Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The field of prenatal diagnosis, once dominated by amniocentesis and karyotype analysis for advanced maternal age, has changed significantly over the last decade with improved screening technologies reducing the use of diagnostic procedures. Much of this expansion has been associated with the development of molecular technologies fostered by knowledge of the human genome. A description of these technologies is presented in Chapter 2 .
Although prenatal testing of the fetus for genetic disorders can have a huge impact on individual families, from a population standpoint, most disorders occur in less than 1% of pregnancies. Based on this, many of the important advances over the last decade have been led by the development of improved screening tests to target individuals at the highest risk for a fetal genetic disorder. In this chapter, we describe the approaches to screening ongoing pregnancies for genetic disease, the modalities available for in utero fetal diagnosis of congenital disorders, and pretest and posttest counseling.
The goal of screening is to detect or define risk for a fetal genetic disease in an asymptomatic low-risk population. As opposed to diagnostic testing, intended to identify or confirm an affected pregnancy, screening is intended to identify pregnancies with increased risk for a specific disorder and patients for whom diagnostic testing may be warranted. An ideal perinatal genetic screening test should fulfill the following criteria:
Identify common or important fetal disorders
Be cost-effective and easy to perform
Have a high detection rate and a low false-positive rate
Be reliable and reproducible
Screen for disorders for which a diagnostic test exists
Be positive early enough in gestation to permit safe and legal options if pregnancy termination based on the testing results is desired
Sensitivity and specificity are two key concepts in evaluating screening test performance. Sensitivity is the percentage of individuals with affected pregnancies who screen positive. Specificity is the percentage of individuals with unaffected pregnancies who screen negative. The reciprocal of specificity is the false-positive rate. Sensitivity and specificity are independent of disease frequency and describe the anticipated performance of a screening test in a population. Alternatively, positive and negative predictive values depend on disease prevalence and are vital in the interpretation of the test result for an individual patient. These latter two values represent, respectively, the likelihood that a person with a positive or negative test does or does not have an affected pregnancy. The impact of disease on positive and negative predictive values is described in Chapter 15 and is shown in Tables 15.7 and 15.8 .
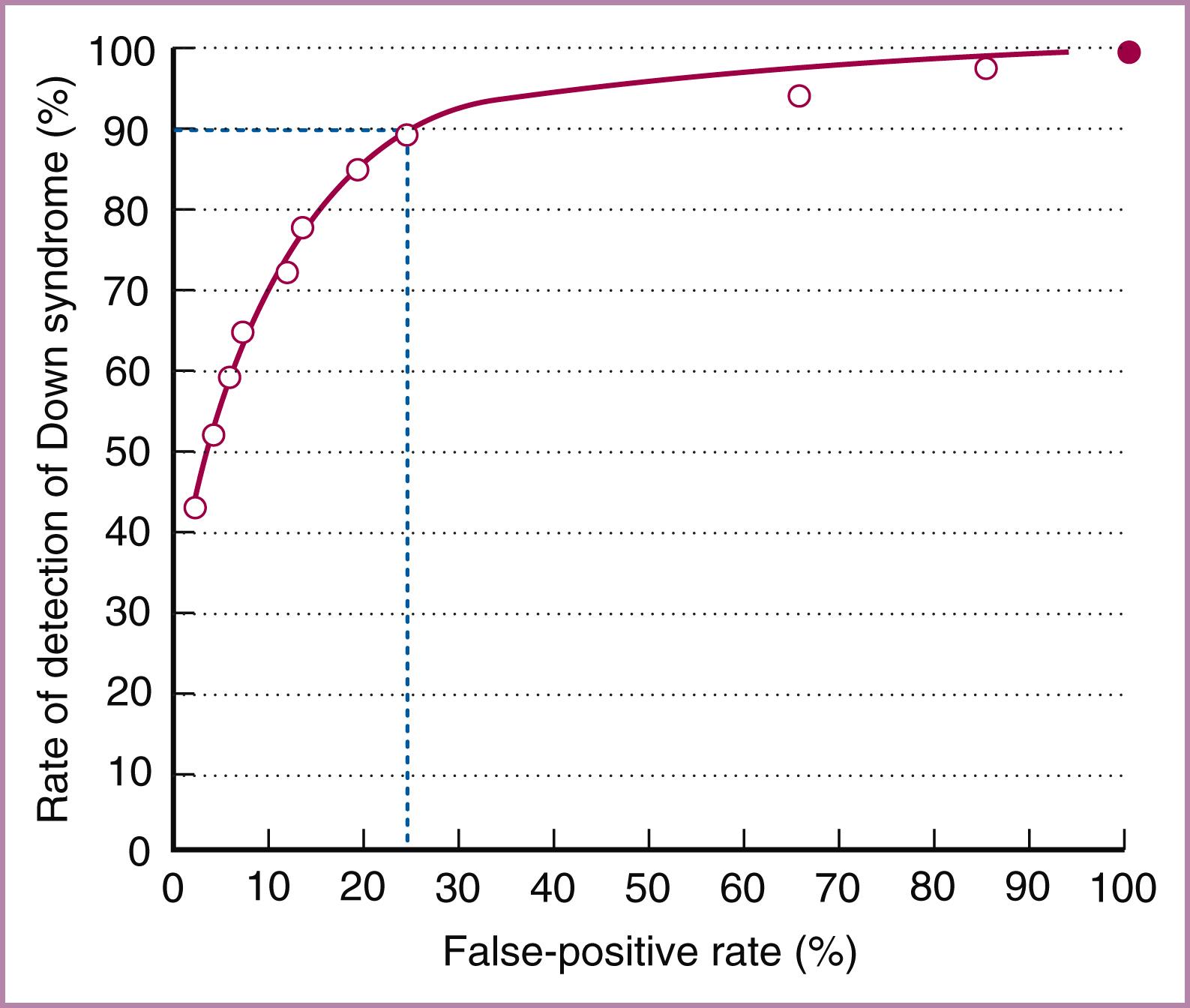
Use of screening tests requires that cutoff values for positive tests be established. Performance of the test depends on this cutoff; for example, an increased detection rate can be obtained by lowering the cutoff threshold, but the concomitant lowered specificity would result in more false-positive results. Table 30.1 shows the performance of second-trimester maternal serum screening for Down syndrome based on various cutoffs. A receiver operating characteristic curve can be used as a statistical method to find the best balance between sensitivity and specificity. A line diagram is plotted with sensitivity on the vertical axis and false-positive rate plotted horizontally ( Fig. 30.1 ). The greater the area under the curve (i.e., the line moving toward the upper left corner), the better the test’s performance, with increasing sensitivity and a reduced false-positive rate.
