Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Primary myelofibrosis (PMF) is a Philadelphia chromosome-negative, clonal, myeloproliferative neoplasm (MPN) that is often but not always accompanied by the driver mutations JAK2V617F , calreticulin exon 9 ( CALR ), and the thrombopoietin receptor, MPL515L/K . PMF is clinically characterized by progressive splenomegaly, cytopenias, and cytokine-driven constitutional symptoms with a propensity for leukemic transformation (20%). Pathologically, PMF is associated with megakaryocyte hyperplasia and atypia, reactive bone marrow (BM) fibrosis, angiogenesis, ineffective erythropoiesis, osteosclerosis, and extramedullary hematopoiesis (EMH), resulting in hepatosplenomegaly. Although there has been exceptional progress in unraveling the complex molecular underpinnings and refinement of the prognostic factors for PMF risk stratification, unfortunately, none of the currently available therapies reliably alters the disease trajectory. Since the last edition of this book, another JAK2 inhibitor, fedratinib, has been added to the therapeutic armamentarium of PMF, which previously included only ruxolitinib. Both these JAK2 inhibitors provide important symptom and spleen relief, but allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative therapy for PMF. This chapter describes the current understanding of the pathobiology, clinical features, and treatment approaches of PMF.
PMF was first reported in the literature in 1879, when a German surgeon, Gustav Heuck, described two young patients with massive splenomegaly, circulating nucleated red blood cells (RBCs), and abnormal leukocytes, which he referred to as “splenic-medullary leukemia.” He noted that this disease differed from chronic myeloid leukemia (CML) due to the presence of bone marrow fibrosis (BMF), osteosclerosis, and EMH. Since then, several other terms have been used synonymously to describe PMF, including angiogenic myeloid metaplasia, myeloid metaplasia with myelofibrosis, and chronic idiopathic myelofibrosis. In 2007, the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) expert consensus proposed the term PMF to standardize the terminology.
In 1951, William Dameshek included PMF among a group of related disorders, which he termed myeloproliferative disorders (MPDs), based on clinical observations of patients with polycythemia vera (PV), CML, and essential thrombocythemia (ET) who developed BM fibrosis and a clinical picture resembling PMF. Additionally, several others described that these disorders originated from a common ancestral cell, reflecting its neoplastic nature. Accordingly, in 2008, the World Health Organization (WHO) modified the terminology from MPD to MPN.
PMF is rare among the MPNs. Epidemiologic studies have reported a variable incidence of PMF across Europe, Australia, and the United States (US), ranging between 0.5 and 1.3 per 100,000 per year. The annual incidence rate of PMF noted in Olmstead County (MN, USA) was 1.5 per 100,000. Recent Surveillance, Epidemiology, and End Results (SEER) database analysis (2000 to 2016) indicates that the incidence of PMF has remained constant. The median age of diagnosis is 69 years, with most diagnosed between 50 and 69 years of age. PMF is rare in the pediatric age-group. Evidence of genetic transmission does exist: a higher incidence has been reported in Ashkenazi Jews than in Arabs, who both live in Northern Israel, and a familial predisposition to MPNs, including PMF, has been identified. Gender distribution of PMF is reported variably, with some studies reporting male predominance while others do not.
PMF in the United States and Europe is epidemiologically similar, but there are differences in other parts of the world, which may be attributed to environmental exposures. These patients may be younger with an accelerated rate of disease progression. In Japan, PMF is considered a rare disorder. However, the incidence of myelofibrosis (MF) among survivors who were close to the hypocenter of the atomic bomb explosion at Hiroshima and Nagasaki was 18 times higher than the general population of Japan, and they became symptomatic approximately 6 years after the bomb blast. Such data indicate a strong link between excessive radiation exposure and development of PMF, which is further substantiated by the high incidence of PMF in patients who received the contrast material thorotrast (which contains 232 Th, a radioactive element with a half-life of 1.41 × 10 10 years). Thorotrast is retained indefinitely by the reticuloendothelial cells, resulting in lifelong irradiation to the liver, spleen, lymph nodes, and BM. Less commonly, chronic exposure to industrial solvents (benzene and toluene) is associated with the development of PMF. Unlike PV, where clusters of patients identified in Eastern Pennsylvania raise the concern for possible environmental effects of waste-coal and Superfund sites, no epidemiologic studies definitively support environmental exposure in PMF.
PMF originates from the pluripotent hematopoietic stem cell, and clonality was first established as early as the 1970s by glucose 6-phosphate dehydrogenase (G6PD) isoenzyme analysis at the post-DNA level, followed by several studies in the 1990s, including an X-linked DNA analysis that revealed a monoclonal X chromosome inactivation pattern in peripheral-blood leukocytes, granulocytes, bone marrow mononuclear cells, and, occasionally, T lymphocytes. The central dogma underlying PMF pathogenesis is based on the concept of a sequential accumulation of acquired genetic or epigenetic alterations within the founder clone population that ultimately drive disease progression and evolution to acute myeloid leukemia. Additionally, the bone marrow niche, megakaryocyte hyperplasia, and the sequent cytokine-induced inflammatory response, as well as aging process, further contribute to the pathobiology of PMF.
Akin to related MPNs, PMF mononuclear cells when cloned in semisolid media generate autonomous erythroid and megakaryocyte colony formation in addition to growth factor-dependent hematopoietic colony growth. Endogenous erythroid and megakaryocyte colonies are a hallmark of abnormal growth of hematopoietic progenitors in vitro, indicative of aberrant hyperactive growth factor signaling. The quest for the genetic basis of autonomous hematopoiesis in MPNs propelled the discovery of JAK2 V671F in more than 90% of patients with PV and approximately 50% of patients with PMF.
JAK2 V617F is a gain-of-function mutation in the autoinhibitory domain of the JAK2 tyrosine kinase, resulting in constitutive activation of JAK2 that promotes signal transducer and activator of transcription (STAT)-mediated gene transcription and the resultant autonomous hematopoietic cell proliferation. In a murine transplant model, BM cells transduced with Jak2 V617F resulted in a two-stage MPN clinical phenotype, with the initial stage closely resembling PV with erythrocytosis and neutrophilia, followed by an early MF-like phenotype comprising anemia, thrombocytopenia, splenomegaly, and bone marrow fibrosis.
Furthermore, a small proportion of PMF patients harbor mutations in the lymphocyte adaptor protein (LNK or SH2B3) or Casitas B-cell lymphoma (CBL) genes encoding negative regulators of JAK-STAT signaling. Loss-of-function mutations in LNK , predictably result in JAK-STAT hyperactivation, and concomitant mutations in JAK2 V617F and LNK are associated with leukemic transformation of PMF. Gain-of-function mutations in CBL are associated with acquired uniparental disomy of chromosome 11q, resulting in homozygous deletion of CBL and the resultant loss of its tumor suppressor function. CBL mutations occur in 6% of PMF patients and are associated with leukemic transformation. The JAK2 V617F mutation is homozygous only in 13% of patients with PMF as opposed to 30% in PV, which is attributed to homologous recombination. Homozygosity of JAK2 V617F in PMF patients is associated with frequent occurrence of unfavorable cytogenetic abnormalities (CGA). Although JAK2 V617F occurs in almost all patients with PV, a sizeable number of patients with ET and PMF are wild-type for JAK2 , which led to the pursuit of identifying other mutations that drive hyperactive JAK-STAT signaling.
In 2006, Levine et al., through exome sequencing, identified a somatic activating mutation in the transmembrane domain of the thrombopoietin receptor MPL (W515L) in 9% (4/45) of patients with JAK2 V617F wild-type PMF. MPL signals the phosphorylation of both MPL and JAK2/TYK2 and subsequently activates STAT3, STAT5, ERK/MAPK, and PI3K/AKT signaling pathways. In contrast to MPL wild-type, the W515L variant promotes ligand-independent JAK-STAT signal activation and confers cytokine-independent growth to 32D, Ba/F3, and UT7 cells. In a murine bone marrow transplant assay, isolated expression of MPL W515L led to fully penetrant lethal MPN (18 days) characterized by marked thrombocytosis, splenomegaly, and increased reticulin fibrosis but not erythrocytosis. These data suggest that the MPL mutation favors the development of thrombocytosis, but JAK2 V617F favors erythrocytosis. Compared with wild-type, PMF patients with MPL 515L/K are older, severely anemic, and more likely to require transfusion support.
The next breakthrough occurred in 2013, when two groups independently reported frameshift mutations in exon 9 of calreticulin (CALR) in patients with ET and PMF. Approximately 25% of patients with ET and PMF harbor insertion/deletion mutations in CALR . Furthermore, 80% of CALR mutations fall into either of the two categories: type 1 (52-bp deletion) or type 2 (5-bp insertion). Retrospective studies show that CALR- mutated PMF patients present with higher platelet counts, low hemoglobin levels, and leukocyte counts, with a lower thrombotic risk and better overall prognosis than patients with JAK2 V617F. Studies also implicate a worse prognosis for type 2 CALR- mutated patients compared to patients with type 1 mutations.
CALR , located in chromosome 19p13.2, encodes a calcium-binding protein in the endoplasmic reticulum (ER), functions as a chaperone protein and maintains calcium homeostasis. The frameshift mutations in CALR exon 9 result in a mutant calreticulin protein with a novel C-terminus sequence lacking the ER-targeting KDEL sequence. The fact that c-MPL is an ER client protein and that CALR mutations occurred exclusively in patients with ET or PMF alluded to the possibility that mutant CALR somehow activates MPL. Consistent with this observation, CALR exon 9 mutation was shown to induce constitutive phosphorylation of JAK2 and activation of STAT transcription only in an MPL-dependent manner.
