Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Enhanced recovery after surgery (ERAS) pathways, introduced more than 25 years ago, represent a paradigm shift in perioperative care that involves integration of evidence-based, multimodal, multidisciplinary interventions that mitigate the undesirable effects of the surgical stress response. Traditional perioperative care has been fragmented with surgeons and anesthesiologists practicing in silos with significant variability in care. Implementation of ERAS pathways have the potential to limit this variability and improve patient safety. In addition, perioperative care pathways reduce postoperative complications and shorten hospital length of stay without increasing postdischarge readmission rates. With reduction in hospital length of stay, ERAS pathways have allowed migration of some surgical procedures from the inpatient setting to the outpatient setting. The ultimate aim of ERAS pathways is to improve patient-reported outcomes, including return to activities of daily living, and to address psychosocial issues (e.g., anxiety, depression, cognitive dysfunction) and enhance patient satisfaction.
Recognition of the generalizability of the ERAS interventions and transferable improvements in postoperative outcomes led them to becoming the standard of care for several surgical procedures. Although initially the ERAS pathways were implemented in relatively healthy patients undergoing elective surgical procedures, there is evidence of potential benefits in high-risk patients (e.g., older and sicker patients with low functional reserve) and those undergoing emergency surgery. Thus, ERAS principles should be applied for all surgical patients.
Typically, an ERAS pathway includes approximately 15 to 20 interventions (also referred to as elements or components ) divided into three distinct phases: preoperative, intraoperative, and postoperative periods ( Table 1 ). Several studies have reported correlation between the overall compliance with ERAS elements and reduced postoperative complications and hospital length of stay. Several elements have now become the standard of care (e.g., preoperative optimization of comorbid conditions, minimal preoperative fasting duration, maintenance of normothermia, intraoperative antibiotic prophylaxis, and venous thromboembolism [VTE] prophylaxis). Also, some of the initially recommended elements (e.g., avoidance of preoperative mechanical bowel preparation, preoperative complex carbohydrate loading, and use of epidural analgesia in minimally invasive procedures) lack definitive evidence and may not be valid in the current rapidly changing perioperative care practices.
| Preoperative |
|
| Intraoperative |
|
| Postoperative |
|
Although the relative contribution of individual interventions is not clear, the use of a minimally invasive surgical approach is one of the core elements as it is associated with a reduced surgical stress response. In addition, avoidance of drains, nasogastric tubes, and urinary catheters is also critical. Furthermore, postoperative elements such as early oral intake and mobilization appear to influence outcomes the most. Thus, interventions that facilitate oral intake and mobilization such as absence of nausea and vomiting, optimal pain control using an opioid-sparing strategy, and avoidance of fluid overload play a major role in enhancing recovery. For the ambulatory setting, procedure and patient selection is one of the key elements that influence perioperative outcome.
Overall, compliance with ERAS pathways may be improved through strong clinical leadership with the desire and ability to change and employment of updated evidence-based pathways.
Because a patient’s preexisting comorbidities can influence postoperative outcomes and quality of recovery, preoperative identification and optimization of comorbid conditions has become the standard of care. Risk stratification can be used to identify patients at high risk for developing postoperative complications and implement specific preoperative interventions to modify perioperative care. For example, assessment and treatment of preoperative anemia, malnutrition, and frailty have been shown to influence postoperative outcomes and hospital length of stay, particularly in high-risk patients undergoing major invasive surgical procedures. Modern preoperative clinics promote global optimization through patient engagement and interdisciplinary collaboration between preoperative anesthesia and surgical teams to achieve shared decision making and better compliance with ERAS components. Ideally, upon scheduling the surgery, the surgeon’s office should refer the patient to this multidisciplinary preoperative clinic.
Prehabilitation is a paradigm shift in preoperative care, which includes cardiopulmonary conditioning, muscle strengthening, and malnutrition correction. Prehabilitation has been reported to reduce frailty and improve postoperative functional status, and consequently shorten hospital length of stay. Prehabilitation allows patients to become engaged in their care. Optimal patient- and procedure-specific approaches are being developed, but their practical application remains challenging because of the need for delaying surgery and cost implications.
Patient and family education and counseling is arguably one of the critical elements. Involving the patient in preoperative decision making, preferably using decision aids, improves transparency, reduces patient anxiety, and can help provide patient-centered care. Psychological preparation and feeling of partnership in perioperative care can optimize patients’ behavioral recovery and has been shown to positively influence postoperative pain levels, early mobilization, and hospital length of stay. Importantly, patient education should include not only the information on the perioperative course and what the patient can expect, but also postoperative goals that must be achieved and factors that must be met for enhanced recovery. It is necessary to emphasize the need for patient participation and instill a sense of responsibility by clearly stating what is expected from the patient. Development of realistic expectations and increased co-operation on the part of the patient facilitates the recovery process.
Prolonged preoperative fasting is frequently associated with hypovolemia, electrolyte imbalance, and hypoglycemia. Fasting can induce a metabolic response that can cause insulin resistance. Although avoidance of prolonged fasting has been emphasized, there is evidence that patients fast for longer than the recommended duration. Current guidelines recommend clear liquids until 2 hours before surgery; however, patients do not even consume water after the evening meal. Thus, patients should be given clear instructions to consume water during the fasting period. For example, patients could be encouraged to drink water before going to bed and just before leaving home on the day of surgery. In fact, recent evidence suggests that the fasting guidelines for clear liquids (i.e., water intake) must be liberalized, and patients should be allowed to drink water if they are thirsty in the preoperative period.
Surgical stress response can cause insulin resistance resulting in hyperglycemia, poor glucose uptake, and muscle degradation, which can delay recovery. Basic science and low-quality clinical evidence suggest that carbohydrate loading (at least 45 g of a complex carbohydrate drink) 2 hours before surgery reduces insulin resistance and is associated with a small reduction in hospital length of stay. However, recent meta-analyses conclude that there are no benefits from preoperative carbohydrate loading compared with placebo in terms of postoperative complication rate and hospital length of stay. Furthermore, there is concern for hyperglycemia and associated adverse outcomes in diabetic patients. Overall, preoperative carbohydrate administration adds unnecessary healthcare costs. Nevertheless, administration of simple cheaper sports drinks with carbohydrates and electrolytes 2 hours before surgery may improve patient satisfaction by reducing preoperative thirst and hunger, although evidence for this is currently lacking.
Avoidance of mechanical bowel preparation in patients undergoing colonic surgery has been recommended for enhanced recovery after surgery. However, meta-analyses of randomized controlled trials and observational trials in elective colorectal surgery found no differences in postoperative complications (e.g., anastomotic leak, surgical site infection, intraabdominal collection, mortality, reoperation, and hospital length of stay) between mechanical bowel preparation and no preparation. Oral antibiotic preparation, either in combination with mechanical bowel preparation or alone, has been reported to reduce postoperative complications in elective colorectal surgery. Overall, the routine use of preoperative mechanical bowel preparation is not recommended in colonic surgery except, based on limited evidence, for patients undergoing an anterior resection with diverting stoma.
Several enhanced recovery pathways recommend the use of alvimopan, a peripheral opioid antagonist, as it has been reported to accelerate gastrointestinal recovery, reduce postoperative ileus, and shorten hospital length of stay. However, the studies reporting such benefits have several limitations including significant heterogeneity with regard to the surgical type and approach as well as perioperative care. Importantly, all of the studies reporting benefits of alvimopan used very high opioid doses in the perioperative period. Thus, the role of alvimopan in current clinical practice that involves an opioid-sparing approach remains controversial.
For ambulatory surgery to be safe and efficient, careful selection of procedures and patients is crucial. Patient selection is a dynamic process that depends on the interaction among the surgical procedure, the patient’s comorbid conditions, and anesthetic technique as well as social factors and the ambulatory setting (i.e., hospital-based ambulatory center, short-stay [23-hour] facility, or free-standing ambulatory surgery center). It is generally accepted that patients with a high burden of comorbid conditions, specifically those with poorly stabilized medical conditions (i.e., American Society of Anesthesiologists [ASA] physical status >3) are not suitable for ambulatory surgery, particularly if the surgical procedure requires administration of general anesthesia. Examples of patients considered ineligible (i.e., ASA physical status 4) include recent (<3 months) new-onset, unstable, or severe angina, myocardial infarction, coronary stents, new-onset or decompensated heart failure, severe valve dysfunction, high-grade atrioventricular block, cerebrovascular disease (transient ischemic attacks/cerebrovascular accident), acute respiratory disease, and end-stage renal disease not undergoing regular dialysis. Other factors that influence decision making include bleeding disorders and use of antiplatelet and anticoagulant drugs. Factors such as age, weight, and presence of sleep-disordered breathing (e.g., obstructive sleep apnea) should not be considered in isolation to determine suitability for ambulatory surgery. The best approach is for the entire perioperative team to develop procedure-specific inclusion/exclusion criteria. This will avoid unnecessary cancellations on the day of surgery.
Although anesthesia-related mortality is extremely low, intraoperative care can influence short-term and long-term morbidity. The residual effects of drugs used to provide general anesthesia (i.e., hypnotic-sedatives, neuromuscular blocking agents, and opioids) can influence 30-day readmission rates. Because the drugs used during general anesthesia have additive or synergistic effects, a minimal number of drug combinations should be used. Also, when possible, drugs should be short-acting and should be administered at the lowest possible doses. The superiority of choice of inhalation anesthesia versus total intravenous (IV) anesthesia is unclear, even in high-risk populations (e.g., cancer surgery patients). Similarly, the role of opioid-free anesthesia (i.e., complete avoidance of intraoperative opioids) remains controversial. In fact, there is concern that the analgesic adjuncts (e.g., ketamine, dexmedetomidine, lidocaine, magnesium infusions) used in an opioid-free anesthesia technique may be detrimental to recovery.
The use of lung protective ventilation strategies (i.e., tidal volumes 6–8 mL/kg, ideal body weight with positive end expiratory pressure [PEEP] 5–10 cm H 2 O) have been shown to reduce postoperative pulmonary complications and thus have become the standard of care. In addition, end-tidal carbon dioxide (ETCO 2 ) values should be maintained around 40 mm Hg, rather than the traditional 30 to 35 mm Hg, as they improve tissue and organ perfusion.
Goal-directed hemodynamic management, which aims to maintain adequate perfusion pressure and oxygen delivery, is imperative in maintaining organ function. Hemodynamic management includes optimizing intravascular volume (fluid and blood management) and rational use of vasoactive drugs such as vasopressors and inotropes. This approach uses both static (e.g., mean arterial pressure) and dynamic (e.g., stroke volume variation and pulse pressure variation) hemodynamic variables.
Although the target mean arterial pressure remains controversial, most accept a value of 65 mm Hg. Although goal-directed fluid therapy guided by cardiac output monitoring was considered an important element of ERAS for all surgical patients, recent evidence has demonstrated that it does not confer benefits in the setting of an ERAS pathway (versus traditional care). Fluid imbalance is reduced within the ERAS pathway because the minimally invasive surgical approach is associated with a lower propensity for intraoperative fluid and blood loss, and patients are encouraged to hydrate during the fasting period as well as resume oral intake immediately after surgery. The goal of intraoperative fluid management is to achieve a “zero” fluid balance. Intraoperative administration of 3 to 5 mL/kg/h balanced crystalloid solution is recommended as a baseline. In addition, blood loss should be replaced with balanced crystalloid solution in the ratio of 1:1.5. The use of goal-directed fluid therapy guided by cardiac output monitoring is appropriate in high-risk patients undergoing major surgical procedures in whom the expected blood loss is greater than 1000 mL.
It is well documented that postoperative nausea and vomiting (PONV) adversely impacts outcomes. PONV reduces the patient’s ability to comply with the critical goals of an ERAS pathway such as early oral intake and mobilization. All patients should receive at least 2 to 3 antiemetics from different classes, either preoperatively or intraoperatively ( Box 1 ). Patients at very high risk (e.g., history of motion sickness, history of previous PONV, high opioid requirements after surgery) should receive 3 to 4 antiemetics.
All patients should receive two to three antiemetics from different classes either preoperatively or intraoperatively.
Patients at very high risk (i.e., history of motion sickness, previous postoperative nausea and vomiting) should receive three to four antiemetics as well as total intravenous anesthesia.
All patients should receive a procedure-specific opioid-sparing analgesic regimen.
Scopolamine transdermal patch (1–3 hours before surgery)
Aprepitant 40 mg PO (1–3 hours before surgery)
Dexamethasone 8–10 mg IV at induction of anesthesia
Dopamine D 2 antagonist (droperidol 0.625–1.25 mg IV or haloperidol 0.5–1 mg IV) at the end of surgery
Serotonin 5-HT3 antagonist (ondansetron 4 mg IV or palonosetron 0.75 mg IV) at the end of surgery
Antiemetic of a class not administered preoperatively or intraoperatively
Promethazine 6.25 mg IV
Ondansetron (4 mg IV or 8 mg orally disintegrating tablet [ODT])
Promethazine 6.25–12.5 mg IV
Prochlorperazine 2.5–5 mg IV
Dimenhydrinate 25–50 mg IV
Ondansetron 8 mg ODT
Over-the-counter antiemetics (e.g., meclizine, dimenhydrinate)
Preoperative PONV prophylaxis could include a scopolamine transdermal patch or aprepitant 40 mg orally, both administered 1 to 3 hours before surgery. Intraoperative options include dexamethasone 8 to 10 mg IV at induction of anesthesia and a serotonin 5-HT3 antagonist (ondansetron 4 mg IV or palonosetron 0.75 mg IV) at the end of surgery. In addition to antiemetic effects, dexamethasone has analgesic and antiinflammatory effects that can facilitate recovery and thus have been increasingly included in ERAS pathways. Of note, a single IV dose of dexamethasone 8 to 10 mg does not seem to influence wound infection and healing.
