizing muscle relaxants may be prolonged in the presence of lithium.
Schizophrenia (Greek for “split mind”) is the major psychotic mental disorder. It is characterized by abnormal reality testing or thought processes. The essential features of the illness include two broad categories of symptoms. Positive symptoms are those that reflect distortion or exaggeration of normal behavior and include delusions and hallucinations. Negative symptoms represent a loss or diminution in normal function and include flattened affect, apathy, social or occupational dysfunction (including withdrawal), and changes in appearance and hygiene. Subtypes of schizophrenia include paranoid type, disorganized type, catatonic type, and undifferentiated type. In some patients the disorder is persistent, whereas in others there are exacerbations and remissions.
The dopamine hypothesis concerning the etiology of schizophrenia suggests the disorder is a result of neurotransmitter dysfunction, specifically dysfunction of the neurotransmitter dopamine. This hypothesis is based on the discovery that agents that diminish dopaminergic activity also reduce the acute signs and symptoms of psychosis, especially agitation, anxiety, and hallucinations. Drugs that affect dopaminergic function by blocking dopamine receptors, especially D 2 and D 4 receptors, have demonstrated the ability to improve a variety of psychotic symptoms, especially positive symptoms. Conventional antipsychotic drugs have broad-spectrum dopamine receptor–blocking properties affecting all dopamine receptor subtypes. As a result, these drugs have significant adverse motor effects. These troubling side effects include tardive dyskinesia (choreoathetoid movements), akathisia (restlessness), acute dystonia (contraction of skeletal muscles of the neck, mouth, and tongue), and parkinsonism. Some of these effects diminish over time, but some persist even after drug discontinuation. Concurrent administration of anticholinergic medication may lessen some of these motor abnormalities. Acute dystonia resolves with administration of diphenhydramine 25 to 50 mg IV.
Newer antipsychotic drugs, also called atypical antipsychotic drugs, have variable effects on dopamine receptor subtypes and serotonin receptors, especially the 5-HT 2A receptor. These newer drugs appear to be quite effective in relieving the negative symptoms of schizophrenia and have fewer extrapyramidal side effects than traditional drugs.
For the anesthesiologist, important effects of antipsychotic medications include β-adrenergic blockade causing postural hypotension, prolongation of the QT interval (potentially producing ventricular dysrhythmias), seizures, elevations in hepatic enzyme levels, abnormal temperature regulation, and sedation. Drug-induced sedation may decrease anesthetic requirements.
Neuroleptic malignant syndrome is a rare, potentially fatal complication of antipsychotic drug therapy that probably reflects dopamine depletion in the CNS. This syndrome can occur anytime during the course of antipsychotic treatment but often is manifest during the first few weeks of therapy or after an increase in drug dosage. Clinical manifestations usually develop over 24 to 72 hours and include hyperpyrexia, severe skeletal muscle rigidity, rhabdomyolysis, autonomic hyperactivity (tachycardia, hypertension, cardiac dysrhythmias), altered consciousness, and acidosis. Skeletal muscle spasm may be so severe that mechanical ventilation becomes necessary. Renal failure may occur as a result of myoglobinuria and dehydration.
Treatment of neuroleptic malignant syndrome requires immediate cessation of antipsychotic drug therapy and supportive therapy (mechanical ventilation, hydration, cooling). Bromocriptine (5 mg orally every 6 hours) or dantrolene (up to 10 mg/kg daily as a continuous infusion) may decrease skeletal muscle rigidity. Mortality rates approach 20% in untreated patients, with death resulting from cardiac dysrhythmias, congestive heart failure, hypoventilation, or renal failure. Patients who have had this syndrome are likely to experience a recurrence when treatment with antipsychotic drugs is resumed, so a switch is usually made to a less potent antidopaminergic drug or to an atypical antipsychotic medication.
Because there are similarities between neuroleptic malignant syndrome and malignant hyperthermia, the possibility that patients with a history of neuroleptic malignant syndrome are vulnerable to developing malignant hyperthermia is an important issue to consider. At the present time there is no evidence of a pathophysiologic link between the two syndromes, and there is no familial pattern or evidence of inheritance in neuroleptic malignant syndrome. However, until any association between neuroleptic malignant syndrome and malignant hyperthermia is clearly disproved, careful metabolic monitoring during general anesthesia is recommended. Note that succinylcholine has been used without problems for ECT in patients with a history of neuroleptic malignant syndrome.
Anxiety disorders are the most prevalent form of psychiatric illness in the general community. Anxiety is defined as a subjective sense of unease, dread, or foreboding. It can be a primary psychiatric illness, a reaction to or result of a medical illness, or a medication side effect. Anxiety is associated with distressing symptoms such as nervousness, insomnia, hypochondriasis, and somatic complaints. It is useful clinically to consider anxiety disorders as occurring in two different patterns: (1) generalized anxiety disorder and (2) episodic, often situation-dependent, anxiety. The γ-aminobutyric acid (GABA) neurotransmitter system has been implicated in the pathogenesis of anxiety disorders.
Anxiety resulting from identifiable stressors is usually self-limited and rarely requires pharmacologic treatment. Performance anxiety (stage fright) is a type of situational anxiety that is often treated with β blockers, which do not produce sedation or allay anxiety but do eliminate the motor and autonomic manifestations of anxiety. The presence of unrealistic or excessive worry and apprehension may be cause for drug therapy. Buspirone, a partial 5-HT 2A receptor antagonist, is a nonbenzodiazepine anxiolytic drug that is not sedating and does not produce tolerance or drug dependence. However, its slower onset of action (several weeks until full effect is reached) and the need for thrice-daily dosing have limited its use. Short-term and often dramatic relief is afforded by almost any benzodiazepine, which is not surprising since these drugs bind to GABA receptors. Other drugs with GABAergic properties such as gabapentin, pregabalin, and divalproex may also be effective in treating anxiety disorders. Supplemental cognitive-behavioral therapy, relaxation techniques, hypnosis, and psychotherapy are also very useful in treating anxiety disorders.
