Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We would like to thank Micah Belzberg, MS4 for his kind assistance with figure creation.
Scalp defects presenting to the neurosurgeon result from a variety of pathologies: trauma, brain neoplasms invading skull or scalp, postoperative bone/soft tissue infections, chemotherapy-related scalp atrophy, eroding scalp neoplasms, irradiated scalp thinning, and effects of repeated scalp incisions. Throughout the centuries, advances in neurosurgery and craniofacial plastic surgery have provided the main foundation for scalp wound management. Prior to this, Hippocrates’ and Galen’s theories relied on suppuration and pus formation for adequate scalp healing; this was the standard of care for nearly 1500 years.
Among the Renaissance “giants” of anatomy and surgery, Johannes Dryander (1536) described eloquent dissections related to the scalp’s multiple layers, along with the brain and its dural envelope. In the 17th century, Augustin Belloste advocated perforating the cranium’s outer table as a method for encouraging granulation tissue formation and subsequent epithelialization. Robinson, in 1908, described the use of skin grafting to intact periosteum, and during World War I, Harvey Cushing attributed a low perioperative mortality to the “ closure of the wounds with buried sutures in the galea. ” When Harvey Cushing was asked about his greatest contributions to neurosurgery, he advocated meticulous approximation of the galea aponeurotica during surgical wound closure, and since then, galeal closure has remained a central dogma of neurosurgery .
Now, in 2019, the burgeoning field of neuroplastic and reconstructive neurosurgery has evolved into a new hybrid specialty, with its purpose being to bridge the sometimes disparate fields of plastic surgery and neurosurgery, as a means to improve outcomes, minimize neurosurgical complications, and improve patient satisfaction. In just a few years time, neuroplastic surgeons have provided several new advances in scalp/skull reconstruction. Three are especially notable: (1) the “pericranial-onlay cranioplasty technique,” described in 2014, which provides a novel method for increased scalp laxity and the newfound ability to avoid the dreaded scalp-dura dissection ; (2) a method termed “component separation of the scalp,” which provides an additional 1-2 centimeters of scalp mobility via the strategic division of subgaleal ligaments (i.e., fibrous bands) , ; (3) the scalp augmentation technique that uses autologous rectus fascia (harvested from a conspicuous abdominoplasty incision) on clean cranial bone with or without periosteum. These advances provide more robust solutions for improving scalp durability compared to the commonly employed split thickness skin graft; at the same time, they improve the dreaded donor site deformity commonly found with the handheld dermatome. , ,
With a surface area of approximately 600 cm 2 , the scalp is a highly vascularized, complex, five-layered structure encasing the skull. Its anatomical boundaries extend posteriorly from the occipital process and superior nuchal line, laterally into the temporal fossa toward the zygomatic arches, and anteriorly to the supraorbital bandeau. The temporal anatomy is especially critical for the neurosurgeon in relation to the commonly performed pterional craniotomy and the requirement to protect the facial nerve. Within the temporal fossa, the fascial network includes three distinct layers. From top down, the superficial temporal fascial layer is contiguous with the galea aponeurosis and provides a safe dissection plane (since the facial nerve neighbors this plane), while the thick deep temporal fascia (encasing the temporalis muscle) is contiguous with the pericranium. Between these two layers is the less distinct layer known as the superficial layer of the deep temporal fascia (or intermediate fascia). Two commonly used techniques, described as the interfascial-subpericranial or subfascial-subpericranial approaches, have been used to reduce injury to the facial nerve branches. ,
The scalp maintains numerous functions vital to both the brain and skull, including protection from trauma, regulating body temperature homeostasis, providing blood and nutrients via its abundant blood supply, maintaining critical skin structures like hair follicles, sweat glands, and sebaceous glands, and allowing for facial expression and tension via direct continuity with the facial aponeurosis (i.e., superficial myoaponeurotic system or SMAS layer). Also, the scalp, with its five distinct tissue layers, is often remembered by way of its popular acronym, ‘‘S-C-A-L-P.’’ From superficial to deep, S kin, Sub- C utaneous tissue, A poneurosis galea, L oose areolar tissue, and P ericranium ( Fig. 126.1 ).