Rescue treatment options for PONV in the recovery room include antiemetics of a class not administered preoperatively or intraoperatively (see Box 1 ). Options for treatment on the wards include ondansetron 4 mg IV or 8 mg orally disintegrating tablet [ODT], promethazine 6.25 to 12.5 mg IV, prochlorperazine 2.5 to 5 mg IV, or dimenhydrinate 25 to 50 mg IV. Postdischarge nausea and vomiting may be treated with ondansetron 8 mg ODT or over-the-counter antiemetics such as meclizine and dimenhydrinate.
Pain management is a critical component of an ERAS pathway. The primary aim should be to facilitate ambulation rather than achieve a certain pain score. Optimal perioperative pain management includes preoperative identification of patients at high risk of postoperative pain. Opioid-sparing multimodal analgesia improves outcomes by reducing the risks of opioid-related adverse events such as nausea, ileus, respiratory depression, and sedation. The number and choice of analgesics used would depend on the balance between the invasiveness of the analgesic technique and consequences of pain as well as the adverse event profile of the intervention ( Table 2 ).
| Analgesic Technique | Indications | Comments |
|---|---|---|
| Acetaminophen | All patients, if no contraindications | Oral administration is preferred Maximum 24-hour dose should be 4 g |
| NSAIDs | All patients, if no contraindications | Contraindications Risk of kidney injury: preexisting renal insufficiency, hypovolemia, major blood loss, hypotension, use of nephrotoxic agents Bleeding and coagulation disorders Hypersensitivity to aspirin or NSAID-exacerbated respiratory disease H/O gastrointestinal bleeding |
| Dexamethasone | All patients, if no contraindications | Primarily for PONV prophylaxis Avoid in patients with HbA1c >10% |
| Ketamine infusion | Opioid-tolerant patients undergoing major painful surgery if non-opioid approaches (e.g., regional analgesia, acetaminophen, NSAIDs) are not possible | No role for single bolus doses Dosing: 0.25 mg/kg IBW bolus followed by 0.1 mg/kg/h infusion |
| Lidocaine infusion | Major open abdominal surgery, if nonopioid approaches (e.g., regional analgesia, acetaminophen, NSAIDs) are not possible | Dosing: 1.5 mg/kg IBW bolus followed by 2 mg/kg/h infusion |
| Dexmedetomidine infusion | No role in pain management | Lack of benefits and concerns of prolonged hypotension and sedation |
| Regional Analgesia | ||
| Epidural analgesia | Major open thoracic and abdominal surgery | Concerns in patients receiving VTE prophylaxis Postural hypotension and delayed mobilization |
| Interfascial plane blocks | Open truncal surgery | Breast surgery: erector spinae plane or pectoralis II blocks or serratus plane block Thoracotomy: erector spinae plane Upper and lower abdominal surgery: erector spinae plane blocks or four-quadrant TAP blocks Lower abdominal surgery: erector spinae plane blocks or TAP blocks |
| Peripheral nerve blocks | Limb surgery | |
| Surgical site infiltration | Most surgical procedures | ± Other regional analgesic techniques |
Unless there are contraindications, acetaminophen and nonsteroidal antiinflammatory drugs (NSAIDs) or a cyclo-oxygenase (COX-2)–specific inhibitor should be administered preoperatively or intraoperatively. In addition, a single intraoperative dose of dexamethasone 8 to 10 mg, IV is recommended. Regional/local analgesic techniques are the core of an optimal multimodal analgesic strategy and thus should be used when possible. However, the role of neuraxial blocks (e.g., epidural analgesia) in the ERAS setting is decreasing because of concerns of delayed ambulation and the availability of alternative analgesic techniques that can provide similar pain relief and recovery outcomes. Patients undergoing major limb surgery benefit from peripheral nerve blocks (e.g., brachial plexus blocks for major upper limb surgery and popliteal-sciatic nerve blocks for major foot and ankle surgery). Patients undergoing torso surgery benefit from interfascial plane blocks (e.g., transversus abdominis plane blocks and erector spinae plane blocks). Surgical site infiltration provides excellent analgesia; however, it is necessary that all layers of the surgical incision are infiltrated meticulously under direct vision immediately before tissue/skin closure. For example, in patients undergoing open abdominal surgery, the peritoneal, musculofascial, and subdermal planes should be infiltrated. The injection solution includes the maximum dose of local anesthetic based on body weight (e.g., for average adults, bupivacaine ∼150 mg or ropivacaine ∼300 mg) diluted with normal saline to a total volume depending upon the incision size, typically 60 to 100 mL.
IV lidocaine infusions have been shown to reduce pain and opioid requirements as well as hasten gastrointestinal recovery after abdominal surgery. The role of a single dose of ketamine (25–100 mg) remains controversial because the benefits over other analgesics (i.e., acetaminophen and NSAIDs or COX-2–specific inhibitors) and regional analgesia is unclear. There are also concerns of adverse effects such as nightmares and sleep disturbances. Nevertheless, a ketamine infusion may be used for opioid-tolerant patients undergoing major surgical procedures. The role of gabapentinoids (i.e., gabapentin and pregabalin) is controversial because of limited benefits and the potential for adverse effects such as sedation, dizziness, visual disturbances, and orthostatic hypotension. When gabapentinoids are used (e.g., surgical procedures with a high propensity of persistent postoperative pain), they should be used with great caution and at the lowest possible dose.
After discharge home, acetaminophen and NSAIDs or COX-2–specific inhibitors should be used on a regular “round-the-clock” or “scheduled” basis. Opioids should be used as “rescue” analgesics on an “as-needed” basis. Using opioids that are not combined with acetaminophen (e.g., oxycodone and tramadol) should alleviate the concerns of acetaminophen toxicity. Patients should also be educated about concerns with opioid use and be familiarized with alternative nonpharmacologic analgesic treatments such as the application of ice, elevation of the operated extremity, music, and cognitive behavioral modalities.
Prophylaxis for VTE has been emphasized because it is one of the common causes of morbidity and mortality. Several professional societies have published guidelines for VTE prophylaxis. The choice of agent is dependent on other aspects of the patient’s overall health such as surgery type, presence of kidney disease, bleeding risk, and analgesic modality (i.e., neuraxial procedures). Mechanical prophylaxis alone may be appropriate in patients with high bleeding risk, while a combination of mechanical and pharmacologic prophylaxis is preferable in patients with high VTE risk. Also, for patients undergoing a major surgical procedure, extended (i.e., more than 3 weeks after surgery) prophylaxis may be necessary. Of note, the guidelines for VTE prophylaxis have been based on evidence from outside ERAS pathways. Early mobilization within the ERAS pathway should reduce the risk of VTE; however, the type and duration of VTE prophylaxis within the ERAS pathway is not well studied.
Because of the increased metabolic demands in the perioperative period and the catabolism associated with surgery, early postoperative oral nutrition is an indispensable component for enhanced recovery. Most patients, except for those with delayed gastric emptying, tolerate early oral intake. PONV, high opioid dose, and fluid overload detrimentally affect oral intake. Thus, early resumption of oral nutrition is the logical continuation of previously stated ERAS components. Surgical factors including the presence of nasogastric tubes, ileus, severe shock, intestinal ischemia, or a high-output fistula may preclude oral nutrition and necessitate other forms of support (i.e., parenteral supplementation).
Early mobilization is considered one of the most important elements as it improves pulmonary and gastrointestinal function, reduces VTE, and avoids loss of muscle mass and function. Furthermore, unimpaired muscle function is essential for hospital discharge and return to activities of daily living. Patient co-operation and early consultation with physical therapists are imperative for effective implementation of this component of the ERAS pathway. The inability to mobilize may be a reflection of poor postoperative pain control, nausea and vomiting, orthostatic intolerance, postoperative morbidity, dependence on IV fluids, lack of facility ancillary support services, or poor patient motivation.
The nursing team plays a crucial role in the successful implementation of ERAS pathways, and they must undergo significant training and education in pathway components to ensure higher compliance rates among patients. Even though important pathway components (e.g., mobilization, diet, pain, education) are prescribed, application for day-to-day practice depends on the nurses. Hospitals can ensure compliance by having continuing education for support staff, adequate staffing, and positive feedback for the nursing teams. Active participation in this type of patient-centered care plan should be tracked and audited by hospital systems.
Postsurgical recovery is measured differently within a hospital organization and by patients. Compliance within the ERAS program has been shown to reduce postsurgical discharge complications and readmission rates. However, because of the positive impact of ERAS pathways on hospital discharge times, the majority of the recovery phase occurs after discharge home, leaving patients and their families feeling vulnerable and unprepared. Patients may see this late recovery phase as taking weeks to months before they return to a physical, emotional, and financial (i.e., return to work) baseline. Challenges remain in accurately measuring functional recovery after surgery and in providing adequate education via digital aids so patients can make informed decisions regarding identification of potential complications and when to seek help or visit the emergency department. Such education would reduce unnecessary visits to the acute care facility. In addition, utilizing digital technology will provide novel ways to track mobility, nutritional status, and mental health, thus promoting positive self-management in the recovery phase.
Given the significant clinical benefits, ERAS pathways are increasingly being implemented worldwide ( Table 3 ). A multidisciplinary approach (i.e., collaboration among surgeons, anesthesiologists, nurses, and physical therapists) is necessary to ensure high compliance rates and achieve maximum benefits. It is clear that several elements are interconnected and collectively influence postoperative outcomes. Fast-track general anesthetic techniques involving opioid sparing and goal-directed hemodynamic management combined with prevention of pain, nausea, and vomiting influence postoperative outcomes.
| Preoperative considerations | Initial Visit (Weeks Before Surgery)
Day Before Surgery
Day of Surgery
|
| Intraoperative considerations | Surgical Considerations
Anesthetic Considerations
|
| Postoperative considerations |
|
Conflicting recommendations and inconsistent education of ancillary staff may be a major factor for the lack of implementation or patchy implementation of ERAS pathways. Although evidence for ERAS elements continues to evolve, some are accepted as dogma without adequate evidence of their efficacy, and others are not beneficial at all or even deleterious to outcomes. However, this is lost in daily practice as originally proposed elements often are regarded as compulsory, irrespective of the evidence for their use. Therefore, it is imperative to identify interventions that are beneficial in improving postoperative outcomes while other interventions may be followed based on local circumstances. This concept of “partial ERAS” should increase implementation and contribute to improved patient outcomes.
Finally, it is essential to institute an audit program to evaluate the success of ERAS pathways and the need for modification. Although hospital length of stay and readmission rates are commonly used to define success of an ERAS pathway, they do not reflect true recovery of a patient; thus patient-reported outcome measures must be emphasized. Furthermore, it is necessary to address early identification and management of complications in the hospital (i.e., failure to rescue) as well as after discharge from the hospital (i.e., days alive and out of hospital).
An essential component of patient care is the appropriate management of fluid and electrolytes. From the molecular to organ level, a patient’s physiology is dependent on their electrolyte and fluid balance. Surgical patients have unique needs that are directly affected by maintenance and resuscitative fluids. The mismanagement of resuscitation and fluids can have disastrous results, as the complex systems in the human body heavily depend on precise homeostasis. The purpose of this chapter is to discuss normal fluid and electrolyte distribution and corrective therapies for pathologies encountered in the surgical patient.
The overall percentage of water to the total body weight is defined as total body water (TBW). Depending on their level of body fat, a patient’s TBW is 50% to 60%. Fat tissue has a higher water content than muscle, and thus obesity and physiologic gender will affect this proportion, with males having around 60% and females 50% to 55%. This water is distributed in the intracellular and extracellular space and is further subdivided as shown in Figure 1 . These proportions may change in the setting of disease; however, in an otherwise healthy 70-kg male, their TBW would be 42L with 28L intracellular and 14L extracellular (two-thirds and one-third TBW, respectively).