The extracellular domain of MPL, especially its sites for N-linked glycosylation (CALR binding site), is necessary for the mutant CALR to drive transformation. While the glycan-binding sites of CALR are crucial for activating MPL, the polypeptide-binding regions and chaperone activity of CALR are dispensable. Additionally, positively charged C-terminal residues of mutant CALR are necessary for activating MPL signaling; mutation of these residues to uncharged glycine residues abrogates its transforming ability. Of note, truncation of exon 9 terminated the transforming potential of mutant CALR , further underscoring the functional importance of the novel C-terminus. Analogously, removal of the 36 most distal residues from the new C-terminus prevented mutant CALR from transforming Ba/F3-MPL cells. However, this Δ36 mutant was still able to bind to MPL, suggesting that binding and activation of the receptor may be distinct processes. The exact pathobiological link between the CALR mutation and the dysregulated JAK-STAT pathway remains an area of ongoing investigation. Collectively, hyperactive JAK-STAT signaling is central to the pathogenesis of PMF.
Approximately 10% of PMF patients are “triple negative,” that is, they lack any of the three driver mutations. Milosevic-Feenstra and coworkers, with whole-exome sequencing, identified noncanonical MPL mutations in 11.4% ( n = 70) and JAK2 mutations in 8.8% ( n = 57) of patients with triple negative ET and PMF. These newly identified mutations were heterozygous, and the mutations in MPL and JAK2 were mutually exclusive. 50% of triple negative patients lacked evidence for clonality, but the presence of germ-line mutations indicated that some of these individuals likely had a hereditary rather than an acquired MPN-like disorder. Based on these findings, sequencing of all coding exons of MPL and JAK2 is recommended during the diagnostic work-up of triple-negative ET and PMF patients.
With the advent of next-generation sequencing (NGS) technologies, the identification of multiple epigenetic mutations has provided further insight into the complex molecular pathogenesis of PMF and offers rationale for the evaluation of novel targeted therapeutics. Table 72.1 lists the common epigenetic mutations recognized in patients with PMF.
| Gene | Location | Mutation | Frequency |
|---|---|---|---|
| Janus kinase 2 ( JAK2 ) | 9p24 exon 14 | JAK2V617F | 65% |
| 9p24 exon 12 | Infrequent | ||
| Calreticulin ( CALR ) | 19p13.2 exon 9 | Type 1 (52 bp del) and Type 2 (5 bp in) | 25% |
| Ten-eleven translocation 2 ( TET2 ) | 4q24 | 17% | |
| Serine/arginine-rich splicing factor 2 ( SRSF2 ) | 17q25.1 | 17% | |
| U2 small nuclear RNA auxiliary factor 1 ( U2AF1 ) | 21q22.3 | 16% | |
| DNA methyltransferase 3 alpha ( DNMT3a ) | 2p23 | 15% | |
| Enhancer of zeste homolog 2 ( EZH2 ) | 7q36.1 | 13% | |
| Myeloproliferative leukemia virus ( MPL ) | 1p34 exon 10 | MPL W515L/K | 5%–10% |
| Casitas B-lineage lymphoma ( CBL ) | 11q23.3 | Exons 8 and 9 | 6% |
| Splicing factor 3B unit 1 ( SF3B1 ) | 2q33.1 | 6% | |
| Isocitrate dehydrogenase ( IDH1 and 2 ) | 2q33.3/15q26.1 | 4% | |
| Lymphocyte specific adapter protein ( LNK ) | 12q24 | Infrequent | |
| Ikaros family zinc finger 1 ( IKZF1 ) | 7p12 | Infrequent | |
| Additional sex Combs-like 1 ( ASXL1 ) | 20q11.1 | 10%–20% |
Translocation ten-eleven oncogene family member 2 ( TET2 ), located on chromosome 4q24, occurs in approximately 15% of patients with myeloid malignancies. TET2 catalyzes the oxidation of 5-methylcytosine to 5-hydroxymethylcytosine (5hmC), which are key intermediates in DNA demethylation. The resultant DNA hypermethylation affects HSC self-renewal and lineage differentiation. Hematopoietic-specific conditional deletion of Tet2 in mice led to global promoter hypermethylation, increased HSC survival, and a propensity towards chronic myelomonocytic leukemia (CMML)-like phenotype. Furthermore, the Tet2-null (Tet2−/−) background in Jak2 V617F mice augments splenomegaly and accelerates the MPN phenotype, suggestive of clonal dominance of Jak2V617F/Tet2−/− double mutants. However, Jak2V617F/Tet2−/− mice did not show increased bone marrow fibrosis or leukemic transformation and resembled chronic phase MF in humans in which TET2 mutations were associated with increasing age and the presence of JAK2 V617F did not predict disease severity or survival. Clonality studies in MPN patient samples have failed to define a consistent temporal sequence of acquisition of TET2 and JAK2 mutations. These two genetic events appear to occur independently of each other, and TET2 may even be acquired late in the progression of an MPN. Taken together, there is no evidence at present to show that restoration of TET2 activity may have disease-modifying effects in PMF.
Isocitrate dehydrogenase 1 and 2 ( IDH1/IDH2 ), located on chromosome 2q33.3 and 15q26.1, respectively, encode NADP + -dependent enzymes that catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate in the Kreb’s cycle. The neomorphic mutant IDH preferentially transforms α-ketoglutarate to 2-hydroxyglutarate (oncometabolite), the accumulation of which inhibits TET2 activity. Approximately 4% (12/301) of patients with PMF harbor IDH1/ IDH2 mutations (7 IDH2 [5 R140Q, 1 R140W, and 1 R172G] and 5 IDH1 [3 R132S and 2 R132C]) and they can co-occur with JAK2 , MPL , and TET2 . In patients with JAK2- mutated PMF, the presence of IDH1/IDH2 mutations portends a poor prognosis in terms of leukemia-free survival (LFS) and overall survival (OS). Furthermore, Idh+/Jak2 V617F+ double mutant transgenic MPN murine model demonstrated augmented blast cell expansion and differentiation arrest, consequently promoting progression to blast phase disease, thereby highlighting JAK2-IDH1/2 cooperativity in the leukemic transformation of MPN. Retrospective clinical studies have also confirmed the relatively poor outcome of this double mutant subgroup of patients on both OS and risk of leukemic transformation.
The enhancer of zeste homolog 2 ( EZH2 ) gene, located on chromosome 7q36.1, encodes a histone methyltransferase (H3K27) and is a catalytic subunit of polycomb repressor complex 2 (PRC2) required for HSC maintenance. PRC2 is a multiprotein enzyme complex (EZH2, SUz12, EED, and YY1) responsible for the trimethylation of lysine 27 on histone H3 (H3K27me3). Additionally, PRC2 can recruit other polycomb complexes, DNA methyltransferases (DNMTs), and histone deacetylases to the gene site, resulting in chromatin compaction and additional repressive activity. Frameshift mutations in EZH2 resulting in truncated protein isoforms or missense mutations within the highly conserved methyltransferase domain suggest a tumor suppressor role of EZH2. In contrast to Tet2, conditional murine knockout of Ezh2 (Ezh2−/−) in Jak2V617F background rapidly induced bone marrow fibrosis and splenomegaly. While Jak2V617F knock-in mice exhibited a PV-like disease, Jak2V617F/Ezh2−/− mice displayed decreased erythroid differentiation and expansion of megakaryocytic precursors, correlating with peripheral blood thrombocytosis. Given that EZH2 encodes an H3K27 methyltransferase, loss of EZH2 function reduced H3K27me3 levels, leading to transcriptional activation of target genes, including two proinflammatory cytokine-like mediators, S100a8 and S100a9, as well as high-mobility AT-hook 2 (HMGA2). Thus, loss of EZH2 transcriptional repression in the background of JAK2 V617F results in a more severe MPN phenotype than JAK2 V617F alone and favors progression to MF. Approximately 13% of patients with PMF harbor an EZH2 mutation, which commonly co-occurs with either JAK2 V617F or additional sex combs-like 1 ( ASXL1 ) mutation. Most of these patients exhibited a high-risk phenotype: leukocytosis, increased blast counts, and massive splenomegaly in addition to reduced LFS and OS independent of the dynamic international prognostic scoring system (DIPSS) risk category and JAK2 V617F allele burden.
ASXL1 mutation occurs in 10% to 20% of patients with PMF, predominantly in those who are triple negative. ASXL1 , located on chromosome 20q11.1, encodes an enhancer of the trithorax group (trxG) and PRC. The PRC proteins (repress) and trxG proteins (promote) regulate gene expression of homeotic genes, such as Hox genes, via histone methylation. ASXL1 mutations result in loss of PRC2-mediated H3 lysine (H3K27me3) trimethylation. Although conditional deletion of Asxl1 in murine hematopoietic stem cells resulted in progressive anemia and leukemia with multilineage dysplasia akin to human myelodysplastic syndrome (MDS) without myelofibrosis, it is of prognostic importance in PMF. The presence of ASXL1 is an independent predictor of poor prognosis, and its presence negates the favorable prognosis of CALR- mutated PMF. Additionally, the acquisition of an ASXL1 mutation (approximately 30%) during ruxolitnib therapy is associated with poor outcome at the time of discontinuation of the drug.
