Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There is a fine balance between hemostasis and hemorrhage in the human body, managed by a complex system of plasma, cellular, and endothelial factors. Coagulation is the normal process occurring when vascular injury results in the formation of a fibrin clot; thrombosis refers to the pathologic formation of clot in response to injury, stasis, and hypercoagulability. Intermittent and continuous dialysis therapies depend on adequate anticoagulation in their extracorporeal circuit (ECC) to maximize circuit and filter longevity, which will increase clearance and lessen costs and nurse time requirements. Insufficient anticoagulation results in decreased filter performance, clotting, and blood loss. Excessive anticoagulation leads to bleeding complications, which occur in 5% to 26% of treatments. Patients with acute kidney injury (AKI) are at risk for hemorrhagic and thrombotic complications. Bleeding can be caused by uremic platelet dysfunction or by the anticoagulants used for dialysis. The activation of coagulation during dialysis can lead to blood loss, estimated to 300 to 750 mL/yr in patients undergoing chronic hemodialysis. This chapter deals with the principles of anticoagulation in the ECC, the disturbances that occur in critically ill patients with AKI, and the importance of the intrinsic pathway, platelet activation, and the fibrinolytic system in affecting ECC patency. This chapter addresses the circuit design, contributions of membrane/blood interaction, and how these factors may be modified, in addition to the anticoagulation techniques for intermittent hemodialysis.
Prevention of blood coagulation depends on a complex interaction of endothelial cells, blood cells, and plasma factors. The balance between procoagulant and anticoagulant activity is a delicate one. This equilibrium is disturbed when blood is forced through an ECC, because the endothelial component is removed temporarily from the mix. As a result, the balance is shifted toward coagulation. There are numerous routes through which activation can occur, with the end result being platelet aggregation and formation of a clot. Shifting back the balance requires addition of an anticoagulant to the ECC, which then can favor bleeding. In critically ill patients with sepsis and multiorgan failure, the imbalance in the normal clotting mechanisms can be extreme, with activation of multiple inflammatory pathways and downregulation of anticoagulant pathways. Activated monocytes and polymorphonuclear cells add to the coagulation cascade via increased expression of tissue factor and production of reactive oxygen species.
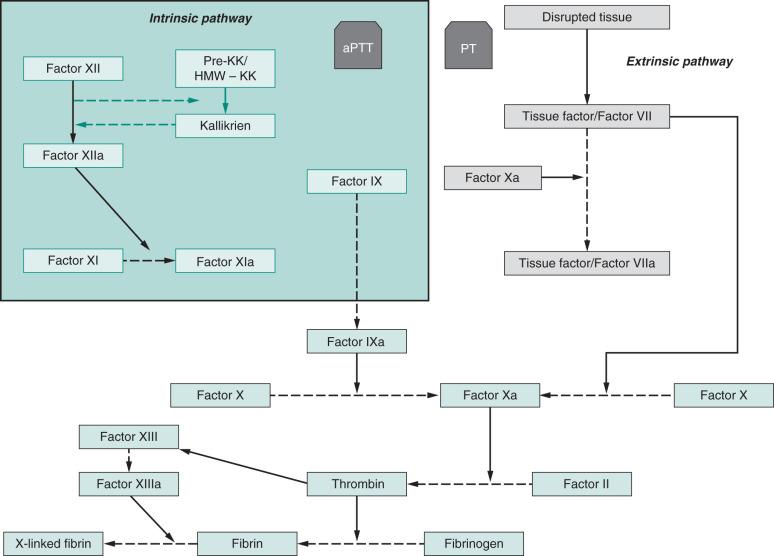
The processes of hemostasis and coagulation involve many functional areas, including platelet function, coagulation enzyme cascades, contact activation, natural anticoagulants, the endothelium, and fibrinolysis. When blood is exposed to the foreign surface of an ECC, two principal mechanisms of thrombus formation traditionally have been considered important: the intrinsic pathway of blood coagulation and platelet adhesion and activation. The traditional theory postulates that the intrinsic pathway in ECC can begin with the “contact activation factors,” such as factor XII, high-molecular-weight kallikrein, and prekallikrein, which occur more readily on a negatively charged surface. However, a recent study looking at blood parameters in continuous venovenous hemodialysis (CVVH) circuits without heparin did not demonstrate any change in plasma levels of factor XIIa-C1 inhibitor complex or the kallikrein-C1 inhibitor complex, findings that would argue against significant involvement of contact activation of the intrinsic pathway in clotting in CVVH. These results were in keeping with those of a previous study, which did not find an increase in contact activation factors in a system using polyacrylonitrile membranes and systemic heparinization. The generation of thrombin can also start with the activation of factor X, either on the surface of activated platelets or by the integrin receptor membrane attack complex 1 (MAC-1) on leukocytes. However it is initiated, it then proceeds through an amplifying series of enzyme reactions, is moderated by multiple negative and positive feedback loops, and culminates in the production of thrombin and the formation of a stable, cross-linked fibrin clot ( Fig. 142.1 ).