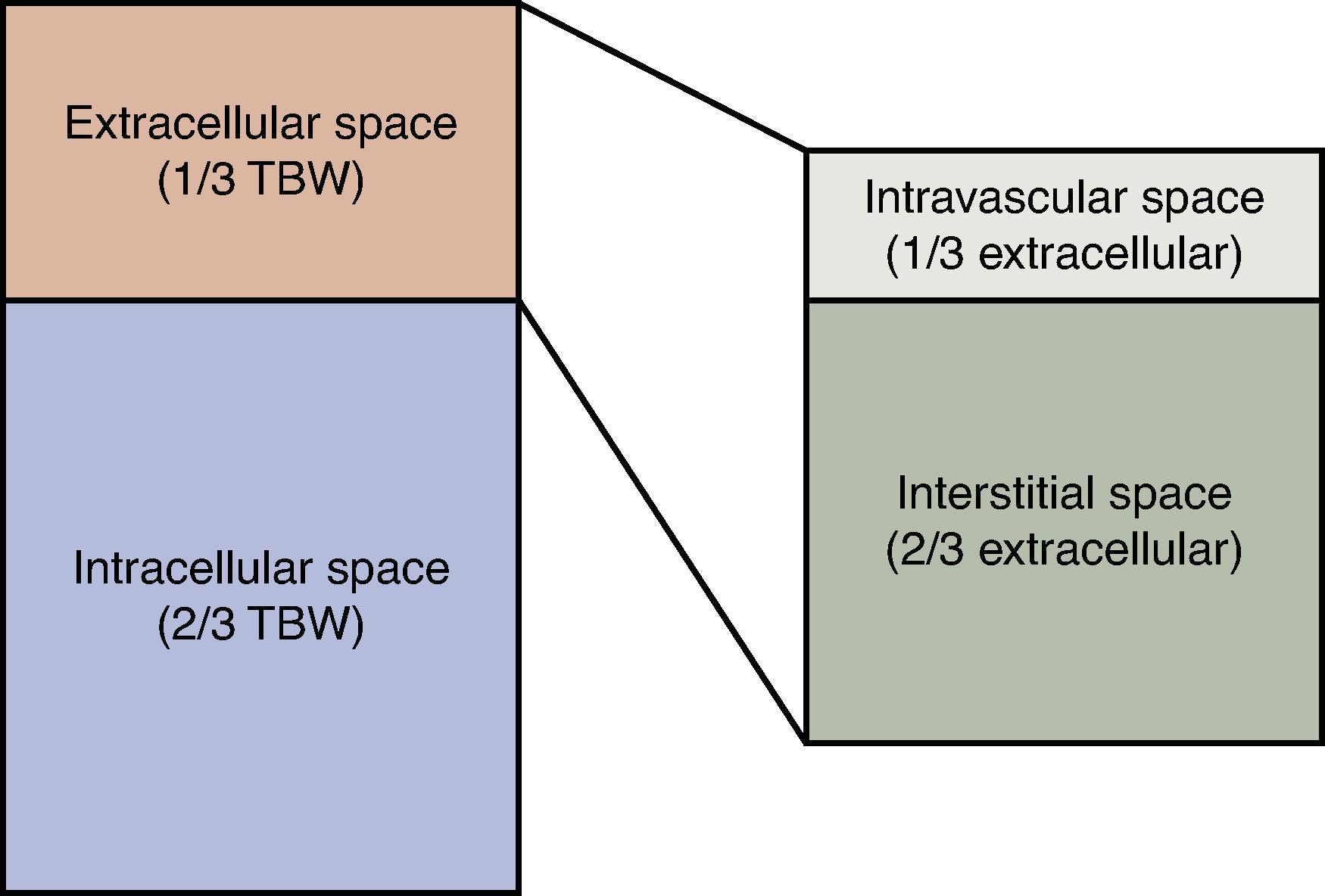
This balance of extracellular and intracellular water content is maintained by the cellular plasma membrane and solutes. The membrane is permeable to water so that the osmolality, or concentration of solutes, is equal between the intracellular, interstation (between cells), and intravascular components.
Although the overall concentration of solutes is equal throughout the body, the membrane and its associated protein channels regulate the permeability of selected solutes, which creates a transmembrane differential. The nonpermeable solutes are referred to as effective osmoles that drive the osmotic forces, which direct water movement across the cell membranes. Lipid-rich membranes allow for simple diffusion across membranes while protein-based water channels, known as aquaporins, allow for direct movement.
Membranes in the capillaries are permeable to smaller solutes such as sodium, potassium, glucose, and low-molecular-weight proteins that are less than 50,000 kDA. These small solutes have no role in effective osmoles to facilitate capillary fluid movement, and instead this is directed by Starling’s law. In contrast to the Frank-Starling curve cardiac contractility, Starling’s Law states that transcapillary water movement results from the balance between oncotic pressure of effective osmoles and hydrostatic pressure from blood flow. This is shown in the following equation:
Here, the efflux of fluid J v is directly proportional to the membrane coefficient and the difference between the capillary hydrostatic pressure P c and interstitial hydrostatic pressure P i . It is inversely related to the reflection coefficient σ and the difference between the capillary oncotic pressure π c and the interstitial oncotic pressure π i . The oncotic pressures are generated by plasma proteins too large to cross the capillary wall, and net movement is created by an imbalance between the hydrostatic forces and water concentration gradients. In the average physiologic state, the hydrostatic pressure will force fluid into the interstitial space while plasma proteins will provide oncotic pressure to draw fluid into the intravascular space. Due to the higher hydrostatic forces in the arteriolar end of the capillary bed, there is a net efflux of fluid. Along the length of the capillary bed there is a sharp drop-off in the hydrostatic pressure resulting in a reversal of the direction of flow in the venous end of the capillary.
Although small solutes do not generally contribute to water movement across capillary membranes, they will function as effective osmoles that direct fluid between the intracellular and extracellular spaces. Given their relative abundance, sodium (Na +) and potassium (K + ) are the primary determinants of osmolality in the extracellular and intracellular spaces, respectively. Due to their charge, they are unable to pass across the lipid-rich membrane, and thus this concentration gradient is maintained by the Na + /K + adenosine triphosphatase (ATPase) pump. This well-described protein will transport three sodium extracellularly in exchange for two potassium molecules intracellularly. This net efflux of positively charged ions creates a net negative cell membrane potential that can be utilized for work. In addition, the manipulation of these ions results in water movement down its relative concentration gradient from the low osmolality compartment to the high osmolality compartment.
Conceptually, osmolality is the inverse of the concentration of water, and thus a higher concentration of solutes and osmolality is fundamentally a lower concentration of water creating a gradient that is inevitably equilibrated. It is because of this that administration of a hypotonic solution will result in decreased extracellular osmolality, so the water will move intracellularly creating swelling. Hypertonic solutions will increase extracellular osmolarity driving water intracellularly and creating cellular shrinking as water moves down its relative concentration gradient. Serum osmolality is maintained in a narrow therapeutic range through osmoregulation. It can be estimated using the following equation:
In this equation, BUN is blood urea nitrogen and the resultant units are mOsm/kg. Normal serum osmolality ranges from 275 to 290 mOsm/kg with a physiologic 1% to 2% variation. Variations beyond this are detected by osmoreceptors in the hypothalamus. Hyperosmolality, or a relative decrease in the concentration of water, drives thirst along with secretion of antidiuretic hormone (ADH) from the posterior pituitary gland. This peptide-based hormone has a myriad of receptors throughout the body with differing effects including the upregulation and fusion of vesicles containing aquaporin channels in the basolateral membrane of the distal collecting tubules. These channels facilitate diffusion of water promoting resorption and increasing urine osmolality. Depending on the serum osmolality and renal function, urine concentration can increase up to 1200 mOsm/kg. ADH release is also stimulated by central baroreceptors in the setting of decreased plasma volume, resulting in increased water reabsorption to restore plasma volume and osmolality homeostasis.
Osmoregulation and volume regulation are regulated processes, with sodium serving not only as the primary regulator of extracellular osmolality but also of volume status. Hormone systems such as the renin-angiotensin-aldosterone (RAA) axis promote volume expansion. Renal hypoperfusion is detected in the juxtaglomerular apparatus by the macula densa within the distal convoluted tubule. The juxtaglomerular cells will secrete renin in response to low sodium in addition to hypoperfusion, which promotes the formation of angiotensin from angiotensinogen. The ultimate result is increased aldosterone production to promote sodium reabsorption in the distal convoluted tubule. The competing axis in sodium regulation is partially enacted through atrial natriuretic peptide (ANP). This hormone is secreted by the cardiac atria in response to increased atrial stretch to increase sodium and volume loss. ANP will promote dilation of the afferent glomerular arteriole, thus increasing renal perfusion in addition to inhibiting sodium resorption and thus water excretion.
Patients can undergo large volume shifts due to preoperative, intraoperative, and perioperative fluid losses and shifts. A healthy normothermic adult with a normal metabolic rate loses approximately 1.5 mL/kg/hr of fluid, while animal models have estimated that evaporative intraoperative fluid loss ranges from 1 mL/hr to 1 L/hr depending on the amount of exposed viscera and type of surgical procedure. Many surgical patients may also arrive to the operative theater dehydrated, whether due to the associated condition that necessitated surgical intervention or potentially due to administration of a preoperative bowel prep and prolonged nil per os status. The components of the various gastroenteric fluids are important to note ( Table 1 ). Furthermore, hemorrhage, ongoing enteric losses, and use of crystalloid in the operative field can all have a role in the volume status changes within the surgical population. While nearly all patients require replacement of these fluids, selecting the appropriate amount, rate, and type of volume replacement is critical to prevent iatrogenic sequelae.
| Fluid | Daily Production (mL) | Na + | K + | Cl − | HCO 3 − |
|---|---|---|---|---|---|
| Saliva | 1000 | 30–80 | 15 | 70 | 30 |
| Gastric | 1000–2000 | 60–80 | –10 | 100 | 0 |
| Pancreas | 1000 | 140 | 20 | 60–90 | 40–100 |
| Bile | 1000 | 140 | 75 | 100 | 40 |
| Small bowel | 2000–5000 | 140 | 20 | 100 | 25–50 |
| Large bowel | 200–1500 | 75 | 30 | 30 | 0 |
The postoperative inflammatory state will often result in a low effective intravascular volume due to cytokine-induced permeability, which results in fluid shifting to the extravascular space. Excessive thirst, dry mucous membranes, oliguria, poor skin turgor, tachycardia, hypotension, orthostasis, and abnormal mentation are all signs that a patient has moderate to severe low effective circulatory volume. Several clinical tools exist to assist with assessing volume status including urine and serum electrolyte levels, acid-base balance, and invasive monitoring equipment.
Urine output remains a mainstay for assessing volume status in an otherwise healthy adult; however, recent studies have shown that morbidity and mortality are not improved when using it for goal-directed therapy. Although a healthy adult should produce at least 0.5 mL/kg/hr and children should produce 1 to 2 mL/kg/hr, the expanded non–water-storing tissue in obese patients may overestimate goal urine outputs. Furthermore, some literature have suggested that, in adults, a perioperative urine output goal of 0.2 mL/kg/hr is tolerated well without significant increased risk of acute renal failure. In addition, patients with renal insufficiency, those receiving diuretics, and those with hyperglycemia and other osmotic diuretics may further alter the accuracy of urine output as a surrogate marker of volume status.
Other markers of intravascular volume depletion include elevated hematocrit, low serum bicarbonate level, elevated base deficit, prerenal azotemia (classically defined as BUN/creatinine ratio greater than 20:1), a fractional excretion of sodium (FENa) of less than 1%, elevated urine osmolality, and a fractional excretion of urea less than 35%. Fractional excretion of urine remains a useful tool in the surgeon’s armamentarium; however, renal insufficiency and use of loop diuretics can interfere with the test’s accuracy. The FENa is as follows:
While the fractional excretion of urea is:
Fluid therapy is generally considered to be divided into two categories based on composition and goal. Maintenance fluids are used to replace normal and insensible fluid losses. These typically contain dextrose to maintain plasma osmolality and mitigate glycogen depletion. In contrast, resuscitative or replacement fluids replace abnormal and excessive losses and correct for water or electrolyte deficits. Patients with large fluid deficits in the immediate postoperative period, injury, or dehydration are usually treated with resuscitative fluids. Common parenteral fluids are shown in Table 2 .
| Fluid | Na + mEq/L | K + mEq/L | Cl − mEq/L | Ca 2+ mg/L | HCO 3 − mEq/L | Dextrose mg/L | pH | Osmolality (mOsm) |
|---|---|---|---|---|---|---|---|---|
| Extracellular Fluid | 142 | 4 | 103 | 5 | 27 | 0 | 7.4 | 280 |
| LR | 130 | 4 | 109 | 2.7 | 28 | 0 | 6.5 | 275 |
| NS (0.9% NaCl) | 154 | 0 | 154 | 0 | 0 | 0 | 4.5 | 308 |
| ½NS (0.45% NaCl) | 77 | 0 | 77 | 0 | 0 | 0 | 4.5 | 308 |
| ¼NS (0.22% NaCl) | 34 | 0 | 34 | 0 | 0 | 0 | 4.5 | 77 |
| Hypertonic Saline (3% NaCl) | 513 | 0 | 513 | 0 | 0 | 0 | 4.5 | 1026 |
| 5% Dextrose in Water | 0 | 0 | 0 | 0 | 0 | 50 | 5 | 278 |
| 5% Albumin | 145 | 0 | 145 | 0 | 0 | 0 | 7.4 | 310 |
| 25% Albumin | 145 | 1 | 145 | 0 | 0 | 0 | 7.4 | 312 |
| 6% Hetastarch in Lactated Solution | 143 | 3 | 124 | 5 | 28 | 990 | 5.9 | 307 |
| Normosol | 140 | 5 | 98 | 0 | 0 | 0 | 6.6 | 294 |
| Plasma-lyte | 140 | 5 | 98 | 0 | 0 | 0 | 7.4 | 294 |
Sensible losses, by definition, can be quantified and occur primarily in urine (∼800–1500 mL daily) and stool (∼250 mL daily). Insensible loses include cutaneous losses through exposed skin and upper respiratory tract. Intraoperatively, insensible losses occur through exposed body cavities as well. The volume of insensible and sensible losses vary greatly depending on (patho)physiologic states including burns, fever, tachycardia, infection, tachycardia, and other hypermetabolic infections. Cutaneous losses will increase by 10% per day for each degree increase in body temperature above 37.1°C. Thoracotomy and laparotomy sites can increase insensible losses as previously noted.