Ikaros is a Kruppel-like zinc finger transcription factor integral to normal hematopoiesis and is encoded by the Ikaros family zinc finger 1 ( IKZF1 ) gene located at 7p.12. Compared to chronic phase MPNs (0.2%), abnormalities in IKZF1 occur at a higher rate (21%) of patients with MPN blast phase (MPN-BP), mostly in association with del 7p. IKZF1 is believed to modulate the expression of lineage-specific genes through chromatin remodeling, which results in effective lymphoid development and tumor suppression. Induced Ikzf1 haploinsufficiency in primary murine stem cells resulted in increased Stat5 phosphorylation and cytokine-dependent growth, suggesting that reduced expression of IKZF1 is sufficient to perturb growth regulation and that IKZF1 loss is an important step in the leukemic transformation in a subset of MPN patients.
RNA spliceosome mutations such as Serine and Arginine Rich Splicing Factor 2 ( SRSF2 P95H) and U2 Small Nuclear RNA Auxiliary Factor 1 ( U2AF1 Q157) occur in 14% and 8% patients with PMF, respectively. SRSF2 mutations cluster with IDH mutations and are independently predictive of poor outcomes in terms of shortened LFS and OS. U2AF1 Q157 occurs primarily in older patients with PMF and is associated with anemia, thrombocytopenia, and inferior survival. In a large series of patients with myelofibrosis that received HSCT, the presence of U2AF1 mutation was associated with poor survival outcomes.
Frequently observed peripheral blood abnormalities in PMF are likely attributed to the EMH inherent to this disease. Abnormal CD34 + cell trafficking, preferential homing to the spleen than the marrow, and continuous proliferation of the malignant clone leads to progressive splenomegaly in PMF. The number of CD34 + hematopoietic progenitor cells constitutively mobilized into the blood is dramatically increased in patients with PMF. The number of circulating CD34 + cells in patients with PMF is 360 times higher than in healthy controls and 18 to 30 times higher than in patients with PV or ET. This striking observation led to the suggestion that the quantitation of CD34 + cells in the peripheral blood might serve as a means of discriminating PMF from other MPNs. A level of 15 × 10 6 CD34 + cells/L in peripheral blood allows differentiation of PMF from PV and ET. The numbers of circulating CD34 + cells tend to increase as the disease progresses, and there is a close correlation between patients presenting with more than 300 × 10 6 CD34 + cells/L of peripheral blood and imminent evolution to leukemia. These findings suggest that CD34 + cell trafficking abnormality in PMF is caused by the inability of these cells to be retained within the BM or their premature release into the peripheral blood.
PMF is characterized not only by the constitutive mobilization of CD34 + hematopoietic cells but also endothelial progenitor cells. Endothelial progenitor cell mobilization predominates during the prefibrotic phase of PMF, while hematopoietic stem/progenitor cell mobilization occurs characteristically in advanced PMF. This perturbed stem cell trafficking likely results in the seeding of extramedullary sites with primitive hematopoietic and endothelial cells, leading to EMH within the liver and spleen, as well as other organs.
Several proteolytic pathways play a significant role in cytokine-mediated stem cell mobilization. Proteases released by activated neutrophils cleave vascular adhesion molecule-1 (VCAM-1) expressed by stromal cells, disrupting the crucial adhesive interaction between VCAM-1 and very late antigen-4 (VLA-4) expressed by HSCs and progenitor cells. The interaction between stromal cell–, endothelial cell–, and osteoblast-derived stromal cell–derived factor-1 (SDF-1 or CXCL12) and the CXC chemokine receptor-4 (CXCR-4) expressed by HSCs and progenitor cells determine the stem cell trafficking pattern. Proteases, including neutrophil elastase, soluble matrix metalloproteinase-9 (MMP-9), and cell-bound MMP-9, have been shown to play a role in the constitutive mobilization of CD34 + cells in PMF patients. Levels of soluble VCAM-1, a degradation product of VCAM-1, are increased in the plasma of PMF patients, which correlates with the number of circulating CD34 + cells. These proteases degrade the chemokine CXCL12 that plays a role in the retention of CD34 + cells within the BM because the degradation products lack the ability to attract CD34 + cells, thereby favoring their mobilization. By contrast, the levels of intact CXCL12 within the spleen are increased, likely supporting the homing and localization of HPC/HSC to extramedullary sites such as the spleen. Furthermore, CXCR-4 expression by PMF CD34 + cells is epigenetically downregulated, which may account for altered CXCL12–CXCR-4 interactions, participating in CD34 + cell mobilization. This downregulation of CXCR4 expression can be reversed in vitro by treatment with chromatin-modifying agents. Reduced CD34/CXCR4 expression was shown to be associated with a shorter interval to development of severe anemia, massive splenomegaly, cytopenias, increased circulating CD34-positive blood cells, leukemic transformation and death.
Furthermore, CXCR4 expression was significantly lower in patients with a higher burden of JAK2 V617F (allele frequency: 75%) compared with patients with a low allele burden, suggesting a dependence upon the JAK2 variant allele frequency. Similar degrees of CD34 + cell mobilization are observed in post-ET and post-PV MF as well. Passamonti and coworkers demonstrated a relationship between the degree of constitutive mobilization of CD34 + and JAK2 V617F allele burden in patients with PV, suggesting that such mobilization may be a consequence of the transition from JAK2 V617F heterozygosity to homozygosity accompanied by granulocyte activation. Agents targeting proteases responsible for constitutive CD34 + cell mobilization may be a possible therapeutic strategy to prevent EMH in patients with PMF.
Most recently, Abdelouahab et al. explored the crosstalk between JAK2 and CXCL12/CXCR4 signaling pathways in MPN. They demonstrated that JAK2 cooperates with CXCL12/CXCR4 signaling to increase the chemotactic response of human cell lines and primary CD34 + cells through signal crosstalk with the phosphatidylinositol-3-kinase (PI3K) signaling pathway, and JAK2 inhibition abrogated CXCL12/CXCR4 signaling in primary MPN cells. These results suggest that inhibition of this signal crosstalk may further contribute to the therapeutic effect (reduction in splenomegaly) of JAK2 inhibitors.
While the cell of origin in PMF is the pluripotent HSC, BM fibrosis is secondary to BM stromal cell reaction. BM fibrosis is characterized by collagen and reticulin fibrosis composed of type III and type I collagen, respectively. While collagen fibrosis appears to be characteristic of MPNs and associated with severe disease, reticulin fibrosis may also occur in nonneoplastic conditions and does not clearly correlate with disease severity. Although the exact pathogenesis of PMF is unknown, it appears to be intimately linked with megakaryocyte proliferation, differentiation, and the elaboration of fibrogenic cytokines from the malignant megakaryocyte, its progeny.
The ability of primitive human hematopoietic stem cells to engraft sublethally irradiated immunodeficient mice is the standard surrogate in vivo assay for human HSCs. Peripheral blood (PB)-derived CD34 + HSCs from patients with PMF are capable of engrafting nonobese nondiabetic/severe combined immune-deficient (NOD/SCID) mice and generating clonal cells of multiple hematopoietic lineages. The differentiation program of PMF PB CD34 + cells in the engrafted NOD/SCID mice differed from that of healthy CD34 + cells as they produced increased numbers of CD33 + myeloid cells and CD41 + megakaryocytes but fewer CD19 + B cells. This predisposition to megakaryocyte hyperplasia was further explored by incubating CD34 + cells from patients with PMF, PV, and healthy human volunteers in the presence of stem cell factor and thrombopoietin (TPO). PMF CD34 + cells predictably induced megakaryocyte hyperplasia in vitro, and these megakaryocytes exhibited a delayed apoptotic pattern secondary to overexpressed antiapoptotic factor B-cell lymphoma-extra-large (Bcl-XL) protein. Therefore the megakaryocyte hyperplasia in PMF is likely attributed to the enhanced ability of CD34 + cells to generate megakaryocytes and the concomitant Bcl-xL overexpression, resulting in delayed apoptosis and megakaryocyte accumulation. The megakaryocyte role in the elaboration of BM fibrosis in PMF is further suggested by megakaryocytic hyperplasia with dysplastic megakaryocytes, increased circulating megakaryocytes, and megakaryocyte progenitors; by the association of BM fibrosis and acute megakaryocytic leukemia; and by the presence of MF in gray platelet syndrome, an inherited disorder of platelet α-granules. It has been hypothesized that megakaryocytes in PMF overproduce cytokines involved in collagen fibrosis, neo-angiogenesis, and osteosclerosis.
In PMF, the levels of platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) were 2.0- to 3.0-fold and 1.5- to 3.0-fold higher, respectively, than in healthy controls. However, fibroblast growth factor (FGF) levels were similar in both, suggesting that PDGF and TGF-β not only promote fibroblastic proliferation but also influence the synthesis, secretion, and degradation of extracellular matrix (ECM) components. TGF-β upregulates the expression of fibronectin and collagen types I, III, and IV involved in ECM synthesis and downregulates ECM-degrading enzymes (collagenase), resulting in ECM accumulation and progressive fibrosis. Additionally, cytokines like lipocalin 2 (LCN2) promote bone marrow fibrosis through osteoblast production. Basic FGF (bFGF) is a potent angiogenic factor and mitogen for human BM stromal cells. It may be released or leaked from dysplastic and necrotic PMF megakaryocytes or platelets. These findings suggest that bFGF may also contribute to progressive fibrosis and pronounced angiogenesis frequently observed in PMF. Investigators have suggested that impaired megakaryocyte emperipolesis might underlie this fibrogenic cytokine release mechanism, which is supported by the correlation of BM fibrosis and the degree of abnormal emperipolesis noted in PMF BM biopsies. Emperipolesis is defined as the random entry of hematopoietic cells into the cytoplasm of megakaryocytes. Impaired emperipolesis of neutrophils and eosinophils in PMF and extrusion of myeloperoxidase-positive granules by the engulfed neutrophils has been reported. Abnormal P-selectin distribution in megakaryocytes likely accounts for the selective sequestration of granulocytes by PMF megakaryocytes.
