Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The major event following neurulation is development of the prosencephalon, which results in the fundamental structure of the central nervous system. The prosencephalon lies rostral to the other major vesicles of the brain—that is, the midbrain (mesencephalon) and hindbrain (rhombencephalon)—and will ultimately form the cerebral hemispheres and diencephalic structures (i.e., the thalamus and hypothalamus). Prosencephalic development peaks between the second and third months of gestation, with the earliest prominent phases in the fifth and sixth weeks of gestation ( Box 2.1 ). The major inductive relationship of concern is between the notochord-prechordal mesoderm and the forebrain (see Box 2.1 ). This interaction occurs ventrally at the rostral end of the embryo; thus the term ventral induction is sometimes used. The inductive interaction influences formation of much of the face as well as the forebrain , and hence severe disorders of brain development at this time also usually result in striking facial anomalies. Development of the prosencephalon is considered best in terms of three sequential events (i.e., prosencephalic formation, prosencephalic cleavage , and prosencephalic midline development ) (see Box 2.1 ).
2–3 months
Prechordal mesoderm → face and forebrain
Prosencephalic development
Prosencephalic formation
Prosencephalic cleavage
Paired optic and olfactory structures
Telencephalon → cerebral hemispheres
Diencephalon → thalamus, hypothalamus
Midline prosencephalic development
Corpus callosum, septum pellucidum, optic nerves (chiasm), hypothalamus
Prosencephalic formation begins at the rostral end of the neural tube at the end of the first month and the beginning of the second month of gestation, shortly after the anterior neuropore closes. Prosencephalic cleavage occurs most actively in the fifth and sixth weeks of gestation and includes three basic cleavages of the prosencephalon: (1) horizontally, to form the paired optic vesicles, olfactory bulbs, and tracts; (2) transversely, to separate the telencephalon from diencephalon; and (3) sagittally, to form, from the telencephalon, the paired cerebral hemispheres, lateral ventricles, and basal ganglia (see Box 2.1 ). The third event, prosencephalic midline development , occurs from the latter half of the second month through the third month, when three crucial thickenings or plates of tissue become apparent ( Fig. 2.1 ); from dorsal to ventral, these are the commissural, chiasmatic, and hypothalamic plates. These structures are important in the formation, respectively, of the telencephalic commissures, the septum pellucidum, the optic nerve chiasm, and the hypothalamic structures. Among the most common anomalies of brain development and indication for neurological consultation before and after birth are disturbances in midline commissural development, primarily those involving the corpus callosum. For this reason, there is a disproportionate emphasis on normal and abnormal telencephalic commissural (especially callosal) development throughout the rest of this chapter.

Major insights into the molecular genetic determinants of forebrain development have been gained in recent years. These discoveries have revealed a highly complex and dynamic interaction between multiple genetic (“driver” and “modifier” genes) pathways and their signaling molecules. The strength, timing, and duration of morphogen expression by these signaling systems must achieve critical thresholds for normal forebrain development to proceed. This signaling network is sensitive to environmental cues, which are thought to play a role in the complex multifactorial pathogenesis of most cases of anomalous forebrain development.
Dorsoventral patterning of the forebrain is dependent the opposing influences of dorsalizing (Notch) and ventralizing (Shh) signals. The earliest steps in prosencephalic development occur under the inductive influences of several nonneural centers located in the adjacent mesoderm, the epidermal ectoderm and the primitive mesenchyme, that express morphogens. Not only do these morphogens shape the early development of prosencephalic structure but they also induce signaling centers (“organizers”) within the adjacent neural tissue that then express the same morphogens in the developing forebrain. The early nonneural signaling centers include the ventral prechordal plate , derived from the prechordal mesoderm, which expresses Nodal, sonic hedgehog (Shh), and bone morphogenic protein (BMP) signaling molecules. The prechordal plate induces development of the hypothalamus and ventral telencephalic midline (through Nodal and Shh signals) but also induces signaling centers in the hypothalamus (producing Nodal and Shh) and ventral midline (producing Shh), which then proceed to express the same morphogens. The Shh signaling pathway is the most important molecular pathway in prosencephalic development and induces developmental events through critical ventralizing molecules . Before secretion of Shh, cholesterol is required for modification of Shh at its C-terminus (an event relevant to causes of holoprosencephaly; see later). Secreted Shh activates a receptor, Patch, which, in turn, leads to activation of several other genes (e.g., GLI2 ) and transcription factors that enter the nucleus to modify gene transcription. Disrupted induction by the prechordal plate underlies the classic forms of holoprosencephaly, including the septo-preoptic phenotype.