Maintenance fluid administration in the pediatric and adult populations can be estimated by using the 4-2-1 rule, which is further delineated later. Abnormally high or low percent body fat and renal failure can result in over and underestimation of a patient’s maintenance fluid requirements. As noted previously, maintenance fluids will contain dextrose; however, their electrolyte composition is variable and allows for replacement of those lost in both sensible and insensible loss. As with all postoperative cares, monitoring for a patient’s response to therapies is advised to avoid iatrogenic injury and failure to rescue. The most common postoperative maintenance fluid for adults is 5% dextrose in one-half normal saline (D5HNS or D5 ½NS) with 20 mEq/L potassium chloride. In children, the standard maintenance fluid is D5HNS or D5 lactated Ringer’s (LR) solution. This is to avoid hyponatremia in the pediatric population. Children younger than 2 years of age often receive D5 ¼NS with 20 mEq/L KCl. The reason for the decreased sodium content is that the infant glomerular filtration rate (GFR) is one-quarter the adult level, and the distal nephrons are unable to effectively concentrate the urine, leading to a difficulty in excreting high sodium loads.
When administering maintenance fluids, utilizing the 4-2-1 dosing regimen allows for a quick estimation of a patient’s hourly fluid requirements. This equation is 4 mL/kg for the first 10 kg, 2 mL/kg for the second 10 kg, and 1 mL/kg for every remaining kilogram. A quick shorthand for any patient in excess of 20 kg is 60 + n where n is the patient’s weight in kg minus 20. For a 70-kg patient, this would be 60 + (70 − 20) = 60 + (50) = 110 mL/hr. When administering D5HNS, this would equate to 203 mEq/day, which is greater than the required 1 or 2 mEq/kg/day by 45%. Patients with normal kidney function can excrete the excess sodium load; however, patients with renal or cardiac dysfunction may have resultant hypernatremia.
Resuscitative fluids are commonly used in the immediate postoperative period and in the setting of hypovolemia. The rate of fluid administration is determined by the severity of deficit, ongoing losses, and the patient’s underlying comorbidities. Normal saline, Plasma-Lyte, Normosol, and LR closely approximate the composition of extracellular fluids; however’ NS and LR are the most commonly used resuscitative fluids. As shown in Table 2 , LR has a pH of 6.5 and provides 28 mEq of bicarbonate (HCO 3 — ) per liter, making it preferential in the setting of acidosis. NS has a pH of 4.5 and does not contain bicarbonate but instead a higher concentration of Na + and Cl — (154 mEq), making it preferential in patients with a metabolic alkalosis. This does mean it can create hyperchloremic metabolic acidosis in the setting of high-volume resuscitation. Large-volume resuscitation should be carried out with isotonic fluid such as NS or LR with cautious monitoring of desired clinical endpoints. Although many have posited that colloid solutions such as albumin, hydroxyethyl starch, and even fresh-frozen plasma (FFP) can provide a theoretical benefit over crystalloid through application of oncotic pressure, there has yet to be a clearly demonstrated benefit in patient outcomes in prospective randomized trials.
Enhanced recovery after surgery (ERAS) programs are evidence-based multidisciplinary pathways designed to accelerate postoperative recovery and shorten time to discharge without compromising safety. ERAS protocols are evidence-based, standardized, utilize preoperative teaching, and have clear goal-directed therapies. Traditionally, colorectal ERAS programs focus on minimizing intraoperative and postoperative IV fluids. Balanced fluid administration is provided intraoperatively through goal-directed therapy that uses vasopressors in addition to fluids and closely monitors hemodynamic parameters. Excessive IV fluid in the perioperative period has been linked to a myriad of complications including delayed return of bowel function and urinary retention.
Although the preponderance of studies in the current literature do clearly suggest that a fluid restrictive strategy can be utilized for major abdominal surgical interventions including colorectal and hepatobiliary operations, there is significant heterogeneity as to which method of goal-directed therapy is optimal. Notable tools under investigation include estimated stroke volume variance, tissue oxygen tension, uncalibrated pulse contour, stroke volume augmentation, and even automated closed-loop systems. Although many of these techniques for providing individualized and optimized fluid resuscitation to patients intraoperatively and in the postoperative period may each focus on different components of complex volume homeostatic regulators, there has been no clear superior technique. Furthermore, with the creation of automated closed-loop systems demonstrating immediate perioperative benefits, the psychology of providers could be a factor in interpreting patient volume status that can in turn impact patient outcomes. Regardless of whichever technique is employed, the main trend in the recent literature of ERAS protocols and goal-directed therapy is to avoid overresuscitation of the patient and to focus on targeted physiologic markers of hypovolemia.
As stated previously in this chapter, sodium is the principal determinant of serum osmolality and free water balance. The normal physiologic range is 135 to 145 mEq/L, and it is predominantly located in the extracellular space. Cell membranes are relatively impermeable to sodium compared with water, making it an effective osmole. Because of the close relationship between water balance and sodium levels, it is vital to recognize a patient’s underlying volume status and overall osmolarity when treatment sodium abnormalities.
Hypernatremia is defined as sodium concentrations in excess of 145 mEq/L and can be subdivided into mild, moderate, or severe. Symptoms of severe hypernatremia are related to central nervous depression due to cellular dehydration. This includes muscle weakness, restlessness, insomnia, lethargy, and coma. Hypernatremia is almost always associated with a hypertonic state but can be related to hypervolemic, euvolemic, and hypovolemic states. Assessment of volume status is an important first step in the recognition of the underlying etiology and its proper management.
Hypovolemic hypernatremia is seen in patients with dehydration and uncontrolled fluid losses. Patients at extremes of age and those with end-stage liver disease are particularly vulnerable. Patients with ongoing sensible loss through nasogastric suction, vomiting, diarrhea, and even those with lactulose administration are at risk. In contrast, euvolemic hypernatremia occurs commonly due to disruption of the renal and neurohypophyseal axis. Any patient who has underlying traumatic brain injury, cerebral hemorrhage, or pituitary surgery with euvolemic hypernatremia should undergo prompt workup for neurogenic diabetes insipidus (DI). In general, any patient with hypernatremia and a urine osmolality less than 600 mOsmol/kg should receive desmopressin (DDAVP). An increase in urine osmolarity of 50% is suggestive of neurogenic DI. Because nephrogenic DI is by definition an inappropriate response to DDAVP, this makes treatment more difficult. In the setting of nephrogenic DI, there are a myriad of possible causes, with management largely depending on the underlying pathophysiology.
Hypervolemic hypernatremia is largely iatrogenic in nature due to fluid resuscitation with hypertonic solutions or mineralocorticoid excess. Treatment of hypernatremia begins with calculation of the free water deficit and estimation of chronicity. The free water deficit in liters (H 2 O) is dependent on the patient’s weight in kg (w) and serum sodium concentration [Na].
Patients with severe hypernatremia, defined as a serum sodium concentration >160 mEq/L or symptomatic hyponatremia should undergo treatment with a 5% dextrose solution in water, or D5W. Those with mild hypernatremia can be corrected gradually with NS. Adults with acute development (<24 hr) should be corrected rapidly, whereas those with chronic hypernatremia (≥48 hr) should have more cautious replacement. Acute hypernatremia in adults can be corrected to near normal levels within 24 hours without significant risk of osmotic demyelination. Chronic hypernatremia should not exceed 0.5 mEq/L/hr with a total change of 10 mEq/L/day, although correcting at rates up to 12 mEq/L/day has been reported without significant sequelae in adults. Furthermore, it is not recommended to correct less than 6 mEq/L/day as this has been associated with increased mortality. It is recommended to obtain serial serum sodium measurements throughout correction until the patient has normalized and has stable serum sodium.
Hyponatremia is subdivided into mild ([Na] 130–135 mEq/L), moderate ([Na] 120–130 mEq/L), and severe ([Na] <120 mEq/L). Use of LR solution perioperatively has been associated with mild transient hyponatremia, which generally does not require intervention. Moderate to severe hyponatremia occurs in approximately 1% of postoperative patients, with 20% of these cases becoming clinically significant. Due to the relative decrease in extracellular solute and osmotic gradient in hyponatremia, severe cases can cause cellular edema. Cerebral swelling can then lead to headaches, lethargy, seizures, and coma. The rate of development of hyponatremia is often related to the clinical manifestations. Severe hyponatremia can be asymptomatic in chronic etiologies such as cirrhosis and heart failure. Furthermore, female physiologic gender, young age, and hypoxia are associated with more severe symptoms of hyponatremia.
The sodium deficit estimation is similar to the calculation of free water deficit using the estimated TBW estimate, which again depends on the patient’s sex (approximately 60% for males and 55% for females).
As with management of hypernatremia, the rate of correction should be closely monitored. Rapid correction of hyponatremia can cause central pontine myelinolysis with permanent spastic quadriparesis and pseudobulbar palsy. Patients with liver disease appear to be particularly susceptible. In this population, there is no reported safe rate of correction. Again, with acute onset severe symptomatic hyponatremia, the sodium should be corrected as quickly as possible. These patients with evidence of neurologic compromise (active seizures, respiratory failure, etc.) should have the sodium corrected by 2 to 6 mEq/L via a 100 mL bolus of 3% hypertonic saline solution over 10 minutes. This can be repeated if clinical improvement is not noted; however, the patient should not exceed a correction of 6 mEq/L over the initial 24 hours. Patients with mild to moderate encephalopathy can undergo infusion of 3% saline at a rate of 1 mL/kg/hr. In general, this will increase the sodium by 1 mEq/L/hr. Patients who are asymptomatic can utilize hypertonic saline and/or furosemide diuresis to facilitate gradual correction.
As with hypernatremia, assessment of osmolality and volume status is a crucial step in the workup and treatment of asymptomatic hyponatremia. In the perioperative period, abnormalities in volume status are relatively common.
Hypovolemic hypotonic hyponatremia is the most common form of hyponatremia in the postoperative setting. Urine sodium levels <20 mmol/L suggests sequestration of isotonic fluids in the extravascular space, loss from the gastrointestinal (GI) tract, or loss from skin. In this unique setting, fluid restriction will worsen the clinical picture, and thus mild to moderate cases are treated with isotonic saline.
Hypervolemic hyponatremia is the least common subtype in the postoperative period. This is most commonly the iatrogenic result of hypotonic fluid administration. It is for this reason that hypotonic fluid resuscitation should only be used for hypernatremia. Treatment for hypervolemic hyponatremia can be accomplished with water restriction and gradual diuresis.
Euvolemic hyponatremia is uncommon postoperatively as it is often the result of inappropriate ADH secretion (SIADH), hypothyroidism, or psychogenic polydipsia. In the postoperative period, there are noted transient increases in ADH due to pain, stress, narcotics, and volume depletion. This transient state is often not sufficient to create hyponatremia. SIADH occurs with traumatic brain injuries, carcinoid and small cell pulmonary lung malignancies, and lung infection. SIADH is often associated with urine osmolality >150 mmol/kg and urine sodium >25 mmol/L. The absence of hypokalemia differentiates it from adrenal insufficiency. Due to the sodium filtration and water resorption in SIADH, isotonic saline will worsen the hyponatremia. Instead, SIADH is treated with fluid restriction (∼1 L/day) and gradual correction of sodium levels.
Hypertonic hyponatremia suggests that another major solute is out of the normal range such as the BUN or glucose. These solutes will create a concentration gradient of water (via oncotic pressure differential) that will then equilibrate extracellularly. It is important to note that in the setting of elevation of another solute that the measured sodium levels can be artifactually decreased due to decreased water concentration in the sample, with nonionic solutes increasing the total extracellular volume. This will decrease the sodium concentration relative to plasma but not relative to plasma water volume, which is the clinically significant concentration resulting in isotonic hyponatremia. Direct ion-selective electrodes used in blood gas analyzers are not as susceptible to this artifact.
Isotonic hyponatremia, or pseudohyponatremia, is associated with hypertriglyceridemia, hypercholesterolemia, hyperproteinemia, and hyperglycemia. Elevations in serum glucose will create a pseudohyponatremia where every 100 mg/dL increase in glucose over 100 mg/dL will artificially decrease the sodium by 2 mEq/L.
Hypotonic hyponatremia occurs in the setting of hypervolemia due to expanded extracellular and interstitial volumes. The relative intravascular depletion results in ADH promoting salt and water retention. This is commonly seen in nephrotic syndrome, cirrhosis potentially with hepatorenal syndrome and hypoalbuminemia, congestive heart failure, and chronic renal insufficiency. Hypotonic hyponatremia is often treated with sodium (1500–2000 mg/day) and fluid (approximately 1 L/day) restriction.
Serum potassium levels are maintained in a narrow range of 3.5 to 5 mmol/L by the kidney. This is regulated via the RAA system. Aldosterone promotes secretion of potassium in the distal tubule. Most of the body’s potassium exists in the intracellular space, and thus transcellular shifts are the primary culprit for development of potassium abnormalities. Metabolic acidosis results in the efflux of potassium into the extracellular space in exchange for intracellular movement of hydrogen to buffer serum pH. Insulin and β 2 receptor stimulation will shift potassium intracellularly.
Hyperkalemia is defined as [K + ] above 5.5 mEq/L either due to translocation of intracellular potassium or total body excess. Due to the dependence on the potassium concentration gradient in maintaining the transmembrane potential, hyperkalemia can cause ventricular arrythmias and death. Electrocardiographic (ECG/EKG) findings in progressing order of hyperkalemia severity are peaked T waves, QRS widening, shortened QT interval, and ventricular ectopy. Acute rises in potassium are not well tolerated, but chronic hyperkalemia, such as those in renal failure, may be asymptomatic. Acidosis promoting transmembrane shifting, rhabdomyolysis, cell lysis, and insulin deficiency are common causes. In the surgical population, ischemia reperfusion injuries especially in the setting of revascularization after 4 to 6 hours of ischemia can cause acute severe systemic hyperkalemia. Other important populations include burns and crush injuries. Spironolactone and succinylcholine can also cause hyperkalemia, and succinylcholine in the setting of burns and crush injuries is to be avoided. Although there exists some debate as to whether LR can exacerbate or promote hyperkalemia, the relative alkalinity and possible dilutional effects may actually assist in the treatment of extracellular potassium.