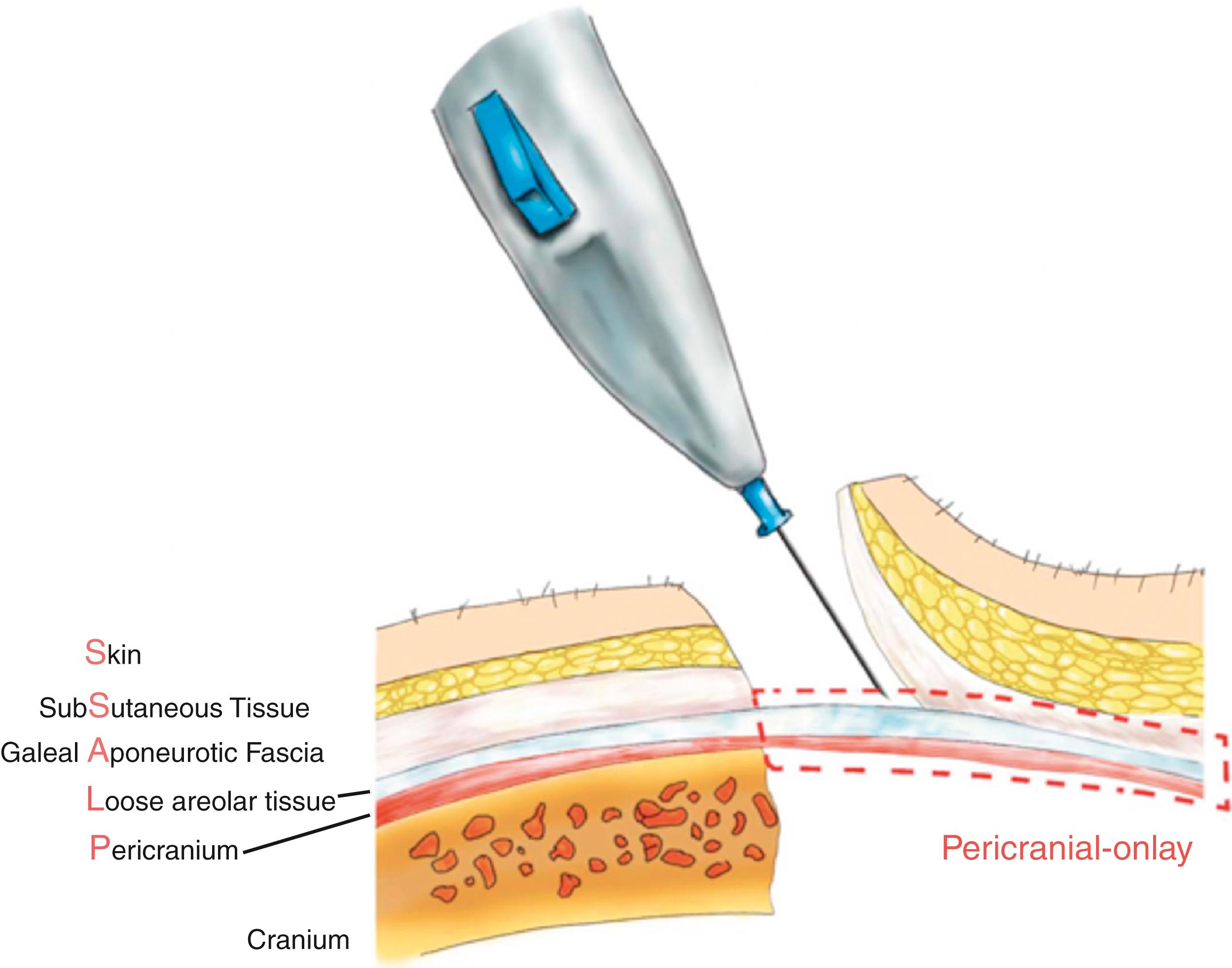
The outer three layers are tightly fixed as a single unit and separated from the pericranium by a simple “loose areolar tissue,” which is avascular and confined by two parallel vascular networks (dermal plexus and pericranium) ( Fig. 126.2 ). To both the neurosurgeon and neuroplastic surgeon, the pericranium is highly valuable, given its robust blood supply. For example, an anteriorly based pericranial flap (based on the supraorbital pedicle) is a keystone flap that is commonly employed to exclude the nasal cavity and intracranial space in the setting of frontal sinus trauma. Furthermore, its robust nature allows for dependable antibiotic delivery and provides an ideal autologous solution in cases of cranial base infection.