The epidermal ectoderm overlying the neuroectoderm expresses BMPs, which induce development of the roof plate and its signaling center in the dorsal midline, which then begins to express BMPs. These BMPs expressed by the roof plate are key dorsalizing molecules and initiate a second major molecular pathway, the so-called nodal pathway. Failure of dorsal induction is believed to underlie the midline interhemispheric phenotype of holoprosencephaly (see later). The transcriptional regulators induced in this pathway include TGIF, TDGFI, and FASTI. The ZIC2 gene plays an important role in both ventral and dorsal patterning of the prosencephalon. Mutations in ZIC2 lead to failure of the SHH signaling pathway, disrupted ventral induction, and thus classical holoprosencephaly phenotypes (see later). In addition, ZIC2 loss-of-function leads to an abnormal differentiation of the roof plate, disturbed dorsal induction of the forebrain, giving rise to the middle interhemispheric (MIH) variant of holoprosencephaly. The clinical relevance of these insights includes the importance of performing mutation analysis of these genes in selected patients with disorders of prosencephalic development (discussed later).
Similar to the BMP sequence of dorsalizing influences discussed above, fibroblast growth factors (Fgf), especially Fgf8, mediate patterning of the anterior neural plate at the rostral tip of the recently closed neural tube, followed by induction of an organizing center that continues to express Fgf8. The inductive interaction between these various signaling systems influences formation of not only the forebrain but also much of the face , and hence severe disorders of brain development at this time may be associated with striking facial anomalies.
The interaction of the different signaling centers inducing forebrain development is complex, with certain morphogens, such as SHH and SIX3, regulating each other’s expression, and cross-regulation between SHH and FGF8 pathways must maintain a delicate balance for normal forebrain development and cleavage to occur.
Disorders of prosencephalic midline development are among the most common clinical indications for consultation by the neonatal neurologist. For this reason, the following discussion will focus heavily on the normal and abnormal events during the phase of prosencephalic midline development.
The most prominent of the midline developments is the formation of the telencephalic commissural system, especially the corpus callosum ( Fig. 2.2 ). The normal telencephalic commissural system comprises the anterior and hippocampal commissures and the corpus callosum (see Fig. 2.2 ). The midline domain encompassing all three developing telencephalic commissures has been termed the commissural plate. When the anterior neuropore closes at the rostral tip of the neural tube, it forms a band of neural tissue across the midline called the anterior neural plate, which in turn forms the lamina terminalis. The upper edge of the lamina terminalis expands in a dorsal direction to form the lamina reuniens a rostral midline region of intense morphogenic patterning that forms the anlage of the telencephalic commissural system (see Fig. 2.2 ).

Development of the telencephalic commissures is complex and certain sequential events are critical for its success. These include (1) establishing a midline organizing apparatus, (2) developing a glial “zipper” and neuronal “sling,” and (3) the arrival and successful crossing of “pioneer” commissural axons at the midline. These events are discussed in more detail later and in an excellent recent review by Raybaud.
An organized midline apparatus capable of releasing factors that attract and repel different neuroglial elements is key. Such a domain, the anterior neural ridge, develops over the outer surface of the anterior neural plate. Where the anterior neural ridge from both sides of the anterior neural plate meets in the midline, a “hem” forms, which becomes a critical organizer for telencephalic development (much like the isthmic organizer at the rhombencephalic-mesencephalic junction; see Chapter 4 ). This anterior neural ridge “hem” secretes important signaling molecules, including Fgf8, which serves as a neuroglial attractant.
In the next phase a midline bridge of glia and neurons develops to serve as axonal guideposts. At ~10 gestational weeks (GW), glial cells migrate from the ventricular and subventricular zones toward the midline where they form a glial “zipper” and establish distinct dorso-ventral regions of transcription factor expression. At ~12 GW these glial cells cross the midline through the meninx primitiva across the future interhemispheric fissure. These glial collections act as intermediate guideposts for callosal axons, forming a “glial tunnel” that secretes chemorepellent Slit2. When the prospective callosal axons approach the midline (see later), they enter the glial tunnel and are channeled toward the opposite hemisphere by Slit2. Soon hereafter these glial elements are joined by migrating neurons to form a transient “neuroglial sling.” These neurons will serve as guideposts for subsequent midline crossing of commissural axons .