Furthermore, the elevation in TPO levels observed in patients with PMF is not due to enhanced synthesis but rather reduced expression of MPL by platelets and megakaryocytes of PMF patients, leading to decreased TPO clearance. In ET and PMF, platelets and megakaryocytes are characterized by lower MPL protein levels, and most of the receptors are immature. Endo-H–sensitive activated JAK2 promotes cell surface localization and enhances protein levels of MPL through stabilization of the mature endoglycosidase H-resistant form of the receptor. But JAK2 V617F may promote receptor ubiquitinylation and proteasomal and lysosomal degradation, resulting in reduced expression of MPL , suggesting that proteasome inhibitors or JAK2 inhibitors may restore platelet MPL levels. By contrast, persistent MPL expression by progenitor or stem cells is linked to myeloproliferation. These observations support a model where a decrease in MPL mass in platelets and megakaryocytes results in increased TPO levels, which act on the primitive stem cells, contributing to myeloproliferation.
Vannucchi and colleagues demonstrated that impaired expression of the transcription factor GATA1 might contribute to the development of myelofibrosis and sought to further define the role of megakaryocytes in the development of BM fibrosis using Gata1 low mutant mouse model. Mutations in the GATA1 functional pathway in human PMF have not been described. However, at the protein level, a large number of the megakaryocytes in the BM of PMF patients are GATA1-negative, suggesting that whatever the genetic defect leading to PMF is, it involves the pathway that affects the posttranscriptional or posttranslational modification of GATA1 in megakaryocytes.
Most recent studies have demonstrated that MPN driver mutations induce a ribosomopathy that decreases GATA1 levels in megakaryocytes, halting their maturation. Gata1 low mice showed high numbers of immature megakaryocytes and severe bone marrow fibrosis, as is seen in patients with PMF. Furthermore, treatment with an inhibitor of Aurora kinase A (AURKA), a protein overexpressed in MF megakaryocytes, rescued GATA1 expression in these cells, reducing marrow fibrosis in animal models, suggesting GATA1 as a druggable target. AURKA inhibition induced polyploidization, features of maturation, and subsequent apoptosis of megakaryocytes from primary MPN patient samples. This concept was tested with the oral AURKA inhibitor, alisertib, in a phase I trial of 25 MF patients in which forced maturation of megakaryocytes and resolution of bone marrow fibrosis in treated patients was associated with clinically significant spleen and symptom responses (NCT02530619).
Using the Gata1 low mouse model, Migliaccio and colleagues demonstrated that increased bioavailability of TGF-β plays a significant role in promoting disease progression in MF. Impaired megakaryocyte maturation coupled with high IL-8 expression, in an autocrine fashion, induces megakaryocytes to increase TGF-β, which in turn is responsible for suppressing hematopoiesis by normal HSCs (leading to bone marrow failure) and promoting an MF-HSC-supporting microenvironment in the spleen, facilitating the transition of pre-MF to overt MF. Although TGF-β inhibition completely rescued MF in Gata1 low mice, it was difficult to target TGF-β due to the lack of specificity and off-target effects. However, the TGF-β1 specific ligand trap AVID200 has made it possible to evaluate this therapeutic concept in the clinic (NCT03895112).
Intrasinusoidal hematopoiesis in the BM is a conspicuous finding in PMF. The BM vascular architecture in PMF is characterized by increased type IV collagen deposits coupled with increased BM microvessel density, resulting in increased blood flow. Vessels from patients with PMF are markedly abnormal and appear as localized vascular nests consisting of numerous short but highly branched and tortuous vessels. Increased BM microvessel density in PMF is probably mediated by α-granules of megakaryocytes as well as several angiogenic growth factors, including overexpressed bFGF and increased serum levels of vascular endothelial growth factor (VEGF). Immunohistochemical methods have confirmed that this increased BM microvessel density in PMF is correlated with increased spleen size and is an independent risk factor for OS.
Osteosclerosis is prominent in PMF, which often manifests clinically as bone pain. Osteosclerosis is a consequence of cytokines released by the neoplastic BM cells or stroma (conditioned and activated) interaction with PMF cells. PMF-associated osteosclerosis can be reversed after HSCT, restoring normal hematopoiesis. In both animal models and patients with PMF, TGF-β and stromal cell-derived osteoprotegerin, a member of the TNF receptor family, play pivotal roles in the development of osteosclerosis. Osteoprotegerin is a decoy receptor for the receptor activator of the nuclear factor kappa-B ligand (RANKL). RANKL is a transmembrane protein expressed on the cell surface of osteoblasts that can be cleaved into a soluble form by proteases. Both soluble and membrane-bound RANKL attach to RANK, a cell receptor expressed by osteoclast precursors to stimulate osteoclastogenesis. RANKL and osteoprotegerin are positive and negative regulators of osteoclast differentiation, respectively. Osteoprotegerin reduces the production of osteoclasts by inhibiting osteoclast differentiation, leading to the development of osteosclerosis. In patients with PMF, it remains unknown if the degree of osteosclerosis is reversed by normalization of levels of osteoprotegerin.
Megakaryocytes are not the only cells capable of releasing cytokines that promote BM fibrosis. Serum levels of macrophage colony-stimulating factor (M-CSF), a cytokine that regulates macrophage development and proliferation, are also elevated in patients with PMF. Monocytes and macrophages from patients with PMF engender and amplify the inflammatory cytokines TGF-β and interleukin-1 (IL-1) over those seen with healthy controls. TGF-β and IL-1 are fibroblast mitogens that promote ECM accumulation. Monocyte adhesion through CD44 appears to induce these fibrogenic cytokines by facilitating monocyte-ECM interaction. The proinflammatory transcriptional factor nuclear factor kappa-B (NF-kB) plays a pivotal role in the elaboration of IL-1 as well as in promoting PMF monocyte adhesion. Most recently, Kleppe et al. through integrated RNA sequencing and ChIP-seq data, demonstrated that the NF-kB-dependent transcriptional network fuels the inflammatory state in MPNs and this transcriptional network in PMF could be successfully targeted by inhibiting bromodomain and extra terminal (BET) proteins of the chromatin reader family and significant anticlonal synergy with combined inhibition of BET proteins and JAK2, which was notable in reversing the MF phenotype of an MPL -driven murine model of MF. This concept is being evaluated in a phase II trial of the oral pan-BET inhibitor CPI-0610 with three cohorts of monotherapy, combination therapy with ruxolitinib in inadequate ruxolitinib responders, and as upfront treatment in JAK inhibitor-naïve patients (NCT02158858). Early signs of clinical activity with spleen and symptom benefit, most pronounced in the combination arm are also coupled with anemia responses and reduction in bone marrow fibrosis in approximately one-third of treated patients.
Abnormal cytokine expression in PMF represents an inflammatory response to the disease phenotype that not only contributes to the development of BM fibrosis, osteosclerosis, and increased BM microvessel density but also PMF-associated constitutional symptoms including weight loss, anorexia, pruritus, bone pain, and night sweats. Increased levels of IL-8 and IL-2R closely correlate with constitutional symptoms, leukocytosis, requirement for RBC transfusions, as well as inferior LFS and OS. These observations evoke the possibility that mutational events leading to the malignant transformation of hematopoietic cells in PMF may also lead to the activation of transcriptional programs that promote hematopoietic cell survival and disease progression. Increased cytokine levels may, therefore, not only be responsible for the numerous epiphenomena that occur as a consequence of these malignant cells but also act on the malignant clone, affecting the proliferation and differentiation in differing microenvironments characteristic of the BM and various extramedullary sites, including the spleen. Ultimately, this may increase the risk of disease progression or leukemic transformation.
Skoda et al., through next-generation sequencing of 104 genes from a cohort of 197 MPN patients, provided insight into the clonal evolution of the clinical phenotype. Somatic mutations were observed in 90% of patient samples, in which 40% harbored mutations other than JAK2 V617F and CALR . JAK2 V617F was the most common mutation (69%), followed by CALR (15%), TET2 (12%), ASXL1 (5%), and DNMT3A (5%). The presence of two or more acquired somatic mutations portended a poor outcome and increased risk of leukemic transformation. By contrast, none of the patients with isolated JAK2 , MPL , or CALR mutations evolved to MPN-BP. However, the rate of acquisition of new mutations was low when assayed from serial samples (two mutations were detected during a total follow-up of 133 patient-years). Most mutations were present at a low allelic frequency from the time of diagnosis, and in the case of TP53 , rapid evolution to blast phase occurred with the loss of the wild-type allele either by chromosomal deletion or uniparental disomy. These data argue against the concepts of hypermutability and genomic instability in MF; however, it supports a complex clonal architecture in MPNs, where some individuals may develop a biclonal disease and others, a linear acquisition pattern.
In 50% of JAK2 V617F-mutated MPNs, the blast cells that represent the progeny of leukemia-initiating clone are JAK2 V617F-negative, suggesting that leukemia originates from a clone distinct from the JAK2 V617F-positive clone. This coexistent JAK2 V617F-negative clone appears to have a higher propensity for leukemic transformation. JAK2 V617F, therefore, does not appear to be a prerequisite for the leukemic transformation of MPNs, suggesting that additional genetic and epigenetic events are required for full transformation to occur. Single nucleotide polymorphism (SNP) array analysis has shown that genomic alterations occur increasingly at the time of blastic transformation and that no single gene or molecular pathway is sufficient to cause transformation. Acquisition of somatic mutations in TET2 , IDH , TP53 , and ASXL1 have all been implicated as genetic events leading to leukemic transformation in MF patients. In general, mutations in TET2 and DNMT3A appeared earlier in the disease course, whereas mutations in ASXL1 , EZH2 , and IDH1 were often acquired after JAK2 V617F.