The pericranium’s dependable perfusion also provides the ability for one to perform a “component separation of the scalp,” as first described by our team. This is especially important for the neurosurgeon to note while performing a craniotomy. Scalps should be assessed for thickness and for tissue viability within both the deep vascular system (pericranium) and superficial vascular system (dermal-subdermal plexus). A thin scalp damaged by irradiation therapy or repeated neurosurgical incisions is not a candidate for component separation. Atrophic scalps, with excessive thinning, are most problematic, due to suturing difficulty and from a blood supply perspective. Risks for incisional scalp dehiscence and wound healing complications are amplified in the setting of cranioplasty and placement of alloplastic materials underneath. Furthermore, scalps closed with unfavorable tension will undoubtedly fail with time, especially when the multiple tissue planes are scarred, and cranial implants are utilized for reconstructing large defects. , , ,
The galea aponeurosis is an important structure to the neurosurgeon, as recognized by Harvey Cushing. Its closure and detailed reapproximation at craniotomy are paramount. The galea bridges the frontalis and occipital muscles and is therefore under constant movement. As such, ineffective suturing at the time of scalp closure can lead to galeal dehiscence, followed by eventual wound dehiscence and secondary infection. , , Many cranioplasty articles that describe high infection rates with alloplastic materials often have conflicting variables related to poor scalp closure and premature galeal dehiscence related to unacceptable tension. The galea is a broad fibrous layer extending from the external occipital protuberance to the eyebrow (connecting frontalis muscle to occipitalis muscle), and then inferolaterally to the superficial temporal fascia and the SMAS (superficial musculo-aponeurotic system). In neuroplastic surgery, wide undermining and local tissue transfer is much preferred over previously described “galeal scoring,” due to the inherent risk of injuring the dermal-subdermal plexus.
Loose areolar tissue (also known as “subgalea”), which accounts for the mobility of the scalp, is greatest in the parietal region. The subgaleal layer separates quite easily from the galeal-skin component above it. Its easy separation and avascularity allows for safe protection of the pericranium/skull during a traumatic avulsive injury (by protective design) with minimal blood loss. In addition, it allows the surgeon to safely perform partial-thickness scalp flap dissections using “component separation.” Based on our clinical experience and cadaver experiments, we described the subgaleal ligaments (quite similar to the true retaining ligaments and fibrous bands of the face), which are widely present around the entire scalp. These “scalp retaining ligaments” are immobile, fibrous adhesions extending from the galea aponeurosis plane down to the pericranium (see Fig. 126.2 ). The separation of these “retaining ligaments” is of great benefit when trying to achieve a tension-free closure during cranioplasty or complex scalp reconstruction.
The anatomy of the temporal/lateral scalp is complex. The temporofrontal branch of the facial nerve runs just parallel to the superficial temporal fascia. The interfascial-subpericranial and subfascial-subpericranial techniques will preserve the innervation of the frontalis muscle.
The subgalea and superficial temporal fascia also house the superficial temporal artery (STA), rendering this layer important in laterally based scalp flaps, most commonly the pterional/temporal/subtemporal/trauma flaps, for preserving pertinent scalp perfusion. At the same time, temporal dissection may easily result in temporalis muscle denervation/devascularization or temporal fat pad atrophy, which may result in the dreaded post-operative temporal hollowing deformity, and often requires adjunctive procedures depending on its severity.
The scalp has a rich vascular system and receives overlapping contributions from the internal and external carotid arteries. The ophthalmic artery and its supratrochlear and supraorbital branches supply the forehead and anterior scalp. The occipital artery and posterior auricular vessels supplies the posterior scalp. The STA, a terminal branch of the external carotid, bifurcates at the level of the superior helix to supply parietal and temporal branches, allowing versatile flap design. All vessels are accompanied by vena commitantes.
Sensory innervation derives mostly from the trigeminal nerve and to a lesser extent the 2nd and 3rd cervical roots, including the supratrochlear (V1), supraorbital (V1), zygomaticotemporal (V2), auriculotemporal (V3), lesser occipital (V3), greater occipital (C2, C3), and occipital (C3) nerves. Importantly, all of these neurovascular structures course along the subcutaneous plane (i.e., superficial to the galeal fascia), which makes superficial scalp dissection tedious.
