Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Liver preservation is one of the most important components of liver transplantation because the efficacy of preservation has an enormous impact on the posttransplant outcome. Especially in wide geographical regions with national organ sharing, liver preservation allows organs to travel great distances. The evolution of organ transplantation was mainly related to the introduction of new immunosuppressive drugs such as cyclosporine, but the development of effective preservation solutions also contributed to a significant extent. The University of Wisconsin (UW) solution is probably the most commonly used static preservation solution; it was developed by Belzer and Southard before it was introduced in 1987. The current standard technique of liver preservation is static cold preservation using hypothermia and special preservation solutions. However, the concept of machine perfusion has challenged the standard technique during the past years, and there is growing evidence that continuous machine perfusion might be superior to the simpler cold storage technique.
The preservation injury is composed of different components that sequentially occur before, during, and after liver preservation. In brain-dead donors the four sources of this injury are (1) prepreservation injury, (2) cold preservation injury, (3) rewarming injury, and (4) reperfusion injury ( Fig. 44-1 , A ). Allografts from donation after cardiac death (DCD) experience an additional warm ischemia injury between cardiac death and cold flushing at procurement ( Fig. 44-1 , B ).
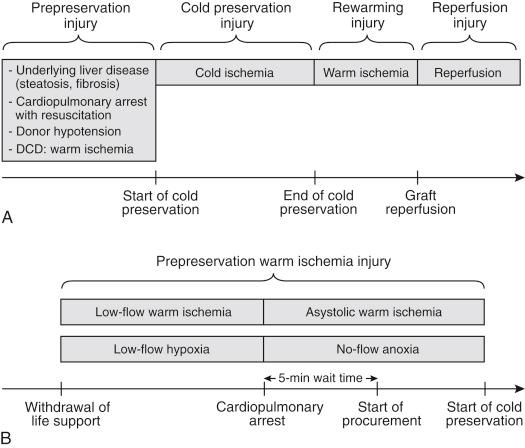
The prepreservation injury can be related to preexisting liver disease, injury associated with brain death or events leading to brain death, and injury during organ harvest. The most common type of preexisting liver injury is related to steatosis, which is often related to obesity or alcohol and drug consumption. Steatosis aggravates the cold preservation injury in hepatocytes and sinusoidal lining cells and increases the magnitude of the reperfusion injury, which is associated with a higher risk for primary graft failure or severe primary dysfunction. Experimental studies have shown that hepatic arterial flow and microcirculation are significantly impaired in steatotic livers. Especially macrosteatosis is an established clinical risk factor for primary nonfunction or dysfunction and posttransplant biliary complications. Although organs with mild macrosteatosis (<30%) are acceptable for transplantation, moderate (30% to 60%) and severe (>60%) macrosteatosis remain a challenging problem in transplantation. Furthermore, the epidemics of obesity and diabetes in the United States in the past 2 decades led to an increased incidence of steatotic donor organs. Because of the significant gap between the supply and demand for donor organs, the use of steatotic donor livers can significantly contribute to reducing this gap. The diagnosis of preexisting liver disease is an integral part of donor organ evaluation. Screening of potential donors by history, physical examination, drug toxicity and liver function test results, imaging with ultrasound or computed tomography scan, and liver biopsy results rules out most cases of preexisting liver disease.
Another important component of prepreservation injury is related to the potential injury that is associated with brain death or events leading to brain death. Trauma leading to brain death is often associated with hypotension or hypoxia, which can lead to warm ischemia of the liver. A significant proportion of donors have cardiopulmonary arrest secondary to trauma, drug intoxication, cardiovascular or cerebrovascular event, or any form of asphyxia that leads to brain death. Donors with an unknown or long down time before cardiopulmonary resuscitation especially experience a significant warm ischemia-reperfusion (IR) injury, which may have deleterious effects on initial graft function in extended criteria donor organs. In addition, the management of brain-dead patients is in some instances suboptimal (e.g., hypotension) before organ donation is considered and the procurement organization is involved.
Injury during organ harvesting may occur because of intraoperative hypotension. This type of injury is mainly attributed to hemodynamic instability of the donor during the procurement procedure rather than to technical misadventure. A donor liver biopsy study demonstrated that one third of donors accepted for transplantation have evidence of prepreservation injury in terms of platelet adhesion to sinusoidal lining cells in biopsies taken at the time of organ procurement. The study found a relationship between the severity of this injury and organ dysfunction after graft reperfusion.
In contrast to organs from donation after brain death donors, livers from DCD donors experience a significant prepreservation injury after withdrawal of life support (see Fig. 44-1 , B ). This injury is related to warm ischemia secondary to cardiopulmonary arrest before procurement. There are two types of warm ischemia after withdrawal of life support. Warm ischemia starts at the time of extubation and is characterized by hypoperfusion and tissue hypoxemia. This type of ischemia is followed by asystolic warm ischemia after cardiac arrest. There is documented evidence that the risk for graft nonfunction and failure is related to the time interval from donor extubation to cardiac arrest, the duration of postextubation hypotension and hypoxemia. Many centers do not proceed with procurement of DCD livers if the total warm ischemia time is more than 30 minutes.