A surprising correlation was observed between the phenotype of preceding MPN and the JAK2 mutational status of the leukemic blasts after transformation. In contrast to JAK2 wild-type MPN-BP in this setting, evolution to BPs with JAK2 V617F-positive blast cells is invariably preceded by the evolution of ET or PV to MF. Because of these observations, the myelofibrotic transformation of ET or PV likely represents an accelerated phase of the initial MPN preceded by genetic changes that result in evolution to MF and eventually BP. JAK2 wild-type leukemia, by contrast, usually arises in patients with chronic-phase PV or ET who have not evolved to MF. Although not universally accepted, some have suggested that these leukemias are therapy-related and are the consequence of the administration of myelosuppressive agents administered during the chronic phase. The reversion from JAK2 V617F to JAK2 wild-type in these leukemias is not due to homologous recombination. Two models have been proposed previously to account for the clonal relationship between JAK2 wild-type BP and its preceding MPN: (1) both the chronic MPN and the MPN-BP arise from a shared pre- JAK2 V617F founder clone and (2) the chronic MPN and MPN-BP arise from two independent stem cells. The model proposed by Lundberg and colleagues also reflects similar models, incorporating a newer understanding of the phylogenetic ordering of mutated JAK2 and CALR with respect to additional acquired somatic mutations ( Fig. 72.1 ). It is possible that each of these models is viable and operates in different patients.
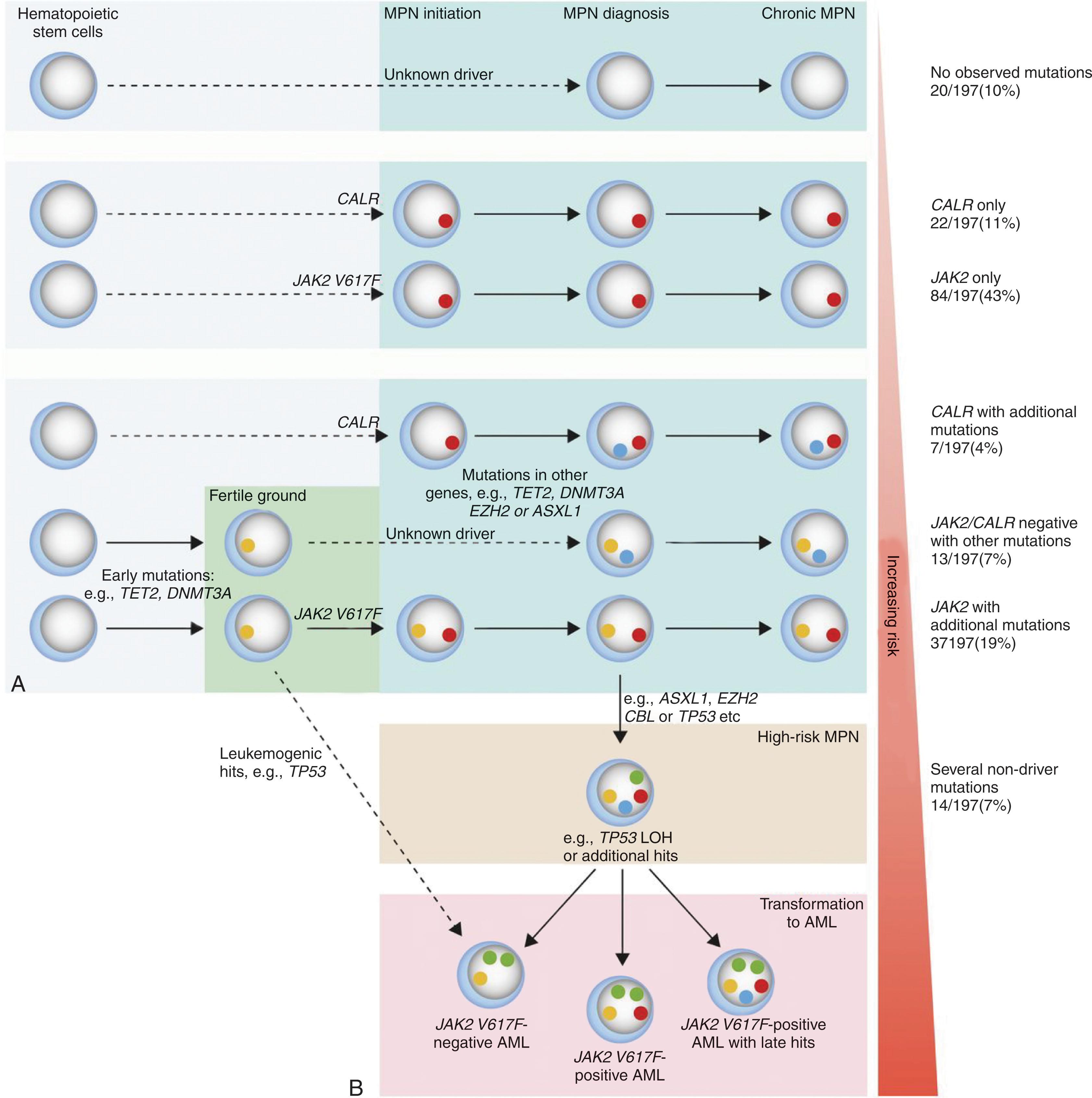
Studies have shown that triple negative patients with PMF have a shorter LFS as compared with CALR- , JAK2 -, or MPL -mutated status. Conversely, CALR-mutated patients are at a lower risk of leukemic transformation compared with triple negative and JAK2 -mutated cases but not with MPL -mutated cases. Optimally, patients should receive a HSCT before the leukemic transformation, as MPN-BP is notoriously resistant to conventional remission induction strategies employed in de novo AML. The median survival after blast transformation is a meager 2.6 months, owing to the patient’s age and aggressive biology of this form of leukemia.
Table 72.2 lists the symptoms and physical findings of patients with PMF at presentation.
| Symptom or Finding | Incidence (%) |
|---|---|
| Asymptomatic | 16–30 |
| Fatigue | 47–71 |
| Fever | 5–15 |
| Weight loss | 7–39 |
| Night sweats | 6–21 |
| Symptoms due to enlarged spleen | 11–48 |
| Bleeding | 5–20 |
| Gout or renal stones | 6–13 |
| Pallor | 60 |
| Petechiae or ecchymoses | 15–20 |
| Splenomegaly | 89–99 |
| Hepatomegaly | 39–70 |
| Peripheral edema | 13 |
| Evidence of portal hypertension | 2–6 |
| Lymphadenopathy | 1–10 |
| Jaundice | 0–4 |
Approximately 25% are asymptomatic and seek evaluation for incidental splenomegaly or an abnormal blood picture detected on a routine medical exam. Symptomatic patients present with symptoms related to cytokine release or splenomegaly. Cytokine-related symptoms include fatigue, fever, night sweats, bone pain, weight loss, sexual dysfunction, insomnia, difficulties in concentration, and reduced Quality of Life (QoL). Splenomegaly-associated symptoms include a dull, heavy sensation in the left upper quadrant, early satiety, diarrhea secondary to splenic pressure on the intestines or symptoms due to a splenic infarct (left-sided abdominal pain radiating to the left shoulder and fever).
Arterial or venous thromboses occur variably in PMF (10% to 12%). Venous thromboses include cerebral venous sinus thrombosis, splanchnic vein thrombosis, deep venous thrombosis, and pulmonary thromboembolism, while arterial thromboses include stroke, transient ischemic attacks, retinal artery occlusion, myocardial infarction, angina pectoris, and peripheral arterial disease. Established risk factors for thrombosis include the cellular phase of PMF, thrombocytosis (platelet count >450 × 10 9 /L), presence of cardiovascular risk factors, Hb greater than 11 g/dL, and JAK2 V617. The prefibrotic phase of MF (pre-PMF), as defined by WHO 2016 criteria, is associated with a higher thrombotic risk than that associated with ET. In pre-PMF, arterial events occurred at a rate of 1.7% patient-year as compared to venous events of 0.6% patient-year. Although pre-PMF behaves like ET and is managed similarly, retrospective studies have shown that survival of patients with pre-PMF is inferior to that of ET but better than patients with overt PMF. Therefore it is essential to distinguish between ET and pre-PMF in order to inform the patients of their prognosis.
Bleeding diatheses in patients with PMF could be either due to a qualitative or quantitative platelet defect with a heterogeneous clinical presentation, from minor petechiae/ecchymoses to life-threatening bleed, including variceal or intracranial bleed. Thus far, no definitive risk factors have been identified to predict bleeding in patients with PMF except severe thrombocytopenia and excessive thrombocytosis, leading to an acquired form of von Willebrand disease. Patients with pre-PMF are at an increased risk of bleeding when compared to ET and bleeding events occurred at the rate of 1.39 and 0.79 patient-year, respectively. Independent predictors of bleeding in pre-PMF include previous hemorrhage, leukocytosis, an acquired form of von Willebrand disease, and aspirin therapy.
Massive splenomegaly is a prominent clinical finding that occurs in 25% to 50% of patients with PMF, which may be attributed partially to EMH. Palpable hepatomegaly is observed in 40% to 70% of the patients. Non-hepatosplenic EMH can occur in the pulmonary, central nervous, gastrointestinal, and genitourinary systems. The symptoms vary depending on the location of EMH: cough, headache, or paralysis resulting from “brain tumors or spinal cord tumors,” small-bowel obstruction, or intractable ascites. Ascites may be due to either peritoneal or mesenteric implants of EMH or portal hypertension, and if secondary to peritoneal EMH, the exudative ascitic fluid frequently contains myeloid, erythroid, and megakaryocytic elements.