Temporal hollowing, a common social stigma associated with neurosurgery, has received little attention until recently. This complication is still prevalent in neurosurgical patients, approaching a 52% incidence in pterional-type surgeries. Undoubtedly, prevention is best, if possible. One strategy employs the use of a myocutaneous scalp flap dissection during the neurosurgical pterional/coronal approach as a means of preventing devascularization and deinnervation of the temporal tissues. Neuroplastic surgery has developed several innovative solutions for those patients seeking facial symmetry following neurosurgery. ,
If the temporal hollowing deformity is isolated to the temporal fat pad and temporalis muscle, then a repeat surgery with titanium screws and liquid polymethylmethacrylate (PMMA) is safe and reliable, and provides a permanent solution. First described by Gordon and Yaremchuk, this approach requires the artistic filling of the temporal fossa and employs strategic screw placement for a “cement-like” rebar effect ( Fig. 126.3 ). In instances where the neurosurgical patient presents with postoperative temporal hollowing and has a coexisting skull defect or bone flap resorption underneath, the neuroplastic surgeon can provide a dual-purpose craniofacial implant, which was first described by our team. This new three-dimensional implant design algorithm, which is material-agnostic (commonly used with PMMA, polyether-ether-ketone [PEEK], and porous polyethylene), allows for two virtual models to be fabricated by computer-assisted design and manufacturing (CAD/CAM) and fused into one alloplastic implant. The inner model replaces the missing cranial bone, while the outer model provides an equal three-dimensional volume restoration of the missing soft tissue, based on the patient’s unaffected contralateral anatomy ( Fig. 126.4 ).

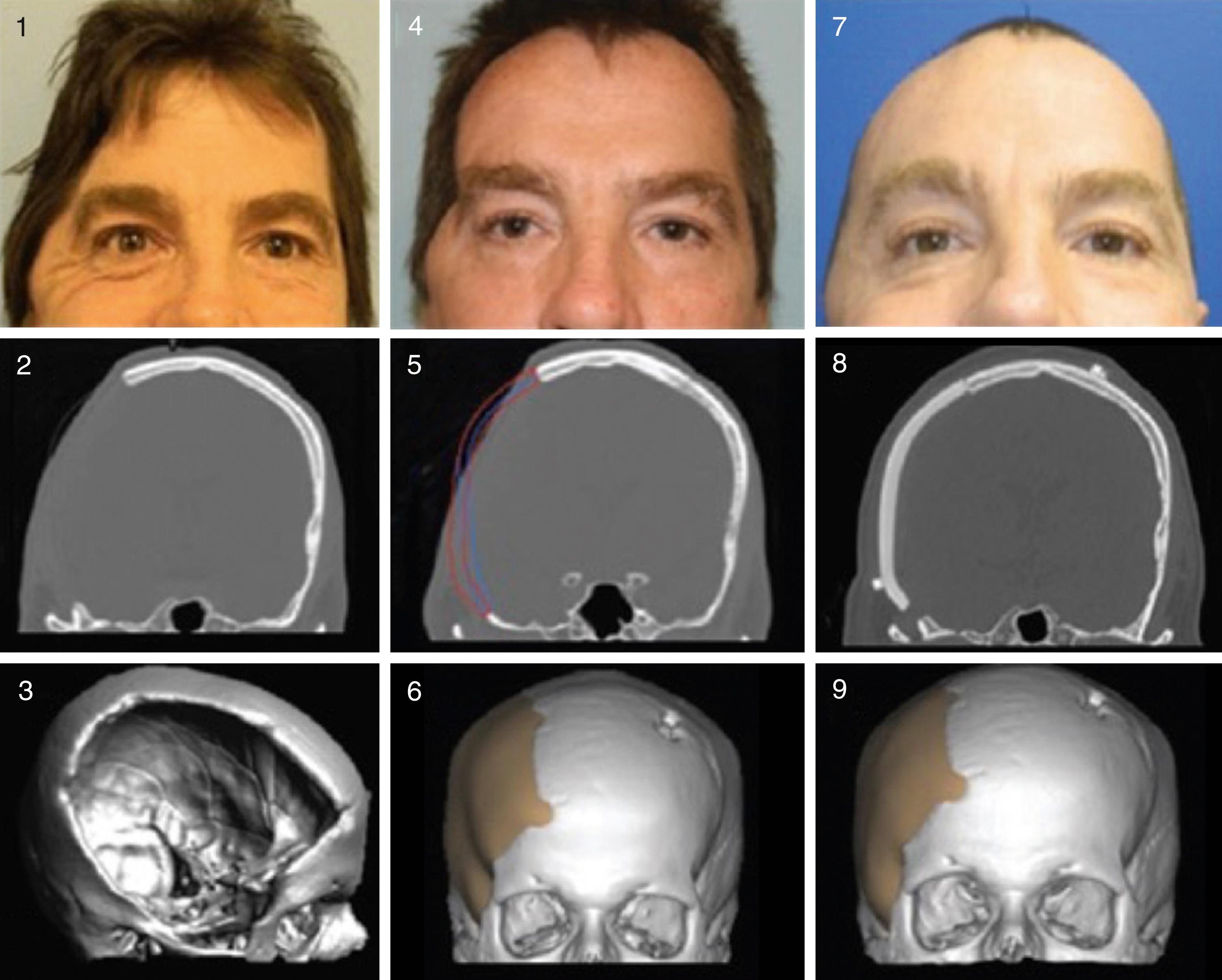
Neurosurgical-associated scalp and skull defects pose a special reconstructive challenge, due to underlying skeletal deficits, rearrangement of the normal scalp anatomy, scalp contraction and corresponding soft tissue deficiency. Adult neurocranial patients should be treated using a multidisciplinary approach in an effort to provide synergistic insight with regard to methods for reconstruction, as similar to breast reconstruction for example. Neuroplastic surgeons can provide improved strategies to achieve symmetric cranial contours without visible irregularities and reduce the risk of postoperative wound complications. A multidisciplinary discussion should be part of the preop planning process, addressing durable options for complicated scalp (with or without concomitant skull) reconstruction. , Careful handling of the delicate scalp, which begins at the time of incision planning and initial scalp dissection, is paramount during all neurosurgical procedures. Any defects of the scalp noted at the time of neurosurgery, or in areas of high tension, should be addressed immediately to prevent future complications. Adjacent tissue transfer, at the time of neurosurgery, is a simple solution that may help prevent scalp dehiscence at a later date. In some cases, scalp dehiscence evolves into a dire consequence, including hardware exposure, bone flap osteomyelitis, or cranial implant infection, which may require one or two additional surgeries. Such dire consequences may be prevented at the time of neurosurgery by proper planning with a neuroplastic surgeon.
