Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
The limitations of currently available biomarkers for screening or prognostic use underscore the importance of identifying “uncorrelated” or “orthogonal” biomarkers associated with novel disease pathways. Most current cardiovascular biomarkers have derived from extensions of targeted physiologic studies investigating known pathways such as tissue injury, inflammation, or hemostasis. By contrast, emerging technologies now enable the systematic, unbiased characterization of variation in proteins and metabolites associated with disease conditions.
Of the emerging platforms for biomarker discovery, perhaps none have garnered more recent attention than proteomics and metabolomics. Proteomics aims to catalogue the entire protein products of the human genome. By contrast, metabolomics attempts to systemically capture smaller biochemical compounds, including simple amino acids and related amines, as well as lipids, sugars, nucleotides, and other intermediary metabolites. Although still in their infancy with respect to other approaches, proteomics and metabolomics offer insight into the full complexity of a given disease ( Fig. 8.1 ). Because proteins and metabolites are downstream of genetic variation and transcriptional changes, they provide instantaneous “snapshots” of the state of a cell or organism. They can change rapidly in response to environmental stressors such as exercise or directly by the ingestion of foods or other compounds. Although the effects of catecholamines and natriuretic peptides on cardiovascular homeostasis are well-established, a growing body of literature suggests unanticipated roles of small proteins and metabolites in the control of biologic functions such as blood pressure and energy homeostasis. Thus metabolomics and proteomics may not only identify novel biomarkers but also provide information on biology and highlight potential therapeutic targets.
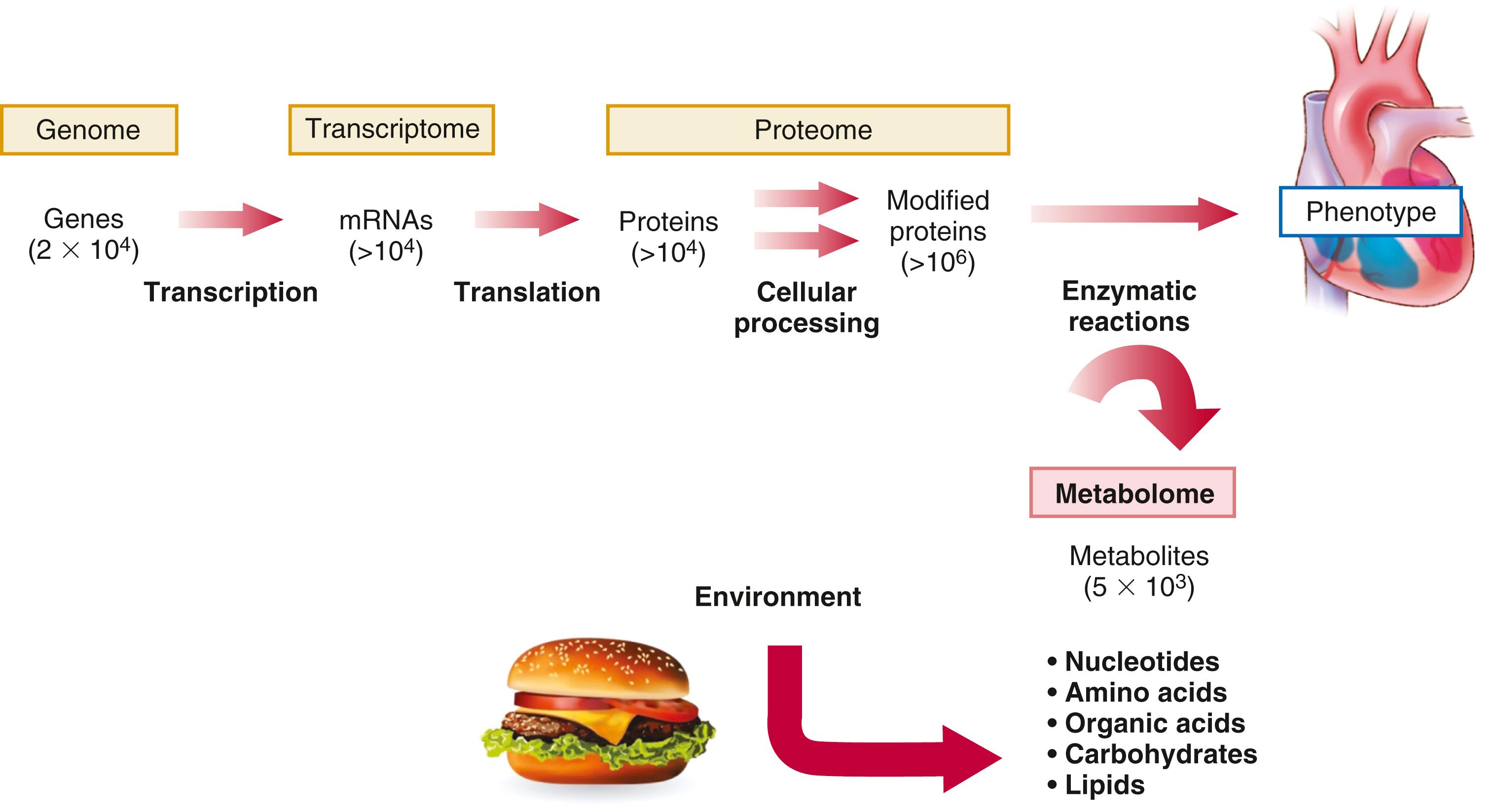
The term proteome was coined in the 1990s with the increasing realization that, although all cells of a given organism contain an equivalent genomic content, their protein content does not represent all possible proteins that the genome can express. Selective gene expression during development and differentiation and in response to external stimuli results in each cell producing only a subset of the encoded proteins at any given time. One can speak not only of the general human proteome but also more specifically about the proteome of tissues such as the heart, of specific cells such as cardiac myocytes, and even of subproteomes that correspond to particular organelles or biologic compartments, such as mitochondria.
