Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Currently there are several imaging techniques used to image the vascular system. These techniques include catheter-based conventional digital subtraction angiography, Doppler ultrasound, magnetic resonance angiography (MRA), and computed tomography angiography (CTA).
Conventional digital subtraction angiography (DSA) has long been the gold standard for arteriography because of its high resolution and rapid image acquisition. In digital radiography, the x-ray signal is electronically detected, digitized, and processed before display. Using subtraction techniques, DSA allows rapid visualization of both the arterial and venous phases and enhancement of the parenchymal phase. Small vessels are well seen. Because image acquisition is rapid, and repeated injections can be made, DSA is invaluable in the performance and assessment of the results of endovascular interventional techniques that are performed at the same time as the diagnostic arteriogram.
Limitations of conventional angiography are its invasive nature, with the requirement for catheter placement; the need for conscious sedation; the length of the study; and radiation exposure. The need for patient monitoring both before and after the procedure and the need for patient recovery time add significantly to the cost of the procedure.
Another limitation is that images are usually acquired in only one plane per contrast injection. Newer developments have helped to overcome this limitation. Newer equipment allows rotational angiography that permits acquisition of an image volume as the image intensifier and x-ray tube rotate around patient. The image volume acquired can be reconstructed to obtain three-dimensional volume-rendered images. This technique is helpful in analyzing complex vasculature and defining vessel branch origins and patterns such as that in aneurysms. The technique is particularly useful in the cerebral circulation. Positioning and timing are more complex in the abdomen. Research in this area is exploring use of this technology for measurement of blood velocity and flow profiles.
Two newer imaging techniques now used for performing angiography are MRA and multislice CTA. These techniques, although representing distinctly different technologies, provide images of the vascular system that are competitive with those obtained from conventional catheter-based angiography. Although the image resolution of these two techniques is less than that of conventional angiography, the image quality is such that these methods have the potential to supplant, and in many instances already do supplant, conventional diagnostic angiography.
Magnetic resonance imaging (MRI) uses radiofrequency waves and magnetic field gradients to generate images. It is characterized by high contrast between soft tissue and flowing blood. The different pulse sequences used during MRI permit the enhancement or reduction of signal from different tissues based on their hydrogen density and their response to varying magnetic field gradients. MRI can be used to obtain static images of the body and images of the vascular system.
MRA affords visualization of flowing blood in vessels with the option of removing background soft tissues. A variety of pulse sequences have been used to perform MRA. These sequences include two- and three-dimensional time-of-flight (TOF), phase-contrast, and, more optimally, three-dimensional TOF gadolinium-enhanced MRA and time-resolved MRA. With MRA, conventional MRI images are usually acquired before and after any contrast administration as part of a complete magnetic resonance (MR) examination.
TOF angiography is performed using a flow-compensated gradient refocused sequence. Data are typically acquired as a stack of two-dimensional slices or as a single three-dimensional volume. In this technique, stationary tissues in the slice or volume of interest are saturated from repeated radiofrequency pulses and have low signal intensity. Blood flowing into the imaging has not been subjected to these radiofrequency pulses and is therefore fully magnetized (unsaturated). When it flows into the volume, it is bright compared with the stationary background tissues. This two-dimensional inflow technique works well in vessel segments running perpendicular to the imaging plane such as relatively normal arteries and veins, including the carotid arteries, the cerebral vasculature, the inferior vena cava, and iliac veins. Selective saturation pulses can be applied, allowing selective saturation of blood entering the imaging volume, such that either the arterial or the venous signal can be suppressed. With this technique, separate images of the arterial and venous system can be acquired.
A limitation of this technique is in-plane saturation, which results in signal dropout when the long axis of the vessel coincides with the scan plane or in the presence of slowly flowing blood in tortuous arteries. Another limitation is turbulence-induced signal loss in and distal to a stenosis. In addition, the relatively long echo times required for gradient moment nulling make TOF imaging sensitive to susceptibility artifacts from bowel gas, implanted metallic objects (e.g., clips), or other air-tissue interfaces. These artifacts are a primary reason for the inaccuracy of TOF MRA. In addition, imaging times are lengthy because vessels need to be imaged perpendicular to the long axis of the vessel and a stack of slices must be acquired. Long acquisition times can lead to artifacts caused by slice misregistration or patient motion. Three-dimensional TOF MRA is used when a small area is being imaged and vessels run in different orientations such as the carotid bifurcation or Circle of Willis. Nearly isotropic volumes may be obtained allowing vessel reformation in any direction. If offers higher spatial resolution and higher signal to noise. Disadvantages include insensitivity to slow flow and saturation effects that limit slab thickness. Imaging times are longer than two-dimensional–TOF techniques and unlike two-dimensional–TOF techniques where motion artifact affect single slices, in three-dimensional–TOF, motion artifact affects all slices.