Although cold preservation injury caused by deleterious effects of hypothermia and ischemia affects all types of liver cells (hepatocytes, biliary cells, sinusoidal lining cells), there is established evidence that sinusoidal lining cells are particularly vulnerable to cold preservation, which is considered to be the main mechanism of this injury. There is established evidence that increased length of cold ischemia translates into inferior outcome after liver transplantation.
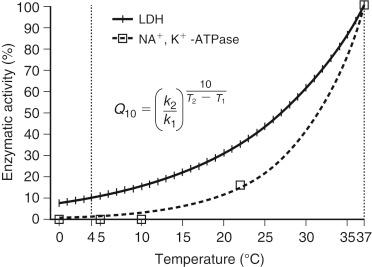
Static cold preservation is the standard method of organ preservation today. The hepatic core temperature reaches equilibrium near 0° C during static cold storage in the ice box. The beneficial effects of cold preservation of organs rely on the principle of suppressing the metabolic rate during the period in which they are not being perfused with oxygen and nutrients. The rationale behind this principle is that most enzymatic reaction rates ( Q 10 = 2) slow down by 50% for every 10° C of temperature reduction ( Fig. 44-2 ). According to this principle, which is also known as van’t Hoff’s rule , cooling at 4° C results in a metabolic rate approximately 10% of that at normal body temperature (37° C).
Van’t Hoff’s rule:
However, the effects of temperature on enzymatic activity are variable. Some complex enzymatic energy-dependent reactions have a Q 10 between 4 and 6 and are completely inhibited at low temperatures. For instance, Na + ,K + -adenosine triphosphatase (ATPase) is completely inhibited at 5° C (0.35% activity), but others such as lactate dehydrogenase, as the key enzyme of anaerobic glycolysis, remain active at approximately 10% at 5° C compared to 37° C (see Fig. 44-2 ). The differences in susceptibility of enzymes at low temperatures account for many of the changes that occur in cold-preserved livers and also have harmful consequences for cellular and subcelluar structures.
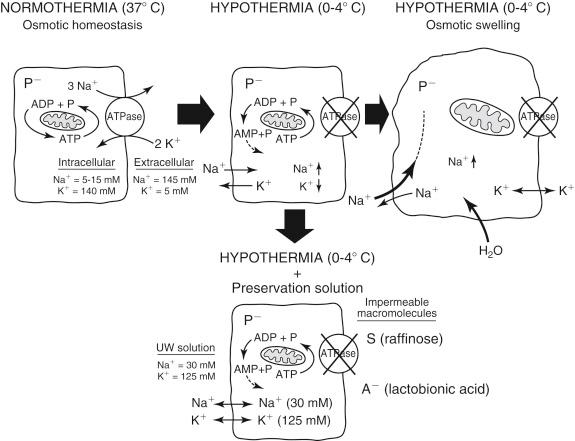
Hypothermia-induced cell swelling is the consequence of impaired function of the membrane-bound Na + ,K + -ATPase pump, which maintains the osmotic homeostasis between the extracellular and intracellular space. Under normothermic conditions the pump excretes intracellular sodium and accumulates potassium in the cell in the ratio 3:2, which generates the cell membrane potential because of the electrochemical gradient ( Fig. 44-3 ). This process is energy consuming and requires adenosine triphosphate (ATP) for normal function. During cold storage the function of the Na + ,K + -ATPase is nearly shut off because of the temperature-dependent suppression of enzyme activity (see Fig. 44-2 ) and the insufficient supply of ATP during cold ischemia. Nonfunction of the pump causes an osmotic equilibrium of sodium and potassium between the intracellular and extracellular space, resulting in passive sodium influx into the cell. The influx of sodium ions is further enforced by intracellular negatively charged proteins (anions). The intracellular hyperosmolarity finally results in water uptake, which leads to cell swelling, formation of protruding pockets, and lysis.
Although cooling of organs reduces the metabolic demand, some metabolic reactions are not stopped at low temperatures. During cold ischemia, liver cells shift from aerobic to anaerobic metabolism (Pasteur effect), which is self-limiting in terms of insufficient generation of ATP. The only source of ATP generation is anaerobic glycolysis. The generation of ATP is nineteenfold higher under aerobic compared to anaerobic conditions. Despite the continuous generation of ATP by anaerobic glycolysis, use of ATP rapidly exceeds production, and ATP is therefore depleted. Normally ATP is regenerated from other phosphorylated nucleotides adenosine diphosphate and adenosine monophosphate, but the energy demand during ischemia results in the dephosphorylation of adenosine diphosphate and adenosine monophosphate with further sequential degradation to adenosine, inosine, and hypoxanthine ( Fig. 44-4 ). Ischemia also promotes the conversion of xanthine hydrogenase to xanthine oxidase, which further catalyzes the degradation of hypoxanthine in the presence of oxygen. A product of this reaction is the superoxide radical, which belongs to the family of highly reactive oxygen species (ROS). During cold preservation this reaction is very slow because of the short supply of molecular oxygen. However, when oxygen is supplied at the time of organ reperfusion, an increased amount of ROS are generated by this mechanism. ROS are highly toxic because they can oxidize any organic molecule and cause cell membrane damage by lipid peroxidation.