The first pioneer axons cross the midline directly through the primordial anterior and hippocampal commissures in the lamina reuniens between 8 and 11 GW. The anterior commissure fibers are the first to cross the midline, at the ventral edge of the lamina reuniens. By ~11 GW the hippocampal (or fornix) commissure forms at the dorsal edge of the lamina reuniens. The hippocampal commissure is formed by fibers of the developing fornix as it crosses the midline. Ultimately, the hippocampal commissure will lie immediately ventral to the splenium of the corpus callosum. Pioneer axons that initiate formation of the corpus callosum are derived from neurons in the cingulate cortex. These commissural neurons develop axons with growth cones capable of reading a dynamic array of guidance ligands, receptors, and chemoattractants, released by specialized glial cells. Between 10 and 11 GW the first pioneer axons from the cingulate cortex cross through the neuroglial sling. Actual pathfinding in search of the midline is performed only by pioneering axons originating almost exclusively in the cingulate cortex. This interhemispheric migration is orchestrated by a complex system of cellular and molecular chemoattractant and repellant signals, as well as cellular adhesion molecules, such as L1CAM . Mutations of the L1CAM gene result in a number of clinical phenotypes, most of which include callosal anomalies (discussed later).
After the pioneer axons have crossed the midline, subsequent waves of neocortical axons cross the midline on the bridging apparatus previously established by the pioneer fibers. At ~14 to 15 GW the first wave of post-pioneer axons cross in the future anterior corpus callosum. Development of callosal axonal pathways goes through several phases, including axonal exuberance, refinement (axonal pruning), stabilization, and myelination. In the exuberant phase , between 20 and 32 GW, an overabundant number of axons cross the midline from most of the neocortical neurons in the brain, resulting in increasing thickness of the corpus callosum. The exuberant phase is followed by a refinement or pruning phase , between 32 GW and 2 months postnatal, during which most of the early-crossing axons are retracted or “pruned.” During this phase of “selective refinement” the cortical neurons retract their callosal axons and differentiate instead into noncommissural projection neurons. These events result in a loss of dorsoventral callosal thickness; in primate models this may result in a three-fold decrease in axon numbers. Only those neurons with axons that have established stable connections with their contralateral target differentiate into commissural neurons; this is the stabilization phase . De Leon Reyes et al proposed that normal callosal development requires an optimal balance between refinement and stabilization; when refinement is deficient, an abnormally thickened corpus callosum results (as in neurofibromatosis type 1), whereas with deficient or delayed (prolonged refinement) stabilization the corpus callosum is decreased in size.
Once across the midline, axons are prevented from back-tracking because of the expression of the chemorepellent protein Slit-2. In the contralateral hemisphere, callosal axons cover long distances to reach their final targets; their growth and guidance are supported by intrinsic neuronal polarity cues and extrinsic gradients of diffusible molecules (e.g., semaphorins). As discussed above, the maturation pattern of these callosal projections involves the transient overgrowth of axons and arbors, some reaching their homotopic target and others overshooting their target and becoming selectively eliminated.
By 12 to 13 GW all of the individual components of the telencephalic commissures are normally present. The arrangement of axons in the telencephalic commissures is highly organized according to their homotopic connections. The anterior commissure contains axons from the more anterior neocortex and amygdala, whereas the hippocampal commissure contains axons from the more posterior neocortex. The axons forming the corpus are arranged in a similar anterior-to-posterior direction ( Fig. 2.3 ). With massive expansion of especially the frontal neocortex and its axons, the corpus callosum expands in the rostrocaudal axis with backward displacement of the splenium. By 20 weeks the corpus callosum is essentially fully developed in its rostrocaudal extent but will subsequently undergo a secondary increase in thickness as myelination proceeds.