There are multiple possible treatment modalities of hyperkalemia, with more aggressive measures depending on symptoms. The general strategies include stabilization of the transmembrane potential, shifting of the potassium intracellularly, and elimination of excess total body potassium. Any patients with EKG changes should receive 1 or 2 g of calcium gluconate intravenously over 2 to 3 minutes with continuous cardiac monitoring as the first step of treatment. Due to the relative abundance of extracellular calcium, the supplementation of this double-charged ion will serve to assist in the restoration of the transmembrane potential and mitigates the risk of fatal arrythmias without treatment of the underlying pathophysiology. Repeat doses can be administered as needed to eliminate EKG changes associated with hyperkalemia. To shift the potassium intracellularly, the serum can be alkalinized via bicarbonate, β 2 agonists such as albuterol, and a combination of insulin and dextrose that can be administered. Again, as with the calcium supplementation, these measures may mitigate the potentially fatal effects of hyperkalemia but do not treat total body potassium excess. This can be managed via use of loop diuretics, hemodialysis, and cation exchange resins such as Kayexalate. These exchange resins are to be used with caution in the surgery patient as oral agents are contraindicated in the obstructed patient and rectal administration is contraindicated in immunosuppression. Furthermore, the sorbitol used in Kayexalate suspensions carries its own risk of bowel ischemia.
Hypokalemia is a serum [K + ] below 3.5 mEq/L and is frequently encountered postoperatively due to nasogastric suctioning and enteric losses. Loop diuretics can also cause hypokalemia. Symptoms of hypokalemia include fatigue, weakness, ileus, and arrhythmias. EKGs can show flattening of T waves and prominent U waves. Given that 98% of the total body potassium is intracellular, small changes in serum concentrations may reflect large changes in total body potassium stores. Hypokalemia is often associated with hypomagnesemia and acidemia, and thus magnesium must be replenished before K + to facilitate adequate serum concentrations. In the setting of acidemia, correction of the pH will exacerbate the issue as hydrogen ions are exchanged for potassium in the functioning nephron. Although enteric and IV replacement of potassium is possible, oral potassium supplements are often poorly tolerated and peripheral IV replacement is associated with allodynia. IV administration should be reserved for moderate to severe hypokalemia and in patients who cannot tolerate oral replacement. Severe hypokalemia can be replaced through central access, although rates should not exceed 20 mEq/hr given the possible risk of arrythmia with concentrated potassium exposure to cardiac tissue. Given the large total body deficit associated with serum abnormalities, serial replacements are often necessary.
Calcium is one of the most abundant electrolytes in the body due to storage in mineralized bone tissue. Serum calcium ranges from 8.5 to 10.5 mg/dL, and this is tightly maintained by bone demineralization, interstitial absorption, urinary excretion, and bone formation. The majority of this homeostatic regulation is controlled by parathyroid hormone and calcitriol. Serum calcium is largely bound by albumin, and thus the active, or ionized portion, must be adjusted to serum albumin levels accordingly.
Although this correction can be useful for a quick estimate of a patient’s ionized calcium, the actual measurement is necessary for patient care and is generally available in most clinical laboratories.
Hypercalcemia is a total serum calcium greater than 10.4 mg/dL or ionized calcium greater than 5.6 mg/dL. This is most often seen in malignancy (such as breast cancer) or in hyperparathyroidism. Other common causes include thiazide diuretics, lithium, familial hypocalciuric hypercalcemia, vitamin A and D overdose, and immobilization. Symptoms include headache, nausea, emesis, altered mental status, lethargy, myalgias, arthralgias, polyuria, and nephrolithiasis. Treatment is indicated in symptomatic and asymptomatic patients with serum concentrations exceeding 14 mg/dL. Treatment begins with normal saline at a rate of 200 to 300 mL/hr to dilute circulating calcium and promote renal excretion. Next, nonthiazide diuretics such as furosemide are given IV to further promote renal excretion and mitigate fluid overload. Calcitonin (4 IU/kg) will inhibit bone and renal reabsorption of calcium. Bisphosphonates are best for long-term calcium control in the setting of enhanced bone resorption.
Hypocalcemia is a serum calcium below 8.4 mg/dL or ionized levels below 4.5 mg/dL. It can be due to increased efflux or decreased influx to the extracellular space. Efflux causes include alkalosis through albumin binding, citrate binding in the setting of massive blood product transfusion, and severe pancreatitis resulting in saponification. Decreased influx is due to hypoparathyroidism and vitamin D deficiency. Hyperphosphatemia and hypomagnesemia also alter calcium homeostasis. Postoperative blood product administration and large volume crystalloid resuscitation can also result in hypocalcemia.
Classically patients with hypocalcemia will first experience perioral numbness and tingling. Patients may also experience hyperreflexia upon facial nerve stimulation (Chvostek’s sign), muscle spasm of the hand with inflation of a blood pressure cuff (Trousseau’s sign), and prolonged QT on EKG. Indications for treatment include symptomatic hypocalcemia, corrected serum calcium below 7.0 mg/dL, or ionized calcium lower than 3.0 mg/dL. Oral replacement for chronic hypocalcemia can be done through calcium carbonate or calcium gluconate at 1 to 1.2 g of calcium per day. Resistant or severe hypocalcemia may require vitamin D supplementation. IV calcium gluconate and calcium chloride are reserved for severe hypocalcemia with tetany, laryngospasm, and seizures. Calcium chloride has three times as much elemental calcium as calcium gluconate and requires central IV access. Magnesium should be replaced concurrently when replacing calcium as in hypokalemia.
Magnesium has a number of roles throughout the body including homeostasis of calcium and potassium in addition to energy metabolism and protein synthesis. Less than 1% of the total body magnesium is stored extracellularly, and serum levels range from 1.4 to 2.0 mEq/L.
Hypermagnesemia is a rare clinical entity and symptoms (such as lethargy) are even less common. Long-term hemodialysis, burns, and trauma are the more common risk factors. In the event that noniatrogenic hypermagnesemia is encountered, it can be managed with saline expansion of the plasma volume and loop diuresis to promote renal excretion. Severe cases can be treated with 1 g of IV calcium gluconate over 5 minutes, with repeat and faster boluses being an option in the setting of cardiac arrest (e.g., 1 g over 2 minutes repeated after 5 minutes). It is worth noting that survival benefits of calcium boluses have not been demonstrated in this setting.
Hypomagnesemia is often seen postoperatively due to dilution. Alcoholics and critically ill patients are at elevated risk. Poor intake and GI losses including diarrhea and biliary/enteric fistulas are other risk factors. Symptomatic hypomagnesemia is rare and often does not occur until levels are below 1.0 mEq/dL. Ventricular arrhythmias including torsades de pointes can occur making monitoring for postoperative hypomagnesemia critical.
Serum phosphate levels range from 2.2 to 4.7 mg/dL and remain critical for many essential cellular processes in the body. Given that phosphorus remains an essential factor in the production of ATP, levels are often altered in critically ill and postoperative patients. Patients with poor nutritional status may also experience deficient phosphate levels. Eighty percent of the total body phosphate resides in bone and less than 1% is intravascular. Thyroid hormone and insulin promote resorption mostly in the proximal tubule, whereas parathyroid hormone promotes excretion.
Hyperphosphatemia occurs when serum phosphate exceed 5.0 mg/dL but is rare in the postoperative period. Patients with renal insufficiency often have a deficiency in 1,25-dihydroxyvitamin D causing both hyperphosphatemia and hypocalcemia. These patients may also experience symptoms from the hypocalcemia. Treatment of hyperphosphatemia includes volume expansion and stimulation of renal excretion via acetazolamide. In severe cases or in the setting of profound renal failure, hemodialysis may assist. Often patients with chronic failure and associated chronic hyperphosphatemia may undergo treatment with phosphate binders such as aluminum hydroxide. Other causes include tumor lysis syndrome, rhabdomyolysis, laxative use, and hypoparathyroidism. Pseudohyperphosphatemia is also described in the setting of multiple myeloma, with immunoglobulins interfering with laboratory evaluation of phosphate levels.
Hypophosphatemia is defined as a serum phosphate level less than 2.5 mg/dL with associated arrhythmias, platelet dysfunction, abnormal glucose metabolism, and cardiopulmonary arrest. Causes include internal redistribution, deficient intake, and excessive loss. Redistribution can occur with increased insulin secretion, epinephrine, acute respiratory alkalosis, or bone hunger. Deficient intake can occur with malabsorption due to GI tract surgery, short gut syndrome, steatosis, vitamin D deficiency, phosphate-binding medications, and chronic diarrhea. Excretion is associated with acetazolamide, hyperparathyroidism, and major hepatic resection.
A life-threatening cause of hypophosphatemia is refeeding syndrome, in which chronically nutritionally depleted individuals receive a carbohydrate load, prompting an insulin surge. This in turn will promote redistribution of phosphate intracellularly, exacerbating hypophosphatemia and depletion of adenosine triphosphate, which can promote cardiopulmonary collapse and death. Any patient at risk of chronic malnutrition must have their phosphate levels monitored closely when they receive nutrition.
Large hepatic resections can also promote hypophosphatemia. Traditionally it was thought that this was due to increased consumption of adenosine triphosphate with liver regeneration; however, emerging evidence exists that the hypophosphatemia of hepatic resections and pancreatic surgery may instead be due to alterations in renal function and the hepatorenal axis. Regardless of the etiology, postoperative phosphate levels should be monitored and cautiously corrected.
Hypophosphatemia treatment begins with the assessment of the etiology. Symptomatic and severe (<1.0 mg/dL) hypophosphatemia is treated with IV phosphate until serum levels exceed 1.5 mg/dL followed by oral therapy; 31 mg of phosphate is equivalent to 1 mmol of phosphate. IV dosing varies, but in general 0.08 to 0.64 mmol/kg can be given at a maximum rate of 7 mmol phosphate/hr. Oral therapy, either as sodium or potassium salt, has variable oral bioavailability and can induce diarrhea that may limit efficacy. The formation can be tailored depending on availability and the patient’s underlying renal function. When serum phosphate is between 1.0 to 1.9 mg/dL, oral therapy is often sufficient. Continuous therapy over 5 to 7 days is often necessary due to the large proportion of extravascular phosphate in total body phosphate levels. Again, due to the inability of patients with renal failure to clear serum phosphate, caution is advised in replacement due to the risk of hyperphosphatemia.
Fluid and electrolyte disorders ( Table 3 ) require prompt assessment and correction in critically ill patients. While IV therapies and electrolyte management have the potential for great benefit in the surgical patient, inappropriate or inaccurate treatment can result in serious harm.
| Disorder | Neurologic | Cardiovascular | Gastrointestinal | Renal | Treatment |
|---|---|---|---|---|---|
| Hyponatremia | Confusion, seizures, coma | Hypotension, hypertension | Salivation | Oliguria | Fluid resuscitation 0.9% NaCl Hypertonic saline + diuretic |
| Hypernatremia | Confusion, seizures, coma | Fluid overload | Thirst | Free water 0.45% NaCl |
|
| Hypokalemia | Fatigue, weakness | Atrial arrhythmias, flat T waves or U waves | Ileus | Nephrotoxicity | Oral/IV potassium Magnesium repletion |
| Hyperkalemia | Confusion, paralysis, areflexia | Ventricular arrhythmias, peaked T waves, prolonged QT, wide QRS | Nausea, vomiting, abdominal pain | Insulin + 50% dextrose 10% calcium gluconate Dial |
|
| Hypocalcemia | Paresthesia, perioral tingling, carpopedal spasms, Chvostek’s sign | Ventricular arrhythmias, prolonged QT | IV calcium gluconate 1,25 Dihydroxy-vitamin D |
||
| Hypercalcemia | Confusion, fatigue, coma | Shortened QT | Abdominal pain | Stones, nephrogenic DI (long-term) | 0.9% NaCl Furosemide |
| Hypomagnesemia | Weakness, cramping, hyperreflexia | Atrial ventricular arrhythmias (torsades de pointes) | Dysphagia | IV Magnesium sulfate |
|
| Hypermagnesemia | Sedation, paralysis, areflexia | Ventricular arrhythmias | Diarrhea | 0.9% NaClFurosemide | |
| Hypophosphatemia | Confusion, seizures, weakness | Heart failure, respiratory failure | Sodium or potassium phosphate | ||
| Hyperphosphatemia | Muscle cramps, perioral tingling, paresthesias | Ventricular arrhythmias | 0.9% NaCl, phosphate binders |
Pediatric surgery falls within the purview of general surgery. Accordingly, it is the intent of this chapter to discuss many of the more common pediatric surgical emergencies from birth to adolescence that are relevant to practicing general surgeons in the community as well as general surgery trainees preparing for board exams. The key management steps for each condition are emphasized. This chapter is not meant to be a comprehensive text of all pediatric surgical emergencies, and we do not focus on complex neonatal congenital anomalies because this group of children is almost exclusively referred to board-certified pediatric surgeons based at major tertiary care referral centers.