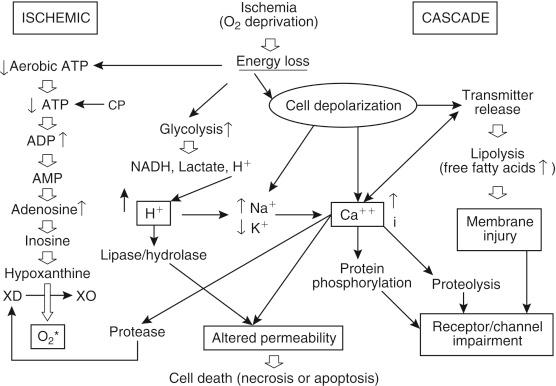
Further deleterious effects during cold ischemia are the development of intracellular acidosis and the rise of intracellular calcium concentration (see Fig. 44-4 ). Intracellular acidosis is mainly attributed to anaerobic glycolysis because lactic acid is the end product of this metabolic pathway. The intracellular accumulation of protons inhibits glycolytic key enzymes (e.g., phosphofructokinase) and blocks the residual anaerobic energy production, which results in further ATP depletion. In addition, the activation of lipoprotein lipases and lysosomal hydrolases by intracellular acidosis causes membrane damage leading to increased permeability. The rise of intracellular calcium concentration is a signal for many ischemic mechanisms leading to cell death. Under physiological conditions the extracellular calcium concentration (1 to 2 mM) is approximately 10 4 -fold higher than the intracellular concentration (0.0001 mM). This gradient is maintained by membrane-bound Ca 2+ translocase and Na + -Ca 2+ exchange mechanisms, both of which are energy dependent. Similar to Na + ,K + -ATPase, both mechanisms are inhibited during hypothermic ischemia because of ATP depletion, which results in massive calcium influx and a rise in intracellular calcium concentration. Other contributing mechanisms are the leakage of intracellular calcium stores. The enormous increase in cytosolic calcium activates Ca 2+ -dependent phospholipases and proteases, which have damaging effects on cellular structures and membranes. These enzymes do not require energy, and therefore their activity is not influenced by ATP depletion. These changes result in impairment of cellular integrity and progress to cell death.
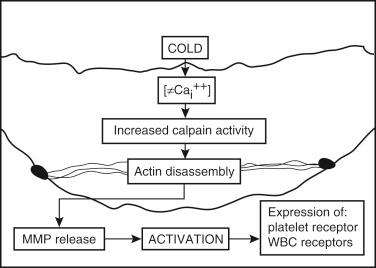
The classical effects of cold ischemia do not completely explain why some organs tolerate cold ischemia better than others do or why UW solution is a more effective preservative for the liver than for other organs. There is convincing evidence that damage to sinusoidal endothelial cells (SECs) is the main mechanism of cold preservation injury in the liver. An experimental study demonstrated that SEC injury was the prominent feature of cold ischemia, whereas warm ischemia affected predominantly hepatocytes. Further evidence comes from a study in which allograft apoptosis was 42 times greater in SECs after 12 hours of cold ischemia than that in hepatocytes. It appears that the described pathophysiological mechanisms of hypothermia and ischemia have more deleterious effects for SECs than for other types of liver cells. Mitochondrial dysfunction, ATP depletion, and ATP-dependent cytoskeletal disruption were features of ischemia injury in hepatic SEC. ATP depletion during cold ischemia results in a rise of intracellular calcium concentration, which activates proteases (see Fig. 44-4 ). This was shown first in animal models and then confirmed in human allografts. One of these important proteases is calpain, a calcium-dependent, nonlysosomal cysteine protease. Other important proteases are matrix metalloproteinases (MMPs), which can degrade all components of the extracellular matrix and play an important role in IR injury (IRI). MMP-2 and MMP-9 were implicated when identified in the effluent of rat and human allografts and by the finding that less MMP release was observed when an efficient preservation solution was used. The Ca 2+ -induced activation of calpain leads to a disassembly of actin stress fibers ( Fig. 44-5 ). This induces the release of MMPs from SECs that are able to degrade the protective endothelial layer of glycoproteins (glycocalyx). Damage to the endothelial glycocalyx is probably the most important mechanism, resulting in a highly exposed and activated SEC surface, as evidenced by increased platelet and leukocyte adhesiveness to the SEC surface. The activation of the SEC surface, as well as the direct damage to SECs during cold preservation, is the prerequisite for platelet and leukocyte adhesion, which may lead to microcirculatory disturbances and cause sterile inflammatory immune response after reperfusion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here