Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The development of prosthetic biomaterial devices has made it possible to treat conditions that otherwise would have resulted in significant morbidity and mortality. Examples of such clinical conditions include but are not limited to aortic aneurysms, hemodialysis access in patients in whom autologous fistulas are not possible, and infrainguinal bypass for limb ischemia. The advent of prosthetic conduits has also resulted in the problem of prosthetic graft infection. The morbidity associated with infected prosthetic vascular grafts is considerable and can be catastrophic, resulting in limb loss, sepsis, and death. This chapter examines the causes, diagnosis, and management of prosthetic graft infection.
Prosthetic graft infection is relatively uncommon, with a reported incidence ranging from 1% to 6%. Graft infections are influenced by the implant site, the indications for operation, and the host's defense status as manifested by comorbid disease. Szilagyi and colleagues' analysis of 2145 patients undergoing a variety of vascular reconstructions—including aortofemoral, aortoiliac, and femoropopliteal reconstructions—revealed an overall graft infection rate of 1.5%. A prospective multicenter Canadian study of repair of unruptured abdominal aortic aneurysms revealed a graft infection rate of 0.2%. A prosthetic graft inserted in the femoropopliteal or femorotibial position has a higher rate of infection, ranging up to 18% in some reports. It has been theorized that the true percentages of prosthetic graft infection may be higher than reported due to the wide range in the time course of presentation, when many affected patients are lost to follow-up.
In recent years, there has been a sharp rise in the use of endovascular interventions for aneurysmal and occlusive vascular disease. Although it is relatively early, reports of infections in endovascular prosthesis are increasing. Extrapolating from the data regarding aortic aneurysm repair, endograft infections occur in around 1%.
Prosthetic graft infections can be classified in one of three ways:
Timing: Early (<4 months after initial implantation) or late (>4 months after initial implantation)
Depth of wound infection (Szilagi classification) :
Szilagyi grade I: Infection confined to the dermis
Szilagyi grade II: Infection involving the subcutaneous tissue but not the graft
Szilagyi grade III: Arterial graft involvement
Site and extent of graft involvement (modified Bunt classification) :
P0: Infection of intracavitary graft (intrathoracic or intra-abdominal)
P1: Infection of extracavitary graft (neck, extremities, subcutaneous tissue of the trunk)
P2: Infection of extracavitary component of graft with intracavitary origin
P3: Infection of prosthetic patch
Graft—enteric erosion
Graft—enteric fistula
Aortic stump sepsis after excision of infected aortic graft
The cause of vascular graft infections can be determined by examining the microorganisms recovered from infected grafts. Although any microorganism can potentially cause a graft infection, Staphylococcus aureus is the most prevalent bacterium. However, over the last 2 decades, Staphylococcus epidermidis has increasingly been identified as the responsible pathogen for a significant proportion of graft infections, predominantly the late-appearing indolent type.
Early-appearing graft infections are more often associated with organisms of higher virulence, including S. aureus . Coagulase-positive strains elaborate toxins that result in a vigorous host inflammatory response, including systemic signs such as fever, leukocytosis, hypotension, and bacteremia with positive blood cultures as well as severe symptoms such as abdominal pain or distension and cellulitis or drainage over the graft. Late-appearing graft infections are caused by less virulent gram-positive bacteria such as S. epidermidis . This organism is indolent and normally harbored in natural skin. It has the ability to adhere to prosthetic material and to secrete a glycocalyx biofilm that insulates the organism from white blood cells (WBCs). Over time, this biofilm induces an inflammatory response that results in perigraft inflammation, which is observed clinically as perigraft fluid, although severe systemic responses are rare. This innocuous presentation has led many to view S. epidermidis infections as inflammatory allergic reactions rather than bacterial graft infections. Additionally, S. epidermidis is not easily cultured or isolated, and ultrasonication is often required to physically separate S. epidermidis from the graft surface in order to culture it.
Other microorganisms such as fungi and gram-negative bacteria can also cause graft infections. Gram-negative bacteria such as Pseudomonas species, Escherichia coli, Serratia, Enterobacter species, and Proteus species are highly virulent and result in dramatic clinical manifestations, such as anastomotic disruption and frank hemorrhage. These organisms induce a substantial host inflammatory response with secretion of potent tissue proteases, which account for the tissue destruction leading to artery-graft dehiscence. Severely immunosuppressed patients are also vulnerable to fungal ( Candida and Aspergillus species) graft infections, an otherwise rare clinical entity in the general population.
The fundamental question concerning the cause of prosthetic graft infections is when exposure to the infection-causing microorganisms occurred. The three major potential mechanisms that can result in prosthetic graft colonization and subsequent infection are
Intraoperative contamination
Hematogenous spread of bacteria
Direct contamination of graft by infection emanating from the skin, soft tissue, gastrointestinal tract, or genitourinary tract
Although all these factors can result in a graft infection, the third mechanism is the least common.