When scalp defects present themselves unexpectedly, neuroplastic approaches are determined based on size, location, depth of defect, bone involvement, wound condition, and patient comorbidities. Additionally, even when the scalp is successfully reconstructed during surgery, the wound healing process plays an equally important role in the restoration of scalp function and continuity. Wound healing depends on biodynamic cellular interactions with various regulatory proteins to accomplish re-epithelialization and scar maturation.
The incision is designed based on several considerations. First, the exposure needed by the neurosurgeon to perform the operation safely, without concern for suboptimal visualization, should be discussed. Second, the incision should be designed so as to assure that no foreign material (plates, screws, hardware, implants) ends up laying directly under the scalp incision. Third, attention should be directed to the four main blood vessels used to assist in flap design, to minimize any concern for impaired perfusion (especially important during repeat surgery). These are the two superficial temporal arterial pedicles and the two occipital arterial pedicles. All other flap pedicles are less dependable and only considered when necessary.
Closed incisional wounds regain approximately 20% of their strength at 3 weeks after neurosurgery, regain 70% of their initial strength at 6 weeks, and ultimately regain 80% of their preinjury strength, a process that takes up to 1 year. As such, we utilize a delayed approach for suture removal, especially in instances of expected wound healing delays. Staples cause a problem, since delayed removal is conflicted by re-epithelization, something that does not apply to sutures. Thus, neuroplastic surgery employs only permanent sutures to the skin at time of scalp closure, in all instances.
There are three distinct, yet overlapping, phases of scalp wound healing pertinent to the neurosurgeon: the inflammatory phase (days 0 to 4), the proliferation phase (day 3 to week 3), and the maturation phase (week 3 to 2 years). Any pre-existing condition, such as diabetes, steroids, adjuvant chemotherapy, may interfere with wound healing biology. When a cranial implant is included at time of scalp reconstruction, this becomes extremely critical, given that even one millimeter of dehiscence could lead to bacterial invasion and major failure related to implant infection. As such, the neurosurgeon should have a low threshold for neuroplastic surgery collaboration when patients have an increased risk for peri-operative complications, most notably in instances of cranioplasty and alloplastic reconstruction. , , Also, when faced with neurosurgical patients battling a poly-microbial infection history, we recommend the collaboration of an infectious disease expert for peri-operative recommendations and strong consideration for titanium mesh as oppose to alloplastic implants.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here