Phase-contrast angiography uses velocity-induced phase shifts, which occur as blood flows through a magnetic field in the presence of flow-encoding gradients. Two images created with opposite bipolar flow-encoding gradients are subtracted from each other; in the phase difference image, the residual phase is proportional to velocity. Stationary tissues do not undergo a velocity-induced phase shift in either image; they are suppressed and therefore subtract completely. The flow-encoding gradients can be in any direction or in multiple directions, depending on the selected flow sensitivity. Phase-contrast angiography can be performed as a two- or three-dimensional gradient refocused sequence and is improved after contrast administration. It has been used in the evaluation of the renal arteries, carotid arteries, and portal veins. It is also used to measure flow velocity. When used with cardiac gating, a time-resolved velocity profile can be generated, providing quantitative measurement of the flow rate.
There are limitations to the technique. The amplitude of the preselected bipolar gradient determines the degree of velocity encoding (VENC). Phase-contrast sequences encode only a specified range of velocities. In occlusive disease, turbulence causes a wide spectrum of rapidly changing velocities, which produce intravoxel phase dispersion and signal loss. Artifactual signal loss at sites of vessel stenosis is common. The sequence is also susceptible to measurement degradation from cardiac, respiratory, and translational motion. Background subtraction can be problematic. Currently, because faster MR angiographic techniques are available, phase contrast techniques are used primarily for the measurement of flow velocity.
Three-dimensional TOF gadolinium-enhanced MRA is the most widely used and useful of the MRA techniques. It does not rely on motion of blood to create flow signal. A contrast agent, typically gadolinium chelate, is given to shorten the T1 (spin-lattice) relaxation time of blood so that it is significantly shorter than that of surrounding tissues. Blood is imaged directly using a T1-weighted sequence. Blood is bright relative to background tissues during the arterial phase of three-dimensional contrast-enhanced MRA, because contrast transiently reduces the arterial blood T1 to less than that of the brightest background tissue (fat). This technique reduces the sensitivity to turbulence, and in-plane saturation effects are eliminated. With this technique, a number of slices are oriented in the plane of the target vessels, permitting rapid imaging of a large field of view covering a large region of the vascular system. Contrast-enhanced MRA is fast and affords high-quality breath-hold and non–breath-hold angiograms. Dynamic contrast-enhanced MRA exploits the transient shortening in blood T1 after the intravenous (IV) administration of a contrast agent using a fast, three-dimensional spoiled gradient echo sequence. This is a first-pass technique, because the contrast agents currently used are extracellular agents and the gadolinium chelate rapidly leaks into the extravascular space. However, repeated slice volumes over the region of interest can be acquired during passage of the contrast allowing images in both the arterial and venous phases. Typical repetition times for contrast-enhanced MRA are less than 5 ms, with echo times of 1 to 2 ms and total scan times of 10 to 30 seconds. The sequences that use T1-weighted gradient echo, T1 fast field echo, or fast low-angle shot have high spatial resolution and a high signal-to-noise ratio. The images can be acquired in a breath-hold fashion and reformatted in any plane. Because intravascular signal is dependent on T1 relaxation rather than inflow or phase accumulation, in-plane saturation and signal loss owing to turbulence are not significant.
During the first-pass arterial phase, imaging is done before vascular contrast equilibration. Using a first-pass technique, steady-state background signal is nearly eliminated. Once the contrast is injected and dynamic imaging is done, however, a second bolus injection of contrast material must be given to repeat the process over, for example, a second region of interest. When the second dose is given, residual soft tissue enhancement can obscure vascular detail. Subtraction techniques, using a precontrast mask, are typically used to remove background tissues. When the second or third dose of contrast is used, subtraction is mandatory. Timing is important in contrast-enhanced MRA. Unlike computed tomography (CT), MRA does not map spatial data linearly over time. With three-dimensional MRI, all the three-dimensional Fourier or K-space data (the information from which the image is constructed) are collected before individual slices are reconstructed. K-space maps spatial frequencies rather than spatial data. Consequently, K-space data do not correspond to image space directly. Different portions of K-space determine the features of the image. The center of K-space, which records low spatial frequencies, affects contrast, whereas the periphery of K-space records high spatial frequencies, which contribute to the fine details, such as edges.