The patient's bacteria are the most common source of bacterial contamination resulting in infection of the surgical site. The resident microflora, usually referred to as the indigenous microflora, consists of a complex mixture of microbial species ranging from nonpathogenic saprophytes to pathogens. Endogenous bacteria are a more important source of surgical site infection than exogenous bacteria.
The patient's endogenous flora from a site close to the prosthetic bed—especially from a colonized site, such as the skin or gut—is a common source of graft infection. Most of the intertriginous areas of the body, such as the axillae, groin, and interdigital spaces of the foot, contain large numbers of eccrine sweat glands and harbor large bacterial populations. Indeed, prosthetic grafts placed in the groin have a well-characterized propensity to develop S. aureus infection. S. aureus bacteria from the groin area may contaminate the wound, resulting in a wound infection, or contaminate the graft surface if it is inadvertently dragged across the skin, resulting in a graft infection. The lymphatics in the groin may be contaminated at the time of surgery, especially if the patient has an open infected wound in an extremity, and these lymphatics can be a source of intraoperative graft infection.
Bacteria can be present in diseased vessels or in the thrombus lining an aortic aneurysm. One prospective study demonstrated that among patients undergoing peripheral vascular bypass surgery, 41% had positive arterial tissue cultures, 68% of which were coagulase-negative Staphylococcus ; but subsequent graft infections were not reported. Therefore, although the positive cultures are intriguing, their significance is unclear. Interestingly, in another study, the aneurysm contents of 216 patients were cultured. Positive cultures were found in 55 patients (25.5%). However, after 3 years of follow-up, only four graft infections occurred (1.9%); among these, only two grew the same organism as the original cultures.
Seeding of an indwelling prosthesis by hematogenous spread of bacteria is an important mode of prosthetic graft infection, although the frequency with which this occurs is unknown. In experimental animals, infusion of 10 million organisms of S. aureus immediately after implantation of a prosthetic aortic graft resulted in a 100% incidence of graft infection. Later studies demonstrated an inverse relationship between the incidence of graft infection and time after implantation, suggesting that graft incorporation and the development of a pseudointima may provide some protection. Vulnerability to infection from bacteremia has been documented at even 1 year after implantation, with 30% of aortic grafts becoming infected after a single bacterial infusion at that time. The anatomic difference between the infected and uninfected grafts was the absence or presence of a complete neointimal lining. Systemic and local antibiotics significantly reduced the incidence of graft infection. The significance of remote infection is unclear, but it has been theorized that transient bacteremia associated with, for example, dental procedures or colonoscopy may account for very late infections.
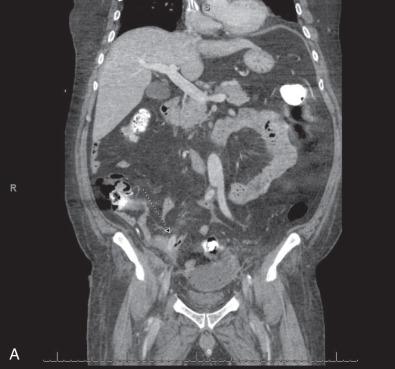
Any direct exposure of the graft to bacterial sources can result in graft infection. Failure of the skin incision to heal after an operation can result in direct bacterial contamination and places the underlying graft at significant risk of subsequent infection. Moreover, hematomas and lymphoceles near or around the graft not only inhibit graft incorporation, thus increasing the duration of susceptibility to infection, but serve as niduses for bacterial growth, which may directly contaminate the adjacent graft. Finally, contamination of prosthetic grafts can occur from contact with adjacent bacteria-containing organs. For this reason, it is prudent to avoid any operation on the gastrointestinal tract at the time of insertion of an aortic prosthetic graft. Rarely, injury to bowel can occur during the placement of an aortic graft. Care must be exercised in tunneling graft limbs cautiously to avoid injury to the bowel, as these injuries can go unrecognized and lead to severe sepsis, peritonitis, and devastating graft infections ( Fig. 59.1A and B ). Should bowel injury with entry into the bowel lumen and spillage of bowel contents occur while a prosthetic aortic graft is being placed, the procedure should be aborted if possible. Of note, one important means of contamination from adjacent organs is the formation of an aortoenteric fistula or erosion (AEF or AEE). With an incidence of 0.4% to 1.6% with open repair and likely less with endovascular repair, AEFs/AEEs are rare. Although the pathogenesis of an AEF/AEE remains unknown, pulsatility of the aorta against the bowel and adhesions between the inflamed aortic graft and adjacent bowel have been postulated as a possible etiology of AEFs and AEEs.