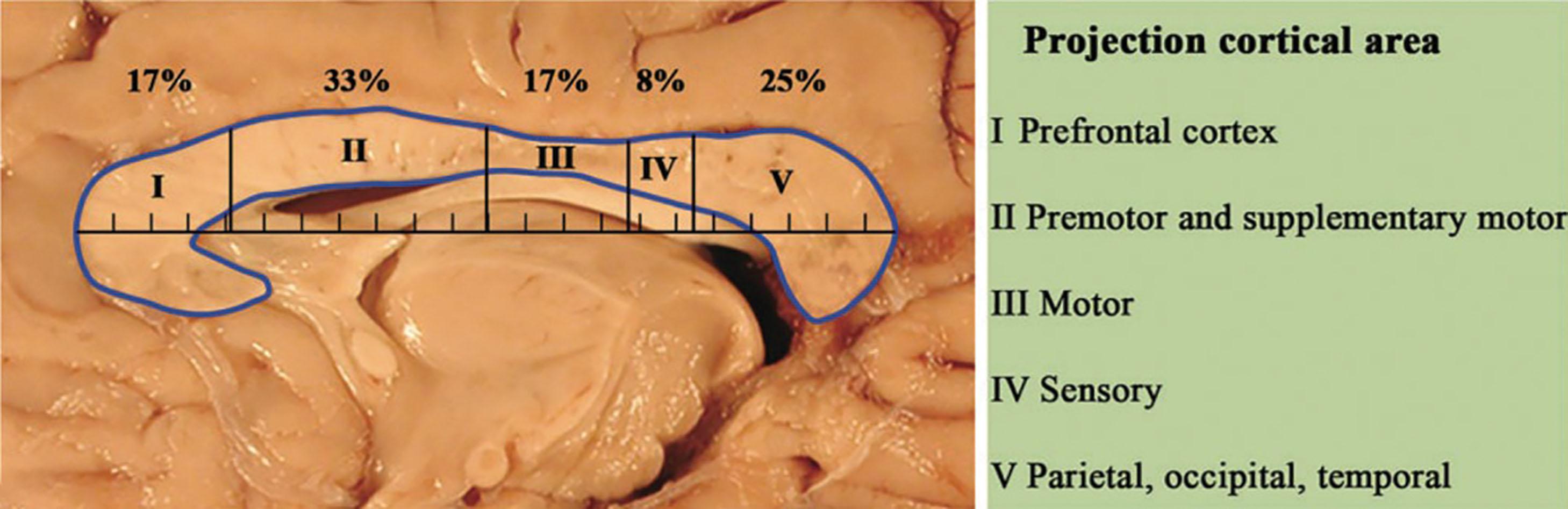
A complex interplay exists between neural and mesenchymal signaling systems of the developing commissural and meningeal structures. In early embryonic development, the mesenchyme surrounding the neural tube forms the neural crest, which runs along the rostrocaudal direction between the surface ectoderm and the neural tube. The neural crest contains a remarkable array of pluripotential cells that differentiate into the meninges, skull, scalp, and parts of the face . The neural crest also plays a significant role in the development of the anterior neural plate (discussed earlier). The neural crest cells are attracted to the anterior neural plate by the morphogen Fgf8, which is secreted by the anterior neural ridge (discussed earlier). The anterior neural ridge also expresses Wnt1 and Wnt3a, which contribute to development of the meninges while also activating processes responsible for cleaving the forebrain. As discussed later, disruption of these complex and coordinated series of patterning events may lead to co-occurring abnormalities in meningeal and medial cortical development, as seen in the association between callosal agenesis, multiple midline (type 2) meningeal cysts with adjacent cortical malformations, and peri-callosal lipomas.
Disorders of prosencephalic development are considered best in terms of the three major events described earlier, namely, prosencephalic formation from the rostral end of the neural tube, prosencephalic cleavage, and midline prosencephalic development ( Box 2.2 ). The spectrum of pathology varies from a profound derangement (e.g., aprosencephaly) to disturbances of midline prosencephalic development (e.g., isolated agenesis of the corpus callosum) that may only be detected incidentally by brain imaging or at autopsy.
Aprosencephaly and atelencephaly are the most severe of the disorders of prosencephalic development. In aprosencephaly , the entire process fails to occur, and the result is an absence of formation of both the telencephalon and diencephalon, with a prosencephalic remnant located at the rostral end of a rudimentary brainstem ( Fig. 2.4A ). In atelencephaly , the anomaly is less severe in that the diencephalon is relatively preserved. The pathogenesis of aprosencephaly-atelencephaly remains unclear but is probably heterogeneous. In most cases, the replacement of the telencephalon by gliomesenchymal tissue, calcifications, and calcific vasculopathy suggests a disruptive mechanism occurring shortly after neurulation, leading to destruction of the primordia before growth and patterning of the prosencephalon occurs. These disorders may result from an encephaloclastic event shortly after neurulation. These anomalies are distinguishable from anencephaly most readily by the presence of an intact, although flattened, skull and intact scalp ( Fig. 2.4B ).

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here