Intravascular T1 signal intensity is determined by the gadolinium concentration at the time the center of K-space is collected. The timing is synchronized so that the midportion of the bolus arrives at the desired site as the center of K-space is being collected. Perfect timing produces maximal arterial signal with minimal venous signal. If central K-space data are acquired too early, while arterial gadolinium is increasing rapidly, ringing or banding artifacts may be generated. Acquiring central K-space data too late leads to reduced arterial signal intensity and enhancement of venous structures. Several methods have been used to obtain proper bolus timing, including simple estimates of the travel time of the bolus from the site of injection to the region of interest. For example, in a healthy patient, the travel time from the antecubital vein to the aorta is approximately 15 seconds; in a patient with cardiac disease or an aneurysm, it is 25 to 35 seconds. To estimate contrast travel time more precisely, a test bolus can be used. In this technique, 1 to 2 mL of contrast followed by a 10- to 15-mL saline flush is injected at the same rate as the planned injection. Multiple single-slice fast gradient echo images of the appropriate vascular regions are then obtained as rapidly as possible—typically, every 1 to 2 seconds for a given period—and the time to peak enhancement (contrast travel time) is determined in the region of interest. A limitation of this technique is the setup time; also, the redistribution of the test bolus to the interstitial space may add to the background signal. Other timing techniques used include MR fluoroscopy. In this technique, two two-dimensional sagittal gradient refocused images are obtained rapidly (<1 second per image) throughout the region of interest. Images are generated in near real time and updated at a rate greater than one image every second. The bolus time is watched, and when it arrives, the operator switches over to the three-dimensional MRA sequence. This technique may be helpful in cases of asymmetrical flow because of asymmetrical stenoses.
Another technique is a temporally resolved method in which multiple three-dimensional data sets are rapidly acquired (over 2 to 8 seconds) without any predetermined timing; injection and scanning are begun simultaneously. The operator then selects the desired image set. Other techniques used to facilitate MRA involve simply scanning faster using parallel imaging techniques and using alternative K-space acquisition techniques.
Respiratory motion causes image blurring, ghosting, and signal loss. In three-dimensional imaging, blurring occurs in the direction of motion, whereas ghosting is more pronounced in the phase-encoding direction. Before the availability of fast imaging systems, MRA techniques were too long to permit breath holding. Breath holding results in improved images in abdominal and thoracic MRA and facilitates the visualization of small vessels, such as the renal arteries. It does not appear to be as critical in the evaluation of the carotids. Most ambulatory patients can hold their breath 20 to 30 seconds, and imaging is usually done on inspiration.
Another MRA technique that is now widely used is a time-resolved three-dimensional MRA technique with very high subsecond temporal resolution and submillimeter spatial resolution, which allows capture of multiple arterial, mixed, and venous phase images during the passage of contrast agent through the vascular anatomy (e.g., TWIST sequence). No test bolus or bolus timing is required with this technique. Imaging is so fast that venous contamination is eliminated. The technique is helpful for monitoring flow through vascular beds. It is helpful in determining flow patterns through cerebral and peripheral malformations and in delineation of shunt characteristics.
There is also renewed interest in non–contrast-enhanced MRA techniques using inflow inversion recovery techniques, and these sequences are an area of research interest, particularly for assessment of the renal arteries.
Patient cooperation is required for optimal contrast-enhanced MRA, because motion and improper breath holding can render the MRA study nondiagnostic. Valuable scanner time is often squandered during attempts to image an uncooperative patient. Patients should be relaxed, and the procedure should be explained to them beforehand. For patients who are particularly anxious, premedication with a sedative such as diazepam or fentanyl may be helpful. The IV catheter should be placed before the imaging is started and before the patient is in the magnet. A 22-gauge IV catheter or larger is placed in the antecubital fossa or below if the arms are to be extended above the head.
Currently, almost all contrast-enhanced MRA studies are performed using gadolinium-based contrast agents. Gadolinium is a paramagnetic metal ion. Paramagnetic atoms or molecules possess unpaired electrons that, when placed in a magnetic field, undergo magnetization (attain magnetic susceptibility). The electrons set up circulating currents in response to the externally applied field. These currents induce an internal magnetization that augments or opposes the external field. When the direction of internal magnetization is the same as that of the external field, the effective field within the object is enhanced. This magnetic field enhancement is known as paramagnetism .