As the saying in pediatric surgery goes, “children are not small adults.” Certainly, as children approach the teenage years, they are more like adults than neonates, toddlers, and school-aged children, but there are many important differences in the physiology and anesthesia of children that are worth noting. First, the normal vital signs in newborns are vastly different than those in adolescents and adults, and the normal ranges change with age (e.g., heart rate 120–180 beats per minute in term infants compared with 60–100 beats per minute in teenagers, blood pressure 70s/40s mm Hg in term infants compared with 100s/60s mm Hg in teenagers). Second, there are important airway issues that are different in children than they are in adults. Besides the obvious smaller size of the airway, orotracheal intubation in children is more challenging in general because of a more prominent occiput, a larger tongue, and a floppier epiglottis. In children younger than 12 years of age, an emergent surgical cricothyroidotomy with placement of either an endotracheal tube or tracheostomy tube is contraindicated because the pediatric airway is less rigid, the glottis is smaller, and there is a nontrivial risk of injury to these structures or to the posterior wall of the trachea. Therefore, needle cricothyroidotomy with jet oxygenation is the emergency airway procedure of choice in young children until they are stable enough for transport to the operating room for conversion to a surgical tracheostomy. Third, there is emerging data that general anesthesia may be detrimental to the developing pediatric brain. Thus, pediatric surgical problems that require general anesthetics but are more elective in nature (e.g., lumps and bumps) in infants should be delayed if possible.
Pediatric appendicitis is the most common acute surgical problem in children. In many ways, it is managed similarly to adults; however, pediatric patients, especially younger age children (2–6 years of age) frequently present perforated. In contrast with adults, ultrasound and MRI have become the preferred imaging modalities in many pediatric emergency departments because they provide comparable diagnostic accuracy while avoiding the potential long-term malignancy risks associated with ionizing radiation ( Fig. 1 ).
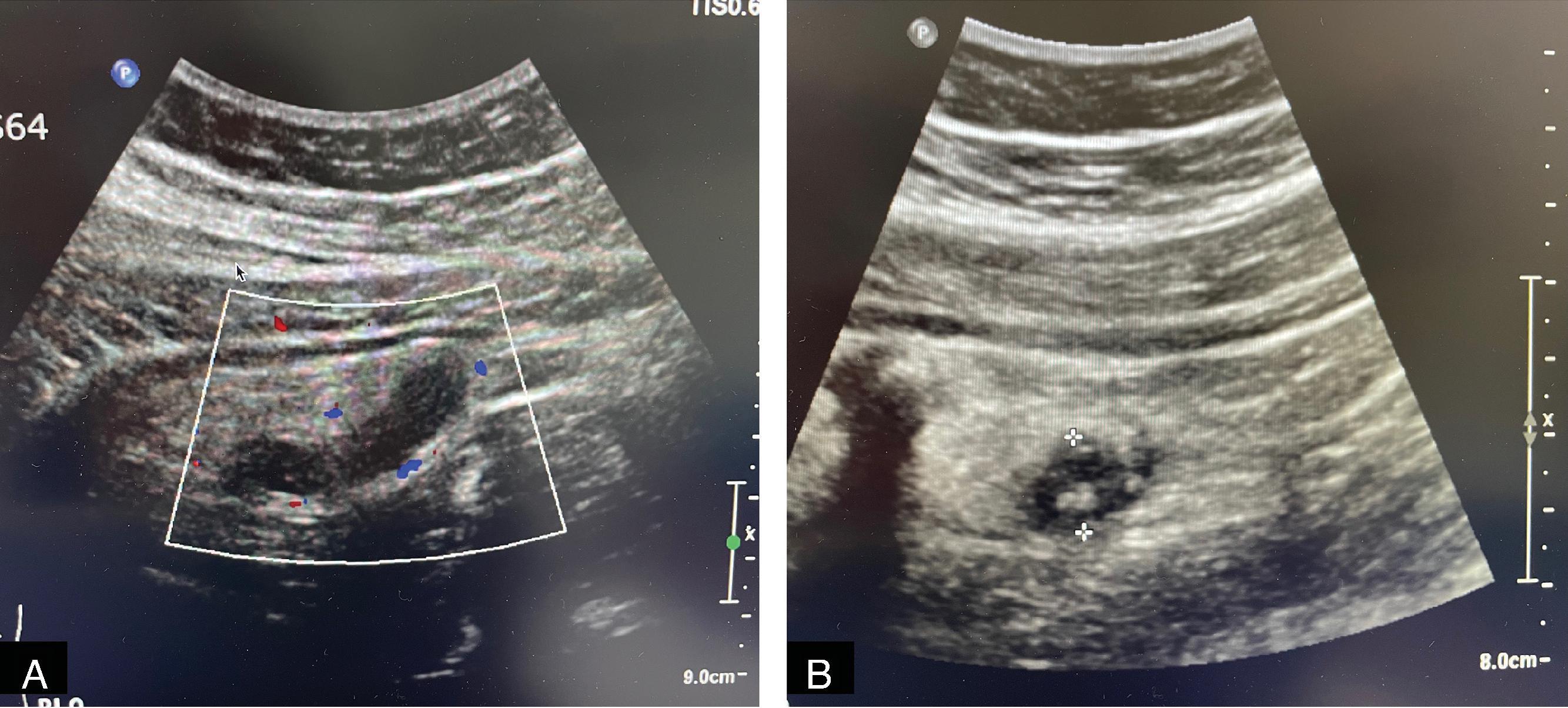
As in adults, laparoscopic appendectomy in children is now the standard of care in the operative management of most cases of acute appendicitis. Despite a smaller working space, conversion of laparoscopy to an open procedure is rarely necessary, even in smaller children. There are now several recent studies that support nonoperative management of acute, simple appendicitis with broad-spectrum antibiotics as a viable treatment alterative in children, demonstrating symptom resolution in 24 to 48 hours in 80% to 90% of cases. Those with a concomitant infection with COVID-19 are usually managed nonoperatively. However, not all children qualify for nonoperative management, such as those with a fecalith, and there is medium-term follow-up data suggesting that overall recurrence rates after discharge may be as high as 40%. As a result, operative management within 12 to 24 hours of diagnosis continues to be the preferred management approach among many pediatric surgeons. Same-day discharge is possible in many children with early disease after appendectomy. In addition, given concerns about recurrent appendicitis, most surgeons view nonoperative management as a bridge to elective outpatient interval appendectomy. In children with known perforated appendicitis who present with symptoms for 5 days or more, patients can often be managed nonoperatively with interventional radiology drain placement (if there is an amenable abscess) followed by an interval appendectomy in 6 to 8 weeks. There is no consensus on the optimal duration of antibiotics for perforated disease, with or without appendectomy, because of the wide spectrum of clinical disease. Antibiotic duration is often based on institution-specific protocols with longer courses used for patients who are febrile, have leukocytosis, are without source control, and/or have a prolonged ileus.
Trauma is the leading cause of mortality and morbidity in children 1 to 14 years of age in the United States. It accounts for more death and disability than all other childhood diseases combined. Unfortunately, there must be increased awareness of nonaccidental trauma (NAT), particularly in younger pediatric patients. Any injury pattern that does not fit the history or a history that keeps changing should alert suspicion for possible NAT ( Fig. 2 ). Injuries or rib fractures in different stages of healing or a delay in seeking medical attention should also alert the surgeon to abuse. Another concerning injury is a scald burn in a stocking distribution without splash marks. In all these cases, the mechanisms for evaluating for child abuse providers in your hospital should be activated. Typically, this usually involves consultation with social work and the child protection team. If there is sufficient concern, then a skeletal survey should be performed to look for evidence of other acute and healed fractures. If there is intracranial hemorrhage demonstrated by cross-sectional imaging of the brain in the setting of NAT, then an exam for retinal hemorrhages by ophthalmology is needed. Once NAT is confirmed, then local government agencies (i.e., child protection services) should be notified to ensure the safety of the injured child following recovery.
Pediatric trauma assessment follows Advanced Trauma Life Support (ATLS) protocols in a similar manner to adult trauma. The one major exception, however, is that chest, abdomen, and pelvis CT scans should be minimized in children, whenever possible, based on evidence-based guidelines. If a child is critically ill, then a CT scan is obviously appropriate. However, imaging in the clinically stable child is approached in a more stepwise fashion starting with trauma radiographs (e.g., cervical spine, chest, and pelvic x-rays) and serum blood work (e.g., CBC, CMP, lipase, PT/INR, PTT, and microscopic urinalysis). If the laboratory studies suggest a concern for bleeding, the lipase is elevated, the AST or ALT is greater than 200 U/L, or urinalysis shows more than 20 red blood cells per high power field, then a CT scan of the abdomen and pelvis are performed. If no abnormalities are identified by clinical exam, radiographs, and blood work in the clinically stable child, continued observation with serial abdominal exams is appropriate.
The use of head CTs in pediatric blunt trauma follows guidelines established by the Pediatric Emergency Care Applied Research Network (PECARN). Depending on the clinical presentation, these guidelines help the surgeon decide whether children with a normal or near-normal mental status based on the Glasgow Coma Scale (GCS) should be imaged by head CT versus close observation for several hours in the emergency department. If repeat imaging is needed after an abdominal initial CT or changing clinical exam, a trauma protocol ultrafast MRI can be helpful to monitor the evolution of intracranial hemorrhage.
The treatment of most pediatric traumatic injuries is similar to those in adults. However, one notable exception in younger children is a stronger preference toward nonoperative management of splenic injuries, regardless of grade of injury by CT. The threshold for splenectomy in non-teenage children is quite high because of the increased risk of overwhelming post-splenectomy sepsis, an infection associated with high mortality. Obviously, if a child is unstable or has ongoing transfusion requirements, then splenic embolectomy or splenectomy may be indicated. An ethical dilemma that may arise in the management of solid-organ injury in children is parental refusal of blood transfusions for religious or other reasons. In these cases, the surgeon is ethically and legally allowed to override the parents in order to save the child’s life.
Inguinal hernias in children are common, especially in the premature neonatal population. They are almost universally indirect hernias resulting from failure of closure of the processus vaginalis during the third trimester of fetal life. Because these hernias tend to increase in size and neonates are not able to communicate symptoms, these hernias should be surgically repaired when safe for the child to undergo anesthesia. If the neonate is in the neonatal intensive care unit, these hernias are often repaired before discharge home. Premature infants who undergo hernia repair under general anesthesia who are younger than 60 weeks corrected gestational age as well as term infants who are younger than 48 to 52 weeks gestational age are required to stay overnight postoperatively for apnea monitoring before discharge.
Inguinal hernias in young children can be repaired using a variety of techniques and approaches. In neonates and infants, many pediatric surgeons now favor repair by laparoscopic-assisted ligation of the internal inguinal ring using a permanent suture. Open inguinal hernia repairs with a high ligation of the hernia sac are also commonly performed, especially in patients older than 1 to 2 years of age and in those with a large hydrocele component to the hernia. Regardless of operative approach, mesh repair is rarely necessary before adolescence because there is almost never a direct inguinal or femoral hernia present in younger children, and the mesh does not grow with the child.
Incarcerated inguinal hernias are much more common in infants than they are in older children and adults. If an infant presents with an incarcerated inguinal hernia without overlying erythema or peritonitis, an attempt at manual reduction of the hernia at the bedside should be performed by an experienced provider. Administering an opioid and placing the child in the Trendelenburg and frog-leg positions can be helpful maneuvers before applying manual pressure. If the hernia cannot be reduced, then an urgent surgical repair should be performed given concerns for strangulation. Successful reductions should be followed with open or laparoscopic inguinal hernia repair before discharge given the high rate of recurrence ( Fig. 3 ). The laparoscopic approach has the additional benefit of being able to fully evaluate for intestinal injury at the time of repair. However, if an open hernia repair is planned, it may be wise to wait 24 to 48 hours to allow for inguinal soft tissue edema to resolve.
Bilious emesis in newborns and young infants is the major symptom of malrotation with midgut volvulus and therefore should always be considered a diagnostic surgical emergency until the condition has been ruled out. Malrotation anomalies of the gut, which are surprisingly common with an incidence estimated to be 1 in 200, are associated with midgut volvulus because of the presence of abnormal right upper quadrant adhesions in conjunction with a narrowed mesenteric base. Seventy percent of children with symptomatic malrotation will present in the first year of life, and of those, 70% occur in the first month (i.e., 49% of all patients with malrotation will develop symptoms in the first month). If an infant presents with bilious emesis and is clinically ill, this alone may be an indication for emergent operative exploration without contrast imaging to save the child’s life.
It is important to understand that a “normal bowel gas pattern” shown on plain radiographs can never be used to exclude malrotation in an infant with bilious emesis under any circumstance. An expeditious workup for bilious emesis always requires an emergent upper gastrointestinal contrast (UGI) study. A positive UGI study warrants emergent laparotomy for presumed volvulus of the entire midgut. A small bowel follow-through study to fully delineate the presence of a small bowel obstruction is not necessary if malrotation is present. Although abdominal CT scans may identify malrotation as an incidental finding in older children and adults with abdominal pain, there is no role for CT as the initial study in symptomatic infants who present with bilious emesis.
There are three key features of the duodenum that are used to exclude malrotation based on a UGI study: (1) crosses to the left of midline (using the spine as the landmark on anteroposterior views, Fig. 4 ), (2) C-loop shape such that the fourth part of the duodenum rises to the level of the pylorus (anteroposterior views), and (3) retroperitoneal course toward the spine (as demonstrated on lateral view). Absence of any one of these components is considered an abnormal UGI and warrants exploration in the symptomatic child. If a UGI series for bilious emesis has ruled out malrotation, a surgical condition distal to the ampulla of Vater may still be present, but there is usually less urgency in the diagnostic workup.