Portal hypertension may be secondary to increased splanchnic blood flow due to splenomegaly and/or intrahepatic obstruction associated with EMH. Complications include ascites or esophageal varices that occur in 9% to 18% of patients with PMF. Occasionally, cirrhosis or evidence of portal or hepatic vein thrombosis has been reported. Nodular regenerative liver hyperplasia occurred in 14.6% of cases and correlated closely with portal vein lesions.
Patients with PMF may sometimes present with documented evidence of pulmonary hypertension (progressive dyspnea, signs of biventricular heart failure, elevated pulmonary artery pressures noted in right-heart catheterization), and acutely worsening hepatosplenomegaly. Many succumb to cardiopulmonary complications within 18 months of their initial diagnosis of pulmonary artery hypertension. Pulmonary artery hypertension in this patient subset is likely secondary to chronic thromboembolic disease, EMH within the lung, and elaboration of fibrogenic cytokines from dysfunctional circulating megakaryocytes, or portal hypertension. Patients with primary pulmonary hypertension may have abnormal blood counts (anemia and thrombocytopenia) and BM fibrosis. They can be distinguished from those with PMF by the lack of high levels of circulating CD34 + cells, leukoerythroblastosis, and absence of driver mutations ( JAK2 V617F, CALR , or MPL ).
Renal EMH is uncommon and may present a challenge for diagnosis. Renal EMH manifests mostly as renal dysfunction and proteinuria/nephrotic syndrome. The proteinuria is caused by concurrent glomerular lesions, which is present in most cases of renal EMH. Such lesions include focal segmental glomerulosclerosis, chronic thrombotic microangiopathy, and fibrillary-like glomerulonephritis. Immune complex deposition with subepithelial electron-dense deposits caused by immune dysfunction of PMF is proposed as a pathogenetic mechanism. Renal EMH may be misdiagnosed as tubulointerstitial nephritis due to the presence of eosinophils and their precursors. EMH should be suspected in cases of kidney masses or enlargement in the setting of renal dysfunction or proteinuria
EMH can rarely involve the skin, manifesting as nontender, pruritic red, pink, or violaceous plaques, papules, or hemangioma-like nodules. These dermal infiltrates are composed of combinations of myeloid, erythroid, and megakaryocytic cells. Occasionally, a Sweet syndrome-like picture may precede an overt PMF diagnosis.
Acute monoarticular inflammation caused by secondary gout related to increased cell turnover is seen in 6% of patients. Other physical findings include pallor, icterus, signs of cachexia, moderate lymphadenopathy, peripheral edema, and bony tenderness.
Careful examination of the peripheral blood smear and BM ( Fig. 72.2 ) permits a ready diagnosis of PMF. Leukoerythroblastosis with teardrop RBCs (i.e., dacryocytes) strongly suggests this diagnosis. Leukoerythroblastosis is characterized by the presence of nucleated RBCs and immature myeloid elements in 96% of cases. Megathrombocytes and megakaryocytic fragments are frequent findings. The reduction in the number of dacryocytes, postsplenectomy, or chemotherapy initiation has led some to invoke splenic fibrosis as an etiology.
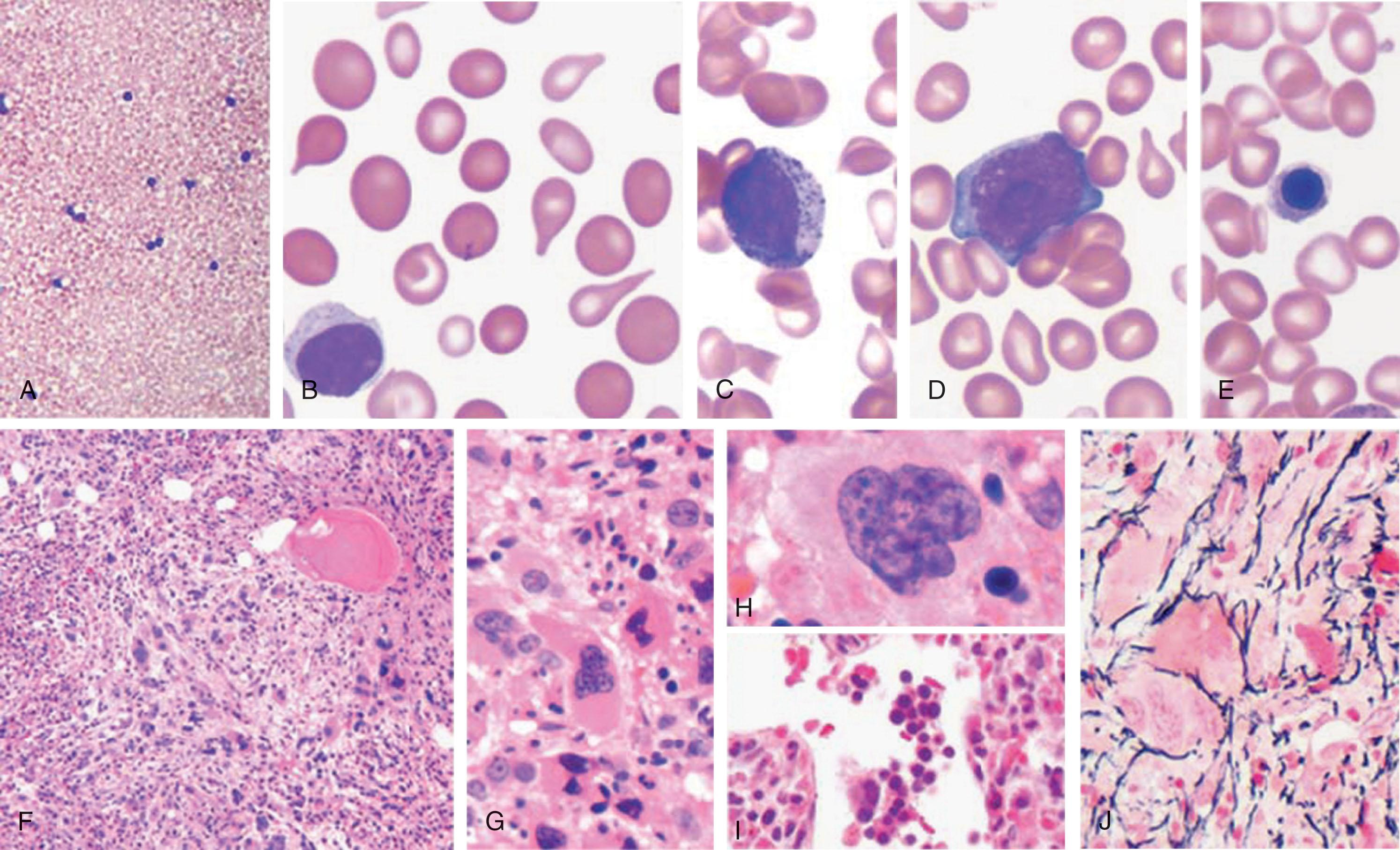
In approximately 60% of patients, hemoglobin levels drop to less than 10 g/dL. The degree of anemia is frequently challenging to estimate through conventional hemoglobin or hematocrit determinations because of dilutional anemia secondary to plasma volume expansion due to massive splenomegaly. Most patients have normochromic, normocytic, and RBC indices. The anemia is a combination of decreased production due to erythroid hypoplasia or ineffective erythropoiesis and shortened RBC survival. Hemolytic anemia in PMF is multifactorial, including hypersplenism, RBC membrane defects resembling paroxysmal nocturnal hemoglobinuria (see Chapter 32 ), or antierythrocyte autoantibodies. About 5% of patients with PMF may develop iron deficiency anemia secondary to blood loss, either due to GI bleed (variceal bleed or duodenal ulceration), or intravascular hemolysis. Occasionally, a patient with PMF may develop an occult malignancy or a site of EMH within the gastrointestinal tract, which may serve as a bleeding source. Unexplained microcytosis (mean corpuscular volume <80 fL) has been reported in PMF and has not been shown to have prognostic relevance. Macrocytic anemia may complicate PMF. Although folic acid absorption is normal, folic acid deficiency may result from increased cell turnover.
Leukopenia can occur in 13% to 25% of patients, and leukocytosis is seen in one third. Occasional blast cells and granulocytes with the pseudo–Pelger-Huët anomaly are frequent findings. The leukocyte alkaline phosphatase (LAP) score is high in more than half of patients, but low in about one-third, and therefore does not add diagnostic value.
Platelet counts of less than 100,000/mm 3 are observed in 31% of patients, and platelet counts of more than 800,000/mm 3 have been observed in 12%. In pre-PMF, almost 90% of patients had platelet counts higher than 500,000/mm 3 and may develop acquired von Willebrand disease, often posing a bleeding risk. Defective platelet function is common, and platelets frequently do not respond to collagen or epinephrine stimulation. A variety of qualitative platelet anomalies have been documented as abnormal using automated platelet function analyzers such as the platelet function analyzer PF100 or light transmission platelet aggregometry. In 15% of patients, features of ongoing DIC are found, including decreased platelet counts, factor V and VIII, and increased fibrin split products. DIC in PMF is rare but may occur after an invasive surgical procedure.
Additional laboratory abnormalities are quite common. Hyperuricemia frequently occurs as a result of increased cell turnover. In one series, nonspecific elevated levels of lactic acid (95%), bilirubin (40%), alkaline phosphate, and serum glutamic oxaloacetic transaminase (50%) were observed. Patients with PMF have decreased levels of total cholesterol, likely secondary to inflammation and cachexia. The ratio of high-density lipoprotein cholesterol to low-density lipoprotein cholesterol is low.