The proteome thus provides information beyond the messenger RNA (mRNA) expression profile of a particular genome. Studies suggest that gene expression often correlates poorly with protein levels. Protein expression depends not only on transcription but also on mRNA stability and rates of protein synthesis and degradation, so the presence or absence of mRNA may not accurately reflect levels of the corresponding protein. Following transcription and translation, proteins may undergo one or more of dozens of potential post-translational modifications (such as phosphorylation, glycosylation, acetylation, or sulfation) at multiple sites which modulate protein function. Subsequent enzymatic and nonenzymatic alterations greatly expand the number of simultaneously existing protein species.
When compared with proteomics techniques, metabolomics technologies focus on smaller compounds, generally less than 2 kDa in size. Metabolites are usually easily separated from protein constituents by simple extraction techniques and precipitation and removal of the proteins. As early as the 1970s, Arthur Robinson and Linus Pauling postulated that the quantitative and qualitative pattern of metabolites in biologic fluids reflected the functional status of the complex biologic system from which they were derived. The term “metabolic profiling” was introduced to describe data obtained from gas chromatographic analysis of a patient sample. This emerging approach to quantitative metabolic profiling of large numbers of small molecules in biofluids was ultimately termed “metabonomics” by Nicholson and colleagues and “metabolomics” by others. Recently, more focused analyses of specific metabolite families or subsets have given rise to new terms such as “lipidomics.”
In terms of applications to human diagnostics, seminal studies of inborn errors of metabolism in infants have served as a key springboard. Millington and colleagues pioneered the use of mass spectrometry (MS)-based methods for monitoring fatty acid oxidation, as well as organic and selected amino acids. Their work culminated in neonatal screening for metabolic disorders, thereby enabling the identification of infants with fatty acid oxidation disorders, organic acidemias, and aminoacidopathies. In certain situations, rapid identification of these disorders triggers intervention in the form of dietary modulation, conferring therapeutic benefits. A global metabolomic or proteomic analysis of more indolent, complex diseases such as atherosclerosis might similarly spotlight pathways for dietary or drug modulation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here