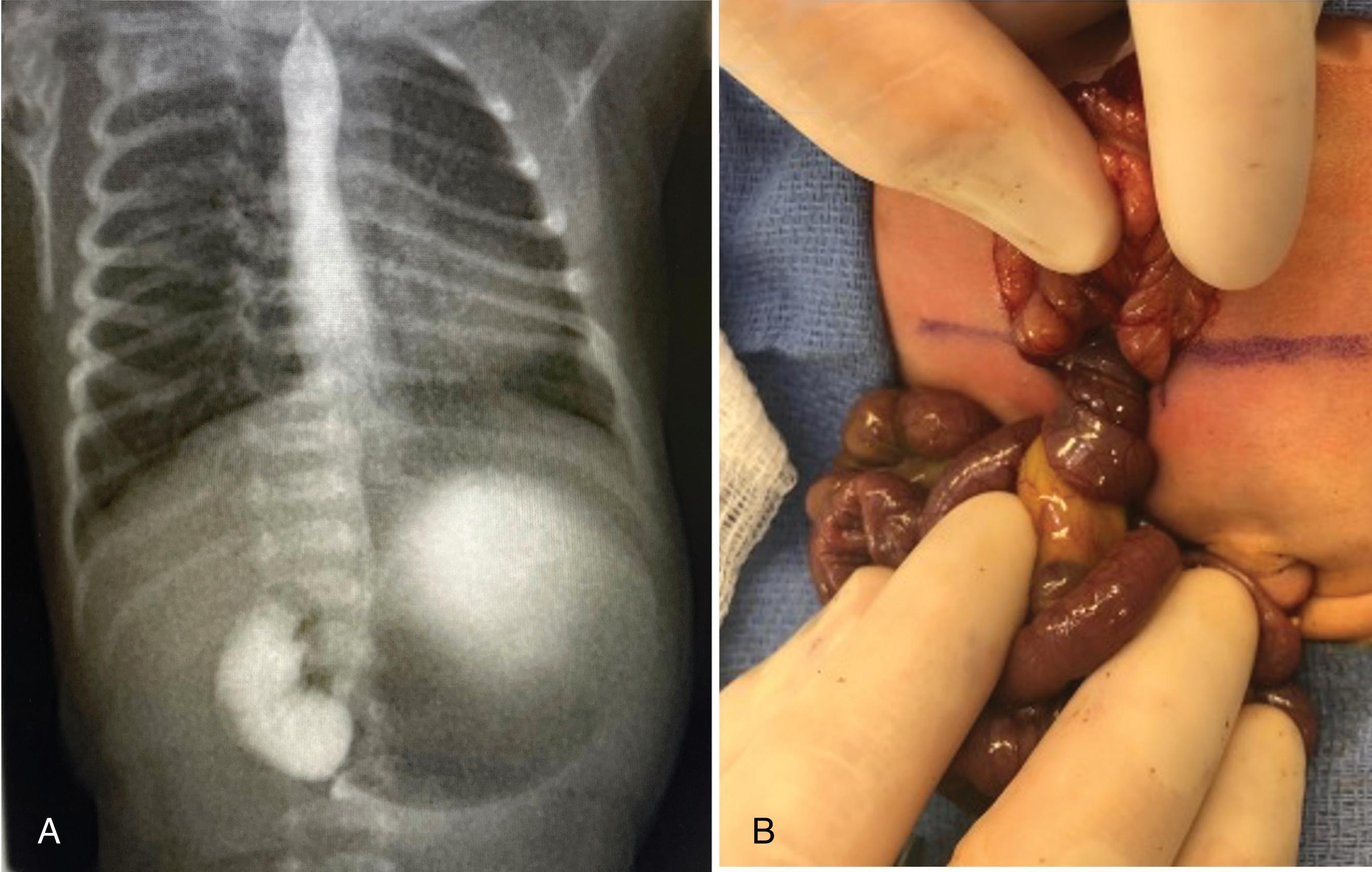
In all cases of symptomatic malrotation with midgut volvulus, an emergency supraumbilical transverse laparotomy is performed because the volvulus must be detorsed as soon as possible before total midgut necrosis ensues. The operation, known as the Ladd procedure, consists of six main steps: (1) detorsing the volvulus by rotating the gut counterclockwise (i.e., “turning back the hands of time”) usually with a 180- to 360-degree twist; (2) lysing Ladd’s bands (bands from the cecum across the duodenum to the right lower quadrant peritoneum); (3) straightening the duodenum, which is often kinked and folded by adhesions; (4) widening the mesentery by opening it like a book and lysing any mesenteric adhesions; (5) performing an appendectomy; and (6) placing the bowel in a position of nonrotation, meaning the colon is on the left side of the abdomen and the small bowel is on the right side. The position of nonrotation serves to keep the small bowel mesentery as wide as possible to minimize the risk of future volvulus. The risk of recurrent volvulus after an open Ladd procedure is less than 2% but is never reduced to that of the normally rotated patient.
Necrotizing enterocolitis (NEC) is a severe inflammatory disease that affects the small and large intestine and occurs in approximately 1 in 1000 live births. NEC is a disease primarily of premature infants and rarely occurs in term infants in the absence of sepsis, congenital heart disease, or respiratory illness. The risk of NEC is highest in premature infants who weigh less than 1500 g and are fed enterally. The overall mortality secondary to NEC is approximately 30%, with a higher mortality in infants requiring surgery (approaching 50%).
The classic presentation of NEC is abdominal distention, bloody nasogastric tube output, and bloody stools. In the history, one will often find that these clinical changes coincided with a change from breast milk to formula or fortification of breast milk to higher caloric density. The workup for NEC includes laboratory evaluation with CBC, electrolytes, and blood gas. Metabolic acidosis and thrombocytopenia should raise increased suspicion for NEC. Radiologic evaluation with abdominal films should be obtained (both an anteroposterior and left lateral decubitus film if the infant will tolerate it). Radiographs will demonstrate pneumatosis, and in severe cases, portal venous gas and/or pneumoperitoneum. Ultrasound is more sensitive than plain films for detecting pneumatosis and portal venous gas, but its role as an aid in surgical decision making remains undefined.
The management of NEC depends on the severity. Historically the severity of NEC was classified using the Bell grading system ( Table 1 ). Those with stage I and IIA disease can be treated primarily with nasogastric tube decompression, bowel rest, and broad-spectrum antibiotics (e.g., piperacillin/tazobactam) provided that the clinical status, serial laboratory tests, and x-rays are closely followed and do not worsen over time. The antibiotic course is typically 7 days from resolution of pneumatosis on plain radiographs, although the exact length of treatment is often individualized.
| Clinical Findings | Radiographic Findings | Gastrointestinal Findings | |
|---|---|---|---|
| Stage I | Apnea, bradycardia, and temperature instability | Normal gas pattern or mild ileus | Mild abdominal distention, stool occult blood, gastric residuals |
| Stage IIA | Apnea, bradycardia, and temperature instability | Ileus with dilated bowel loops and focal pneumatosis | Moderate abdominal distention, hematochezia, absent bowel sounds |
| Stage IIB | Metabolic acidosis and thrombocytopenia | Widespread pneumatosis, portal venous gas, ascites | Abdominal tenderness and edema |
| Stage IIIA | Mixed acidosis, coagulopathy, hypotension, oliguria | Moderate to severely dilated bowel loops, ascites, no free air | Abdominal wall edema, erythema, and induration |
| Stage IIIB | Shock, worsening vital signs and laboratory values | Pneumoperitoneum | Bowel perforation |
The decision to operate for NEC (20%–40% of cases) can be difficult. The only absolute indication for operative management is pneumoperitoneum. However, many children will undergo a laparotomy in the absence of pneumoperitoneum because of deteriorating clinical status or worsening imaging findings despite medical management. In these cases, there is a high suspicion for bowel necrosis. Relative indications to operative management include abdominal wall erythema/cellulitis and radiographic evidence of portal venous gas and/or a persistent fixed loop of dilated bowel. Surgery for NEC consists of a transverse superumbilical laparotomy, exploration of the intestine and colon, and removal of all necrotic bowel while salvaging all healthy or questionable bowel. If there is a lot of ischemic bowel without frank necrosis, a spring-loaded silo should be placed with plans for a second look operation in 24 to 48 hours. If the area of pathology is well demarcated, most pediatric surgeons would create a proximal ostomy and distal mucous fistula before abdominal wall closure. The ostomies can be reversed after 6 weeks or more have passed if the child weights at least 3 kg. It is essential that the infant tolerate feeds before surgery (to ensure no proximal stricture from NEC), otherwise a small bowel follow-through should be performed before reversal of the ostomies. A contrast enema and/or mucous fistula study should also be performed as well to rule out distal strictures before placing the bowel back into continuity.
It is important to distinguish NEC from spontaneous intestinal perforation (SIP), another intestinal disease that affects low-birthweight premature infants. SIP has been associated with indomethacin administration and/or early postnatal steroids and presents as pneumoperitoneum secondary to a focal area of terminal ileal perforation. In contrast, babies with NEC have more widespread, patchy intestinal ischemia and necrosis with perforation as later stages. Accordingly, neonates with SIP will often lack the signs of shock initially that are almost universally observed in neonates with ischemic bowel secondary to NEC. Surgical management of SIP and NEC are similar in that necrotic intestine should be resected and ostomies created. However, in very-low-birthweight neonates (<1000 g) in whom SIP is highly suspected, placement of a bedside lower quadrant peritoneal drain (using a vessel loop or ¼-inch Penrose drain) is considered appropriate first-line therapy. After the intestinal perforation heals spontaneously and the clinical condition of the neonate improves, the peritoneal drains and antibiotics can be discontinued after 7 to 10 days. For those managed with a drain, the surgeon should be prepared to do a laparotomy if there is no clinical improvement in 24 to 48 hours.
Hypertrophic pyloric stenosis is relatively common, occurring in approximately 1 in 300 live births. The male-to-female ratio is 4 to 1. Pyloric stenosis is not a congenital disease. Instead, it typically presents in infants between 3 and 6 weeks of age (range, 2–10 weeks) with progressively worsening, nonbilious emesis that ultimately becomes projectile in nature. Because of the proximal obstruction and emesis, they often present severely dehydrated as evidenced by sunken fontanelles and dry mucous membranes. On physical exam, one may be able to feel an epigastric, olive-shaped mass, but this usually requires decompressing the stomach with a nasogastric tube and pain medications to be successful.
Ultrasound has become the gold standard for diagnosis of hypertrophic pyloric stenosis ( Fig. 5 ). Pyloric stenosis is confirmed when the pyloric muscle thickness is 4 mm or more and the pyloric channel length is at least 16 mm. Typically, the sonographer will also observe the inability of fluid to pass through the pylorus during the entire exam. On occasion, a UGI is performed when serial ultrasound exams show equivocal findings with gastroesophageal reflux disease and pylorospasm still in the differential diagnosis. All infants with hypertrophic pyloric stenosis should have a complete metabolic panel obtained. The metabolic panel will often demonstrate a hypochloremic, hypokalemic, metabolic alkalosis.
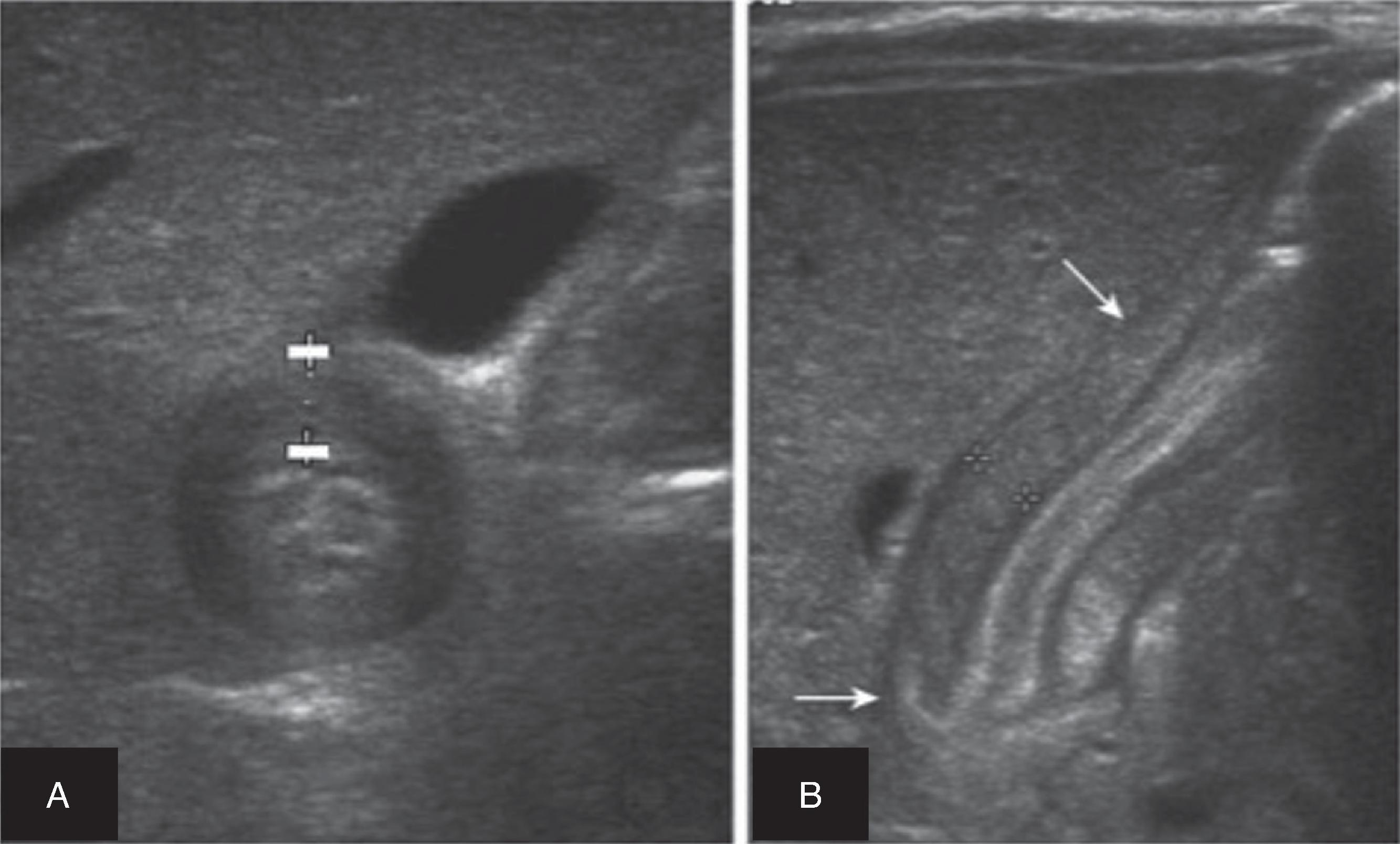
Surgery for hypertrophic pyloric stenosis is never a true emergency. These infants must first be resuscitated to address the dehydration and electrolyte abnormalities. These infants should receive one or two 20 mL/kg boluses of normal saline in addition to 1.5 × maintenance rate of D5/NS with electrolytes checked every 6 to 12 hours until normal. Infants with bicarbonate greater than 30 mEq/L have a diminished respiratory drive and are at risk for postoperative apnea and possible respiratory arrest. Therefore, they should not undergo anesthesia until the metabolic alkalosis is corrected. The endpoints suggestive of a successful preoperative resuscitation include wet diapers, a chloride level of 90 to 100 mEq/L, and a bicarbonate level of 30 mEq/L or lower.