Many abnormalities have been described in uremic platelets, including decreased thromboxane A 2 , abnormal intracellular calcium mobilization, increased intracellular cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), and abnormal aggregability. Platelets undergo transient morphologic changes secondary to circulating through the ECC. These changes are consistent with primary, reversible aggregation and activation. Platelet activation can occur because of interaction with the activated clotting cascade (e.g., thrombin) or from contact with the ECC. Platelet activation and adhesion in the ECC and on dialysis membranes do not require von Willebrand factor (vWF). The reaction either occurs directly on the foreign surface or is promoted by various adsorbed plasma proteins, including fibrinogen.
Once platelet adhesion occurs, the platelet activation sequence follows, which also can be initiated by thrombin. The important stages in this sequence include shape change, aggregation, secretion of thromboxane B 2 (which is involved in activating other platelets), elaboration of contents of alpha granules and dense granules, platelet surface membrane modification, and, ultimately, platelet contraction and fusion. The platelet membrane modification provides a surface on which procoagulant reactions are facilitated. Platelets contribute substantially to thrombus formation in the ECC, and increased levels of thromboxane B2 and factor III thromboglobulin can demonstrate their activation during dialysis. The increase in thromboxane B 2 is higher in heparin-free dialysis compared with heparin dialysis. Adherence can occur quickly when platelets are exposed to the ECC foreign surface. Multiple studies have shown that patients with a higher baseline platelet count require more heparin to achieve comparable filter life and that lower platelet counts are associated with a decreased risk of filter clotting.
The fibrinolytic system has as its main enzyme plasmin, an active fibrin protease that is produced from plasminogen. There is an increase in fibrinolytic activity during dialysis, secondary to an increase in the endothelial release of tissue plasminogen activator (tPA). It is believed that the blood contact with the dialysis membrane provides the stimulus for this reaction. There is no evidence that this response limits clotting in the ECC. However, in the study by Bouman already mentioned, baseline levels of factor XIIa-C1 inhibitors and kallikrein-C1 inhibitor complexes were lower in the subgroup of patients with early increased thrombin generation, which would be consistent with decreased fibrinolysis. Confusing these results was the presence of a trend toward increased levels of plasmin-antiplasmin complex (normally associated with increased fibrinolysis) in the patients with early clotting. The authors hypothesized that these levels of plasmin-antiplasmin complexes may have been more a marker of coagulation than of fibrinolytic activity. Alternatively, it was speculated that these same elevated complex levels may have reflected a higher baseline thrombin generation, which then could have activated endogenous anticoagulant protein C and resulted in less coagulation in the CVVH circuit. Factor XIIa and kallikrein are believed to be important in the activation of fibrinolysis. Factor XII can activate fibrinolysis by activating prekallikrein (and with subsequent urokinase-type plasminogen activator), causing the prekallikrein-driven generation of kallikrein (leading to increased tPA), and by directly activating plasminogen. Activated protein C also promotes fibrinolysis because of its inhibitory effect on plasminogen activator inhibitor. Thus decreased levels of activated protein C with sepsis may tip the balance toward coagulation; however, this effect may be offset by the consumption of coagulation factors associated with disseminated intravascular coagulation (DIC), which would favor bleeding.
The extrinsic pathway begins with factor VII, which depends on the release of the tissue factor thromboplastin to have an effect on its natural targets, factors IX and X. Thromboplastin is released from injured vessel walls and is expressed by activated monocytes, usually in response to endotoxins, cytokines, hypoxia, advanced glycation end products, growth factors, and oxidative stress. However, the extrinsic pathway and its mechanisms have not been found to be important in the formation of ECC thrombi (see Fig. 142.1 ).
Natural anticoagulant systems are in place and include antithrombin III (ATIII), a serine protease inhibitor. Its actions, which are potentiated by heparin and heparin sulfate (located in vessel walls), include inhibition of thrombin; factors Xa, IXa, XIa, and XIIa; and kallikrein. Multiple studies have shown subnormal levels of ATIII in critically ill patients, and low levels have been associated with filter failure. A retrospective study of septic, ATIII-deficient patients undergoing continuous renal replacement therapy (CRRT) showed reduced filter clotting when heparin anticoagulation was supplemented with ATIII. Protein C, in reactions catalyzed by protein S, inactivates factors Va and VIIIa, thus limiting thrombin generation. Protein C is activated by the binding of thrombin to vascular endothelial-associated thrombomodulin. Decreased levels of activated protein C with sepsis may tip the balance toward coagulation (however, as mentioned previously, this coexists with DIC-associated consumption of coagulation factors, which favors bleeding). In a study of patients with severe sepsis undergoing CVVH, treatment with continuous intravenous recombinant human activated protein C removed the need for additional anticoagulation. Filter life compared favorably with that observed with the use of unfractionated heparin.
A hypercoagulable state exists to some extent secondary to uremia. Patients with end-stage renal disease and those who are critically ill with acute kidney injury/uremia have intrinsic clotting system activation. They have increased levels of procoagulant factors VII and VIII, decreased levels of coagulation inhibitors such as ATIII and proteins C and S, and impaired fibrinolysis. Dialysis can be associated with the release from endothelial cells of other procoagulant substances such as vWF, 6-keto-prostaglandin F 1a , and tPA. There is also a large dialysis-associated increase in the levels of prothrombin and thrombin-antithrombin complexes (TAT), even more so when anticoagulation is suboptimal or withheld.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here