Gadolinium decreases both spin-lattice (T1) and spin-spin (T2) relaxation times. Because gadolinium is toxic in its natural form, it is chelated with ligands, such as gadopentetate dimeglumine, gadoteridol, or gadodiamide, to form MR contrast agents. These agents are extracellular and pass from the intravascular compartment into the interstitial space in a matter of minutes. Measurements of T1 shortening at different cardiac outputs have shown that injection rates greater than approximately 2 mL/s do not increase T1 shortening. Signal intensity increases asymptotically as the injection rate increases, with negligible increases seen beyond a rate of 4 to 5 mL/s.
Most contrast-enhanced MR studies are performed with 0.1 mmol/kg of gadolinium or a double dose of 0.2 mmol/kg, or with a set volume of 15 or 30 mL with an injection rate of 1.5 to 2.5 mL/s. Contrast can be injected manually or, preferably, with a power injector. Either technique is followed rapidly by a 15- to 20-mL saline flush.
With contrast-enhanced MRA, once arterial data are collected, the sequence is repeated to capture the venous and equilibrium phases. With longer scanning times, the patient may take a short breath before repeating the sequences.
From their introduction, these agents were found to have a high safety margin and a low rate of adverse effects. It has been estimated that approximately 6 million doses of gadolinium-containing contrast agents are administered annually. However, in the late 1990s a new disease was described, and in 2006 was shown to be associated with the use of gadolinium-containing contrast agents. This disease, nephrogenic systemic fibrosis, has a low incidence; there are only several hundred cases reported in the literature. It is characterized by fibrosis of the skin, connective tissue, and internal organs, eye changes, and flexion and extension contractures. There is currently no consistently successful treatment, and it is potentially fatal. The majority of cases have occurred in patients with end-stage renal disease, chronic kidney disease, acute kidney injury, and acute renal insufficiency of any severity because of hepatorenal syndrome, and in patients requiring dialysis or those in the perioperative liver transplantation period. These patients and patients with glomerular filtration rate (GFR) of 30 mL/min per 1.73 m 2 or less are at high risk. It is recommended that gadolinium-based contrast agents be avoided in these high-risk patients and alternative imaging methods be used unless the information cannot be obtained with noncontrast MRI or other methods. It is also recommended that all patients undergo screening for renal insufficiency before receiving gadolinium-based contrast agents and package insert doses not be exceeded. The risk in patients with normal renal function is unknown, and to date there have been no reported cases in patients with normal renal function.
Three-dimensional contrast-enhanced MRA produces a contiguous volume of data. In most body MRA studies, the volume is asymmetrical (e.g., 400 × 300 × 64). The slice is typically viewed interactively using a computer workstation. Thin multiplanar reformatting (MPR) can be performed, and 1- to 2-mm slices can be viewed in multiple planes (axial, sagittal, and oblique).
Because the thin sections do not display the entire vessel, the maximum-intensity projection (MIP) processing technique is often used. With this algorithm, the user first selects the volume or portion of the volume to be evaluated. The algorithm then generates rays perpendicular to the viewing plane, records the maximum intensity of any voxel encountered along that ray, and assigns that maximum value to the corresponding pixel in the output image. This process results in images similar in appearance to conventional angiograms.
The MIP algorithm has some limitations. A major problem occurs when stationary tissue or structures within stationary tissue have a higher signal intensity than the vessels of interest. This can occur in the presence of crossing vessels, hemorrhage, fat, metallic susceptibility artifacts, or motion artifacts. This results in mapping of these extra signals into the projection image, producing a discontinuity in vessel signal that mimics vessel stenosis. Reducing the thickness of the MIP subvolume to exclude as much extraneous tissue as possible can mitigate this limitation. Underestimation of vessel diameter is another limitation of the technique.
Subtraction techniques are also used to improve vessel visibility. These techniques are performed by a complex subtraction of precontrast and postcontrast raw data sets and are routinely used in contrast-enhanced MRA of the extremities. Subtraction is relatively fast and there is good bone removal from the subtracted images. It is important to note that vessel calcifications are not well seen with standard MRI/MRA techniques and calcifications typically appear as dark regions of signal void. Metallic objects such as metallic stents produce artifacts seen as a signal void, limiting assessment of stent patency.