A variety of immunologic abnormalities have been reported in PMF, including the presence of ANAs, elevated titers of rheumatoid factor, direct Coombs test positivity, lupus-type circulating anticoagulants, hypocomplementemia, BM lymphoid nodules, and increased circulating immune complexes. In one series of 50 patients with PMF, increased quantities of circulating immune complexes were detected in 39% and found to be associated with increased disease activity as manifested by increased transfusion requirements, bone pain, and fever. Some investigators have suggested that abnormalities of the complement system may be significant in the disease progression of PMF, and others have hypothesized that low levels of C3 may predispose these patients to develop serious bacterial infections.
A remarkably high incidence of monoclonal gammopathies has been reported in PMF, with some series reporting 8% to 10% of benign gammopathies. Several case reports mention concurrent plasma cell or B-cell dyscrasias with PMF.
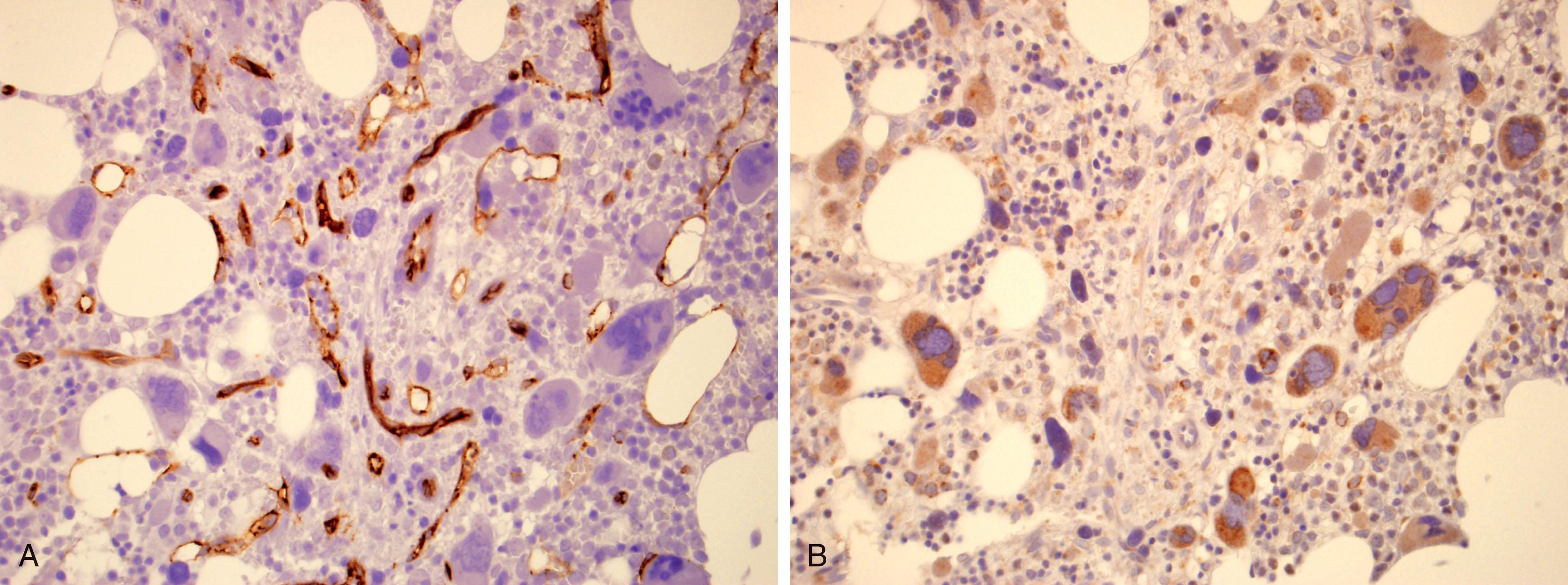
Successful BM aspiration is unusual, accomplished only in 5% to 10% of cases, with a dry tap in 50% of cases. A BM biopsy is an absolute diagnostic requirement for PMF to determine the presence of atypical megakaryocytes, myeloid hyperplasia, assess the amount of residual hematopoietic cellular tissue, and establish the degree of BM fibrosis. BM biopsies in PMF are typically hypercellular with atypical megakaryocyte morphology and myeloid hyperplasia. The atypical features of the megakaryocytes are paramount in establishing the diagnosis of PMF. Such features include hyperchromasia, high nuclear to cytoplasmic ratio, cloud-like nuclear contour, and tight clustering. Naked megakaryocytic nuclei and open sinuses are fairly common, especially in advanced stages. Although BM fibrosis is expected, prefibrotic PMF lacks this finding (see below). BM fibrosis and osteosclerosis were seen in 67% and 54% of cases, respectively. The characteristic morphologic features include patchiness of hematopoietic cellularity and reticulin fibrosis, with some microscopic fields being cellular and others void of hematopoietic cells. The amount of reticulin may vary from field to field. Additionally, BM biopsies reveal a substantial increase in vascularity. BM microvessels are more tortuous and branched, and the increased microvessel density correlates with increased VEGF expression by megakaryocytes ( Fig. 72.3 A and B ).
A rare histologic variant, MF with fatty BM , is characterized by myeloid hypoplasia associated with fairly complete fat substitution, mimicking the BM of aplastic anemia. These patients frequently have areas of clusters of densely aggregated hematopoietic elements exhibiting the histopathologic characteristics of PMF and large numbers of hematopoietic progenitors circulating in their peripheral blood. This variant of PMF is likely caused by the abnormal trafficking of hematopoietic cells from the BM to extramedullary sites, consistent with the osteosclerosis observed in patients with PMF, increased thickness of some bone units with new lamellae, and focal areas of woven bone. There is a net decrease in osteoclast number and conversion of trabecular pillars into plates.
Currently, pre-PMF is recognized as a distinct type of MPN that is characterized by the absence or presence of minimal BM reticulin fibrosis. Pre-PMF may pose a diagnostic challenge, as clearly differentiating overt PMF from ET may be difficult. However, the myeloid hyperplasia and the tight clustering of megakaryocytes are quite specific for pre-PMF. Progressive fibrosis is frequently observed in such patients as the disease evolves. Based on a careful histomorphometric evaluation of the BM, a progressive fibro-osteosclerotic process paralleled by an increase in numbers of small megakaryocytes with irregular perimeters and megakaryocytes with naked nuclei has been observed during disease evolution. The clinical and morphologic findings of patients with the pre-PMF have been further characterized. Although a steady progression to BM fibrosis has been demonstrated in patients with the prefibrotic phase, fibrosis may remain static or diminish in the more advanced stages of PMF.
Different pathological scoring systems have been used for grading BM cellularity and fibrosis, with the aim of staging and documenting the progression of the disease. The European Consensus Criteria recognized the importance of age-dependent decrease in cellularity ( Table 72.3 ). Grading of MF was simplified using four easily reproducible categories, including differentiation between reticulin and collagen. A consensus was reached that the density of fibers must be assessed in relation to the hematopoietic tissue, which is especially important to avoid a false impression of reduced fiber content in fatty or edematous BM samples after treatment. The progression of BM fibrosis in PMF is accompanied by the expression of subsets of collagenases that is independent of JAK2 V617F status.
| Grading | Description a |
|---|---|
| MF-0 | Scattered linear reticulin with no intersections (crossovers) corresponding to normal bone marrow |
| MF-1 | Loose network of reticulin with many intersections, especially in perivascular areas |
| MF-2 | Diffuse and dense increase in reticulin with extensive intersections, occasionally with focal bundles of collagen, focal osteosclerosis, or both |
| MF-3 | Diffuse and dense increase in reticulin with extensive intersections and coarse bundles of collagen, often associated with osteosclerosis |
a The quality of the reticulin stain should be assessed by detection of normal staining in vessel walls as internal control. The degree of myelofibrosis should be assessed by disregarding lymphoid nodules and vessels and by disregarding fibers framing adipocytes. Areas of prominent scleredema or scarring should be included in the overall grading of myelofibrosis. Fiber density should be assessed in hematopoietic areas.
Morphologic examination of the spleen frequently reveals follicular atrophy in the white pulp ( Fig. 72.4A ) with foci of EMH in the sinusoids of the red pulp (see Fig. 72.4B ), where megakaryocytes, myeloid elements, and nucleated erythroid elements are seen. The extramedullary hematopoietic cells belonging to each of the myeloid lineages can be distributed in the spleen diffusely or limited to macro nodules. The predominance of immature granulocytic forms is associated with a dismal prognosis. Pathologic examination of the liver reveals hematopoietic cellular elements within the sinusoids. Sinusoidal dilatation is a common finding, as well as prominent intrahepatocyte and Kupffer cell hemosiderin deposition. A marked increase in the hepatic reticulin network has also been observed.
Approximately 50% of patients with PMF harbor karyotypic abnormalities at the time of diagnosis. It is essential to perform appropriate cytogenetic analyses to exclude rare cases of CML with associated BM fibrosis. Because of BM fibrosis, it is often difficult to obtain an optimal number of metaphase cells for cytogenetic analysis. In the past, a substantial percentage of patients with PMF had a “dry tap” or were uninformative, which made the cytogenetic analysis challenging. Although cytogenetic analysis of PMF remains time-consuming and laborious, chromosome studies are now informative in 100% of patients from unstimulated peripheral blood specimens. This success is due to the presence of large numbers of circulating immature mitotic hematopoietic cells (including CD34 + cells) combined with the use of an MPN specific interphase FISH panel and array CGH+SNP that allows for identification of recurrent and novel genomic changes in virtually all patients with MPN ( Fig. 72.5 ). There is a high concordance rate (92%) between conventional cytogenetics and interphase FISH for the 12 most frequent chromosomal abnormalities detected in PMF.