The best way to avoid graft infection is to prevent it in the operating room and during the postoperative period. It has been shown that the cutaneous microflora of patients who are hospitalized for any length of time undergo change. It is unclear whether this is caused by the underlying illness or the use of antibiotics. Thus the preoperative stay, if any, should be as short as possible to avoid colonization by resistant bacteria. Prophylactic antibiotics have been shown to decrease the incidence of graft infections. Most authors recommend the administration of a first-generation cephalosporin approximately 30 to 60 minutes before incision, with continuation of the scheduled antibiotic dosing for the first 24 hours after surgery. If the operation lasts for an unusually long time, additional doses should be administered to maintain adequate circulating levels. Although bacteremia is noted in 4% to 8% of angiographic procedures, it is usually asymptomatic. Antibiotic prophylaxis is not routinely recommended in endovascular procedures except for patients at high risk (e.g., repeated intervention within 7 days, prolonged indwelling arterial sheaths, prolonged duration of procedure) and for interventions where aortic or peripheral endograft placement is planned. There is no evidence to support the continuation of prophylactic antibiotics until indwelling lines, such as central venous or urinary bladder catheters, are removed. Indeed, the prolonged use of postoperative antibiotics may even be detrimental in some patients, leading to antibiotic-associated colitis or the development of other resistant infectious organisms.
The use of antibiotic-impregnated or soaked grafts has been proposed as a potential method to reduce the risk of prosthetic graft infection. In particular, rifampin, which has a higher affinity for collagen and gelatin coatings of grafts as compared with other antibiotics, has been the most extensively studied. However, randomized controlled trials have shown mixed results, with lower short-term infection rates in patients treated with antibiotic-impregnated grafts. This is attributable largely to infection reduction in patients in whom a groin incision was made, although no benefit has been demonstrated in long-term follow-up. The latter finding may be due to the development of bacterial resistance to rifampin or gradual loss of the rifampin coat over time.
Finally, meticulous surgical technique is vital in preventing wound and graft infections. The graft must be handled carefully, with minimal if any contact with the patient's skin, even after skin has been prepped prior to the start of surgery. With no strong evidence favoring iodophors versus chlorhexidine preparations, the decision regarding skin prep should be driven by patient factors, costs, and potential side effects. Adhesive drapes, such as Ioban, are commonly used to aid in preventing contact between the prosthetic graft and the skin, although there is no clear evidence demonstrating a reduction in the incidence of wound or graft infection with the use of adhesive drapes with or without iodine impregnation. However, if the adhesive plastic drape is separated from the skin during the operation, the infection rate increases. Simultaneous gastrointestinal procedures should be avoided to prevent intraoperative contamination; if an unplanned enterotomy occurs, graft implantation should be postponed if possible. Irrigation should also be used to remove all blood from the peritoneal cavity, because blood acts as medium for bacterial proliferation. If irrigation of a wound is performed, every effort should be made to evacuate the fluid. Wounds should be closed in layers, with efforts to minimize dead space using the least amount of suture material. Incisions should be covered with clean dressings, although there are new data suggesting some benefit with the use of negative-pressure wound dressings in high-risk patients or incision locations (e.g., groin), However, these data remain inconclusive, with other studies failing to show a significant effect of specialized dressings.
Graft infections can result in limb loss, systemic sepsis, and sometimes death, even in the setting of correct diagnosis and treatment. Therefore prompt diagnosis is essential to avoid or minimize complications. The consequences of a missed diagnosis in this setting can be catastrophic. Clinicians should maintain a high index of suspicion for graft involvement in any patient with a prosthetic graft presenting with local or systemic signs of infection, and every attempt should be made to confirm or exclude the diagnosis by either imaging or operative exploration.
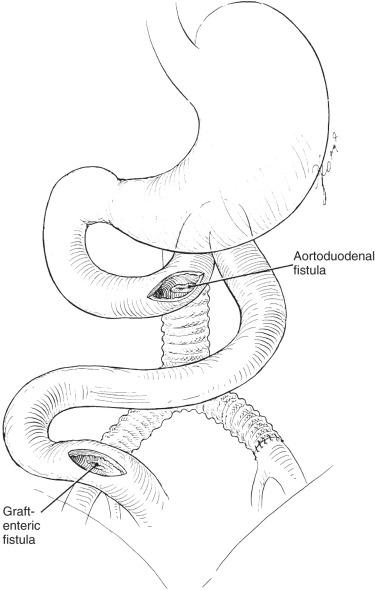
The clinical presentation of graft infection is influenced by the anatomic location of the graft and the time of presentation. Infection of an infrainguinal graft frequently appears as cellulitis, soft tissue infection, drainage tract, or pseudoaneurysm. Conversely, an intraabdominal graft infection may appear as systemic sepsis or as an ileus or abdominal distension with or without tenderness. Occasionally an aortic graft infection can result in an aortoenteric fistula, the first sign of which is a herald gastrointestinal bleed. A patient with upper gastrointestinal bleeding and an aortic graft must be presumed to have an aortoenteric fistula until proved otherwise ( Fig. 59.2 ).
Early graft infections, usually caused by virulent organisms such as S. aureus , can manifest with pain, erythema, induration, or purulent drainage from the graft site. Patients may have signs of systemic sepsis (tachycardia, tachypnea, hypotension, and fever) or infective endocarditis (splinter hemorrhages, septic emboli, etc.) with positive blood cultures. A late graft infection, usually caused by indolent organisms such as S. epidermidis, often appears as a healing complication such as a seroma or pseudoaneurysm or as late graft thrombosis with no anatomic reason. Systemic signs of illness, such as fever, are often not present.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here