Panic disorders are qualitatively different from generalized anxiety. The patient typically experiences recurrent and unprovoked episodes of intense fear and apprehension associated with physical symptoms and signs such as dyspnea, tachycardia, diaphoresis, paresthesias, nausea, chest pain, and fear of impending doom or dying. Such episodes can be confused with, or indeed caused by, certain medical conditions such as angina pectoris, epilepsy, pheochromocytoma, thyrotoxicosis, hypoglycemia, and cardiac dysrhythmias. Several classes of medications are effective in reducing panic attacks, including SSRIs, benzodiazepines, cyclic antidepressants, and MAOIs. These drugs have comparable efficacy. Psychotherapy and education increase the effectiveness of drug treatment.
Eating disorders are traditionally classified as anorexia nervosa, bulimia nervosa, and binge-eating disorder ( Table 29.8 ). Bulimia nervosa and binge-eating disorder are more common than anorexia nervosa. All these disorders are characterized by serious disturbances in eating (fasting or binging) and excessive concerns about body weight. Eating disorders typically occur in adolescent girls or young women, although 5% to 15% of cases of anorexia nervosa and bulimia and 40% of binge-eating disorders occur in boys and young men.
| Anorexia Nervosa |
| Body mass index <17.5 kg/m 2 |
| Fear of weight gain |
| Inaccurate perception of body shape and weight |
| Amenorrhea |
| Bulimia Nervosa |
| Recurrent binge eating (twice weekly for 3 mo) |
| Recurrent purging, excessive exercise, or fasting |
| Excessive concern about body weight or shape |
| Binge-Eating Disorder |
| Recurrent binge eating (2 days/wk for 6 mo) |
| Eating rapidly |
| Eating until uncomfortably full |
| Eating when not hungry |
| Eating alone |
| Guilt feelings after a binge |
| No purging or excessive exercise |
Anorexia nervosa is a relatively rare disorder, with an incidence of 5 to 10 cases per 100,000 and a mortality rate of 5% to 10%. Approximately half of deaths result from medical complications associated with malnutrition, and the remainder are due to suicide. The disease is characterized by a dramatic decrease in food intake and excessive physical activity in the obsessive pursuit of thinness. Bulimic symptoms may be part of the syndrome. Weight loss often exceeds 25% of normal body weight, but patients perceive that they are still obese despite this dramatic weight loss.
Marked unexplained weight loss in adolescent girls is suggestive of anorexia nervosa. Among the more serious medical complications seen in these patients are those that affect the cardiovascular system. Such changes include a decrease in cardiac muscle mass and depressed myocardial contractility. Cardiomyopathy due to starvation or to abuse of ipecac (used to induce vomiting) may be present. Sudden death has been attributed to ventricular dysrhythmias, presumably reflecting the effects of starvation or associated hypokalemia. ECG findings may include low QRS amplitude, nonspecific ST-T–wave changes, sinus bradycardia, U waves, and a prolonged QT interval (another possible association with sudden death). Hyponatremia, hypochloremia, and hypokalemia can be present along with metabolic alkalosis from vomiting and laxative and diuretic abuse. Amenorrhea is often seen in patients with anorexia.
Physical examination reveals emaciation, dry skin that may be covered with fine body hair, and cold, cyanotic extremities. Decreased body temperature, orthostatic hypotension, bradycardia, and cardiac dysrhythmias may reflect alterations in autonomic nervous system activity. Bone density is decreased as a result of poor nutrition and low estrogen concentrations, and long bones or vertebrae may fracture as a result of osteoporosis. Gastric emptying may be slowed, which leads to complaints of gastric distress after eating. In addition, starvation may impair cognitive function. Occasionally patients develop a fatty liver and abnormal liver function tests. Renal complications may reflect long-term dehydration resulting in damage to the renal tubules. Parturient women are at increased risk of delivering low-birthweight infants. Anorexic patients are often anemic, neutropenic, and thrombocytopenic.
Treatment of patients with anorexia nervosa is complicated by the patient’s denial of the condition. Pharmacologic treatment has not been predictably successful, but SSRIs that are effective in treating obsessive-compulsive disorder, particularly fluoxetine, may have some value. Most therapy involves medical management of the malnutrition-related symptoms and signs, dietary counseling, and family and/or individual psychotherapy.
There is a paucity of information relating to management of anesthesia in patients with this eating disorder. Preoperative evaluation is based on the known pathophysiologic effects of starvation. Electrolyte abnormalities, hypovolemia, and delayed gastric emptying are important preanesthetic considerations. There is a risk of perioperative cardiac dysrhythmias. Experience is too limited to permit recommendations regarding specific anesthetic drugs, muscle relaxants, and anesthetic techniques.
Bulimia nervosa is characterized by episodes of binge eating, purging, and dietary restriction. Binges are most often triggered by a negative emotional experience. Purging usually consists of self-induced vomiting that may be facilitated by laxatives and/or diuretics. In most patients this disorder is chronic, with relapses and remissions. Depression, anxiety disorders, and substance abuse commonly accompany bulimia nervosa.
Findings on physical examination suggestive of bulimia nervosa include dry skin, evidence of dehydration, and bilateral painless hypertrophy of the salivary glands. Resting bradycardia is often present. The most common laboratory finding is an increased serum amylase concentration, presumably of salivary gland origin. Metabolic alkalosis due to purging is frequently seen. Dental complications are common, especially enamel loss from repeated vomiting and exposure of the lingual surface of the teeth to gastric acid.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here