Three-dimensional volume rendering is another postprocessing technique that allows separation of overlapping structures. In many instances, this rendering may provide additional information over standard MIP images.
The carotid arteries can be well seen with contrast-enhanced MRA ( Fig. 15.1 ). The short circulation time of the carotid circulation (4 to 6 seconds) makes timing critical; otherwise, venous overlap of the images results. Both long and short imaging times have been used to overcome this problem.
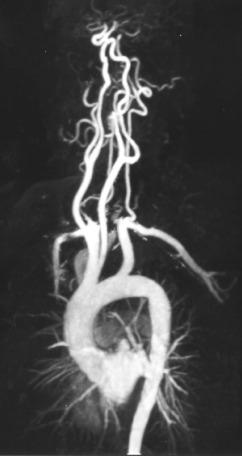
High-resolution imaging with short echo time is recommended. A major problem that impedes accurate stenosis assessment is the occurrence of high-speed turbulent jets at the site of stenosis. Specialized coils are recommended. The origins of the vessels from the aorta should be included in the imaging plane. Numerous studies have shown a high degree of correlation between stenosis measurement with contrast-enhanced MRA and DSA. MRI has also been used to evaluate atherosclerotic plaque in the carotid arteries.
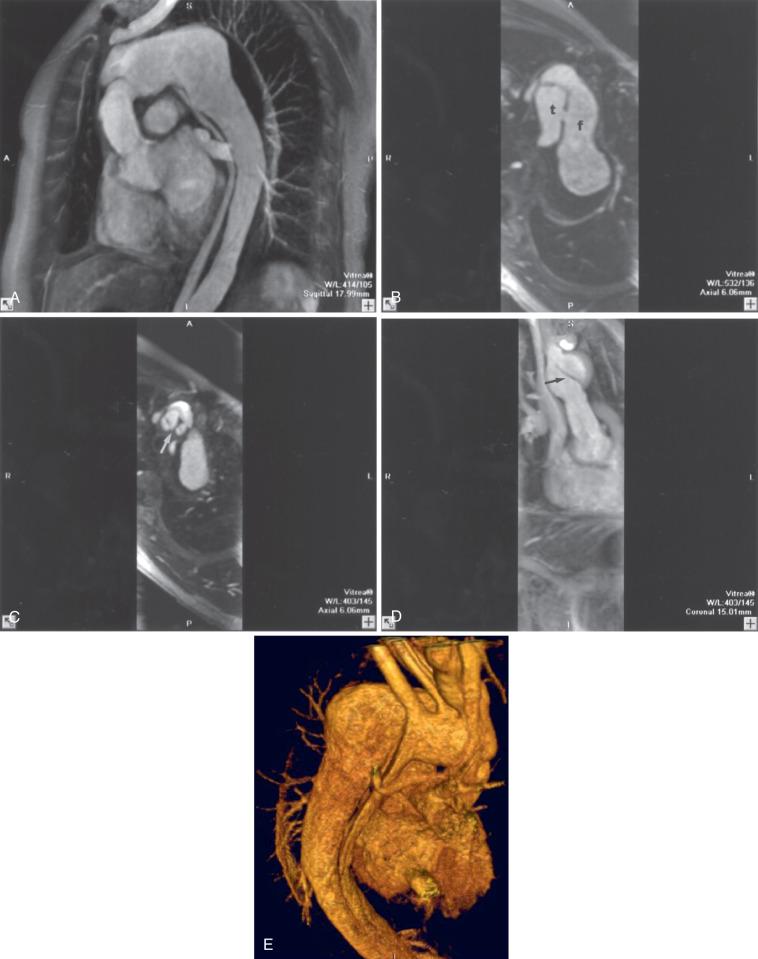
Contrast-enhanced MRA is used routinely to evaluate the thoracic aorta. It is the standard of practice for following the status of dissections and monitoring aneurysm enlargement ( Fig. 15.2 ). Imaging is usually performed in the oblique sagittal plane or coronal plane. In aortic dissection, the origins of the brachiocephalic vessels should be included. A phased array body coil is recommended when the aneurysm is confined to the thoracic aorta. The ascending, descending, and abdominal aortas are evaluated. A full evaluation of the aorta should include multiplanar cardiac-gated black blood imaging of the aortic wall to detect intramural hematoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here