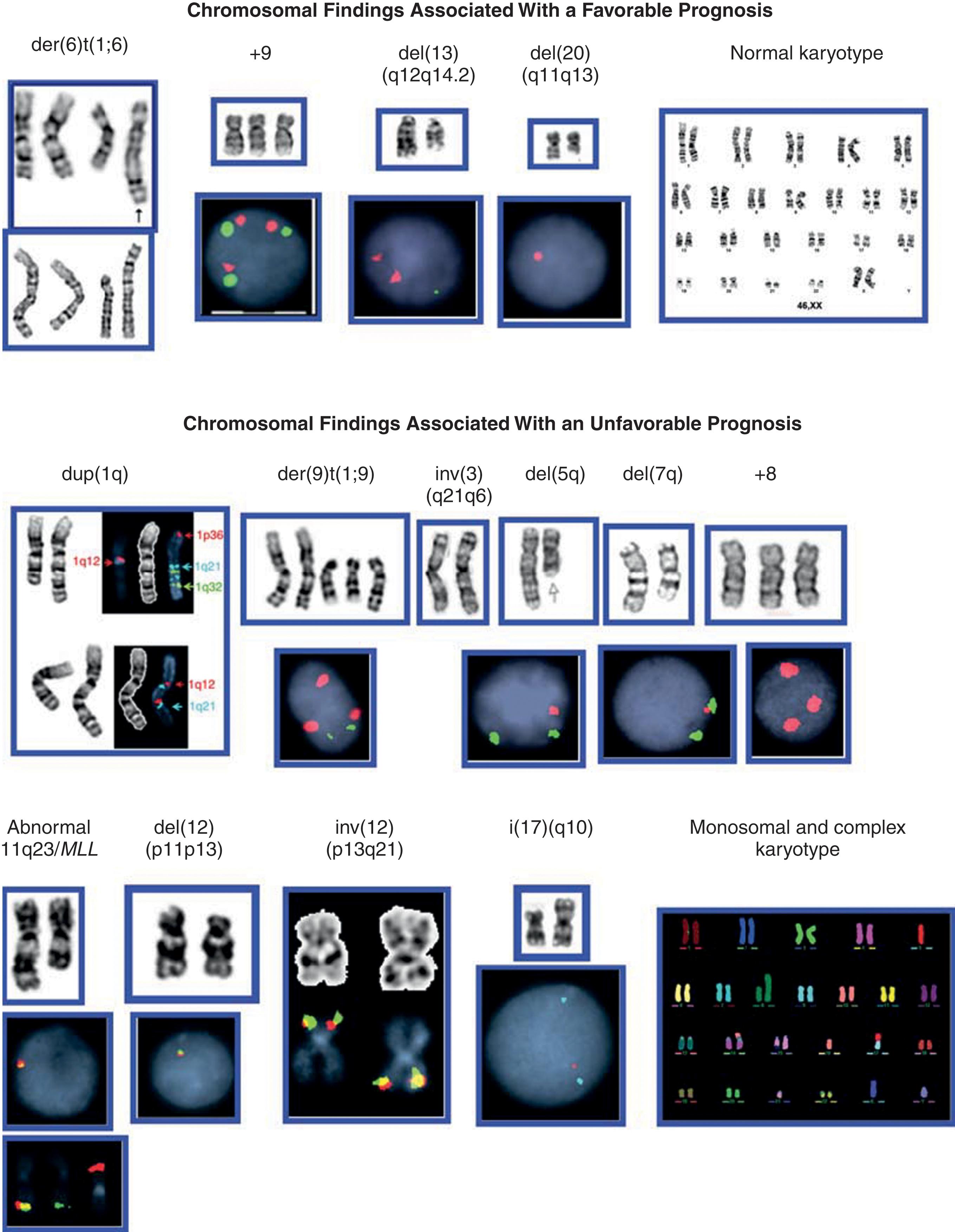
Among the Philadelphia chromosome-negative MPNs, PMF has the highest rate of chromosomal abnormalities at diagnosis. Deletions of the long arms of chromosomes 13 and 20, trisomy 8, and abnormalities of chromosomes 1q, 7q, and 9p constitute more than 80% of all chromosomal abnormalities detected in PMF (see Fig. 72.5 ), although none is specific to PMF. The deletion of the long arm of chromosome 13 is more frequent in PMF than in PV. Fine FISH mapping has defined the commonly deleted region as 13q13.3–q14.3, encompassing RB1 , D13S319 , and D13S25 loci. As mentioned in Chapter 70 , del (20q) and +9/+9p are more frequent in PV than in PMF. Multiple copies of 9p result in trisomy/tetrasomy or amplification of JAK2 , and each of these abnormalities has been reported in PMF. A 2.7-Mb region on chromosome 20 spanning D20S108 (proximal) and D20S481 (distal) is deleted in all Philadelphia chromosome-negative MPNs. A different region is deleted in other myeloid malignancies, but a common 1.6-kb region may constitute the major site responsible for the loss of heterozygosity.
With disease progression from PV/ET to MF, the frequency of CGA is increased up to 100%. Longitudinal studies over a period of 16 years have demonstrated that an increased number of cells with +1q is associated with disease progression. Moreover, loss of 17p13, which leads to a deletion of TP53 in patients with +1q, is associated with perturbations of the TP53 pathway, which contributes to PMF progression. The chromosomal abnormalities observed are similar to those seen at diagnosis of PV/ET or PMF, but through subclonal evolution, they may become very complex. The number of genomic alterations is two or three times greater in the blast phase than it is in the chronic phase. Specific regions on 1q ( MDM4 ), 12p ( ETV6 ), 17p ( P53 ), and 21q ( RUNX1 ) are frequently altered and associated with disease progression. The use of comparative genomic hybridization techniques suggests that genomic aberrations are much more common than what has been previously indicated by conventional cytogenetic analysis and occur in the majority of cases. Even among cytogenetically normal patients with PMF and array CGH+SNPs, show at least one genomic lesion in 87% of patients. 9p cnLOH occurs in approximately 18% of PMF patients while a gain of 9p and/or cnLOH 9p is present in 88% of cytogenetically normal patients with de novo PMF suggesting that genes located on 9p may play a crucial role in the pathogenesis of PMF. In the majority of patients, CGAs are detected in cells that harbor driver mutations, and single cell analysis has demonstrated that an increased frequency of chromosomally abnormal colonies in JAK2V617 homozygous progenitor cells may be due to a hyper-recombination phenotype induced by JAK2V617F . Others have reported that chromosomal abnormalities such as del(20q), +8, +9 can occur before acquisition of JAK2V617F indicating that clonality can also precede acquisition of JAK2V617 .
Mutational analysis is invaluable in the diagnosis and prognostication of PMF. In PMF, the proportion of patients with JAK2 V617F in granulocytes has been reported to range from 35% to 95%. The detection rate for JAK2 V617F is much higher for patients with post-PV MF (91%) than PMF (45%) or post-ET MF (39%). In PV, a high allele burden of the JAK2 V617F has been associated with an increased risk of evolution to MF. Such differences in allele frequencies are attributed to the variable sensitivity of the diagnostic techniques and differences in the case-mix of the reported series (i.e., the proportion of primary PMF and secondary (SMF) cases). JAK2 V617F PMF is associated with older age at diagnosis and a history of thrombosis and pruritus. A common JAK2 germ-line haplotype (46/1) that is identified by the rs12343867 SNP was found to influence susceptibility to developing PMF regardless of JAK2 mutational status.
Gain-of-function mutations of MPL, MPL W515L , and MPL W515K are present in approximately 5% of patients with PMF, 1% with ET, but none with PV. MPL mutations may occur concurrently with JAK2 V617F, suggesting that these alleles may have functional complementation in MPN. In all cases, MPL W515K/L VAF is in excess of the JAK2 V617F VAF. The molecular profile at the time of leukemic transformation of PMF is distinct with a predominance of epigenetic and RNA splicing mutations ( TET2 , IDH1/IDH2 , ASXL1 , and SRSF2 ) as opposed to de novo AML in which mutations in the signaling receptor tyrosine kinases ( KIT and FLT3) are common.
The number of circulating cells expressing the CD34 antigen, a phenotypic marker of hematopoietic stem, progenitor cells, and endothelial cells, in patients with PMF is reported to be ≥300-times higher than healthy controls and 18- to 30-times higher than in patients with PV or ET. The clinical utility of the cytofluorimetric measurement of CD34 + cells as a diagnostic marker of PMF is hampered by the observation that a small number of subjects with PMF exhibit a normal number of CD34 + cells in the peripheral blood. Cases with very mild disease or absent or slight reticulin BM fibrosis account for the majority of such patients. High values of CD34 + cells (>200 × 10 6 /L) have been proposed as an indicator of an accelerated phase of the disease.
The characteristic radiographic features of PMF include a diffuse increase in bone density and increased prominence of the bony trabeculae. This increased bone density may be patchy and can produce a mottled appearance. Such abnormalities have been reported in 25% to 66% of patients with PMF. Noninvasive imaging of BM is a promising means of evaluating the BM cellularity and distribution in PMF. Magnetic resonance imaging (MRI) can portray the conversion or reconversion of fatty to cellular BM. Fibrotic BM is easily distinguished from cellular BM by its strikingly low signal intensity with all pulse signals. The BM patterns in the proximal femurs of PMF patients are reported to correlate with the clinical severity of the disease. BM MRI has been used to differentiate PMF from ET, where the BM adipose tissue is preserved, but not so in PMF.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here