Once all electrolyte abnormalities have been corrected, laparoscopic pyloromyotomy has become the operation of choice among many pediatric surgeons (although a right upper quadrant or periumbilical pyloromyotomy can also be performed). The laparoscopic approach is conducted through three laparoscopic incisions: one with a port at the umbilicus and two 3-mm stab incisions without ports on either side of the abdomen. The pylorus is carefully stabilized with a grasper, and the muscular layer is incised and split from the stomach to where the muscle softens on the duodenal side. One should see bulging mucosa underneath the myotomy, which indicates a complete myotomy. A leak test is then performed by injecting 30 to 60 mL of air through an orogastric tube to demonstrate that air passes through the pylorus and that no air escapes through the exposed mucosa. Although there have been numerous published studies on the optimal feeding protocol for these infants following pyloromyotomy, ad lib feeding with a 60-mL limit may be the easiest and best approach. Most children can be discharged from the hospital within 36 hours after tolerating at least two consecutive feeds.
The two most infamous complications of pyloromyotomy are an incomplete myotomy and a mucosal perforation. An incomplete myotomy occurs when the myotomy is not carried far enough on to the stomach side and presents as ongoing projectile emesis. This requires a return to the operating room for revision. A mucosal perforation can be managed with either closure of the perforation and buttressing with omentum or, more commonly, repair of the mucosal perforation, closure of the myotomy, and then rotation of the pylorus to perform a repeat myotomy 180 degrees from the first. If not identified intraoperatively, these infants can become septic and must undergo emergency operation with peritoneal washout.
Ileocolic intussusception is a condition in which the terminal ileum slides distally and telescopes into the adjacent right colon, leading to obstruction and impaired blood flow to the affected segment. Although rare in adults, it is a common cause of abdominal pain in older infants and toddlers. Most children present with intermittent abdominal pain and vomiting, while others may be inconsolable or lethargic. The history often describes distinct periods in which the child may keep his or her legs curled up followed by periods of relatively normal activity and minimal pain. Some patients may also have “currant jelly” stools as a result of sloughing of intestinal mucosa at the site of the intussusception. Because most intussusceptions in children are caused by viral-mediated mesenteric lymphadenopathy serving as the lead point, there may also a history of a recent viral illness (either respiratory or gastrointestinal). Physical exam findings may reveal abdominal tenderness and possibly a palpable abdominal mass.
The workup for possible intussusception often starts with an abdominal plain film to look for evidence of a small bowel obstruction. However, if there is increased suspicion, an abdominal ultrasound exam should be ordered to look for the obstructing mass. CT scans can be performed but are rarely required to establish the diagnosis and expose the child to unnecessary ionizing radiation. The ultrasound exam will demonstrate a classic target sign ( Fig. 6 ), which represents the terminal ileum intussuscepting into the cecum. If the ultrasound exam is positive or equivocal, then proceeding with an air-contrast enema can be both diagnostic and therapeutic. The only contraindications to performing an air-contrast enema for suspected intussusception are peritonitis, free air, and hemodynamic instability.
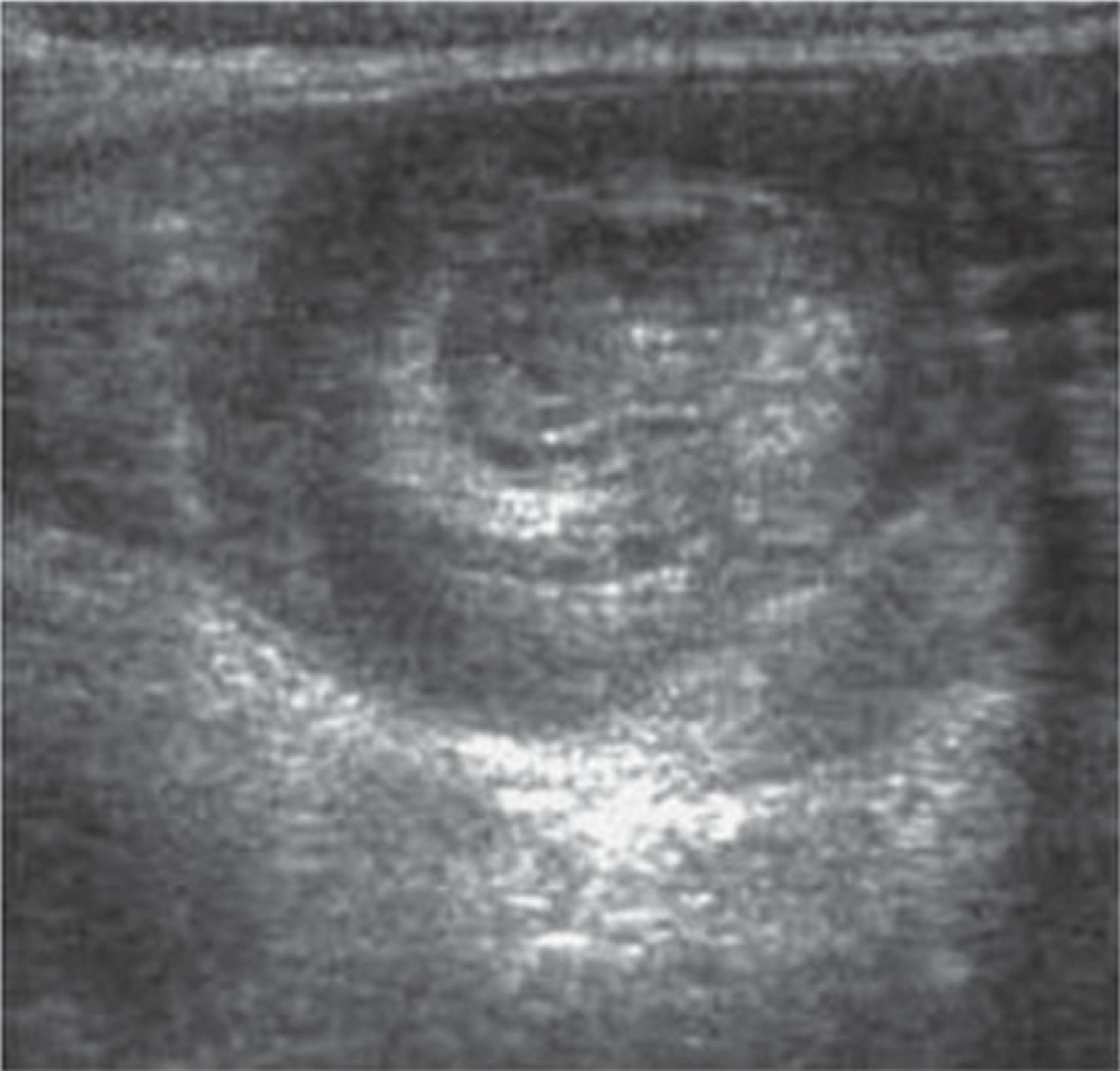
An air-contrast enema performed by a pediatric radiologist is the first-line treatment of choice in younger children in stable condition and is associated with a 70% to 90% success rate. The colonic pressure should never exceed 120 cmH 2 O pressure during the study, and the surgeon should be immediately available in the rare event (1%–2%) that a perforation occurs. Successful reduction is suggested by resolution of the obstructing mass followed by reflux of air into the terminal ileum. Once reduced, children are observed for a short period (range, 4–24 hours) based on institutional protocols and then discharged once tolerating a regular diet. Family should be warned that the overall recurrence rate is 5% to 10%, with most recurrences evident in the first 2 to 3 days postreduction. If the intussusception recurs after successful reduction, the air-contrast enema can be repeated. However, if it recurs after three reductions, then most surgeons would proceed to the operating room for definitive reduction and to determine whether a pathologic lead point may be the etiology. Pathologic lead points, which may include a Meckel diverticulum, duplication cysts, colonic polyps, colonic lipomas, and lymphoma, are more common in children older than 4 years of age.
If radiologic reduction fails or the patient has peritonitis, surgical reduction should be performed. This can be done by laparoscopy or laparotomy ( Fig. 7 ). In the open approach, the classic teaching is that the surgeon should carefully milk or push the intussuscepted ileum out of the cecum as opposed to pulling it out, which is thought to pose a higher risk of perforation. If the bowel is necrotic or cannot be reduced, it should be resected. During the operation, one should confirm whether there are any pathologic lead points that would require resection. In cases of lymphoma, the disease should be treated medically.
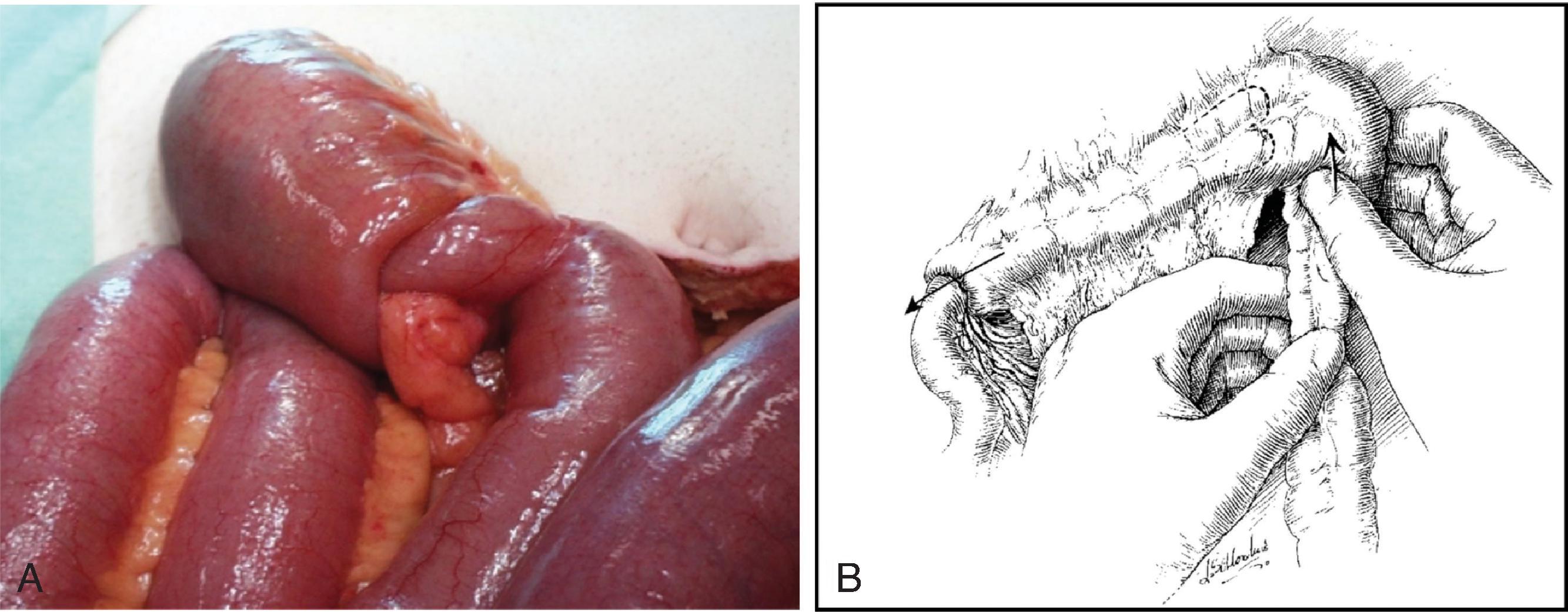
This chapter discusses the contemporary diagnostic workup and management of children presenting with common surgical emergencies. All general surgeons and trainees preparing for board exams must be familiar and comfortable with handling these pediatric surgical problems and the various nuances in their care when compared with those present in their adult counterparts. Although many of these surgical emergencies are now managed by full-time, board-certified pediatric surgeons, adult general surgeons in the community setting will inevitably encounter many of these cases over their careers, and if appropriately managed, can make a substantial impact on minimizing morbidity and can potentially save a young child’s life.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here