| Triple-Screen Cutoff | Detection Rate (%) | FPR (%) | Quadruple-Screen Cutoff | Detection Rate (%) | FPR (%) |
|---|---|---|---|---|---|
| 1:200 | 57 | 4.3 | 1:200 | 60 | 3.5 |
| 1:250 | 61 | 5.6 | 1:250 | 64 | 4.5 |
| 1:300 | 64 | 6.8 | 1:300 | 67 | 5.5 |
| 1:350 | 67 | 8.1 | 1:350 | 69 | 6.5 |
| 1:400 | 69 | 9.3 | 1:400 | 72 | 7.6 |
Pretest patient education is an important component of screening since understanding the concept of risk modification is difficult for many patients. This should include an explanation of the significance of a positive or negative result, and posttest reporting of the absolute predicted risk rather than simply positive or negative will further help in interpretation. Viewed from the patient’s perspective, reporting tests as positive or negative (i.e., above or below the laboratory cutoff) can be confusing and misleading. For example, if the laboratory cutoff for a positive result is chosen as 1:250, a test result of 1:249 would be reported as “screen positive” and may lead to a choice of a diagnostic test, whereas a result of 1:251 would be reported as “screen negative” and may provide greater reassurance than intended.
The impact of a positive screening test depends on the pretest (a priori) risk of an affected pregnancy. Likelihood ratios are the likelihood that a specific test result would be expected in a patient with the target disorder compared with the likelihood that the same result would occur in a patient without the target disorder; that is, likelihood ratios are basically a ratio of the probability that a test result is correct to the probability that the test result is incorrect.
For binary risk factors that are either present or absent, likelihood ratios are determined by comparing the frequency of positive tests in a population of affected pregnancies with the frequency of a positive test in unaffected pregnancies. This is calculated as the sensitivity of the test divided by its false-positive rate. For tests that use continuous variables (e.g., serum marker measurements), likelihood ratios are calculated by comparing gaussian distributions of the results from normal and affected pregnancies. Once a likelihood ratio is determined, it can be used to modify the a priori risk ( Table 30.2 ). If more than one likelihood ratio is available and if these are independent of each other, they can each be used separately to modify risk. In this way, multiple factors (e.g., individual serum analyte results and ultrasound findings) can be simultaneously used to modify the a priori risk (e.g., maternal age).
| A priori risk for Down syndrome (age or serum screen) | 1:1000 |
| Positive ultrasound marker for Down syndrome | |
| Rate in Down syndrome population (sensitivity of marker) | 10% |
| Rate in general population (FPR of marker) | 1.0% |
| Likelihood ratio (sensitivity/FPR) | 10.0 |
| Adjusted risk for Down syndrome | |
| (A priori risk × likelihood ratio) × 1⁄1000 × 10 | 1:100 |
The association of Down syndrome with advancing maternal age was first reported in 1909. It was not until 50 years later that karyotype analysis was developed and correlated the Down syndrome phenotype with an extra G chromosome. Based on recommendations from a National Institute of Child Health and Human Development (NICHD) consensus conference in 1979, invasive testing for Down syndrome began being offered routinely to women age 35 years or older at delivery, consistent with a second-trimester risk of 1:270 or higher (liveborn risk of 1:380). The age of 35 seemed a natural cutoff because women age 30 to 34 had a risk of 1:880, and the risk for women age 35 to 40 was almost fourfold higher. Other factors initially considered in determining this threshold included the relative risk of invasive testing compared with the risk of trisomy 21, the availability of resources capable of performing amniocentesis and karyotype, and the cost-to-benefit analysis.
The risk for Down syndrome is now recognized to be continuous, which emphasizes the arbitrary nature of an absolute age threshold of 35. In addition to maternal age, the risk for trisomy 21 depends on the gestational age at which testing is performed, as only 69% of first-trimester and 76% of second-trimester Down syndrome pregnancies are viable ( Table 30.3 ). In addition, although the risk for Down syndrome increases with age, 70% of affected pregnancies occur in women younger than 35. For these reasons as well as because more refined risk analysis has been developed, maternal age alone is no longer used as an independent indication for invasive testing.
| Maternal Age (year) | Gestational Age | Liveborn | ||
|---|---|---|---|---|
| 12 weeks | 16 weeks | 20 weeks | ||
| 20 | 1/1068 | 1/1200 | 1/1295 | 1/1527 |
| 25 | 1/946 | 1/1062 | 1/1147 | 1/1352 |
| 30 | 1/626 | 1/703 | 1/759 | 1/895 |
| 31 | 1/543 | 1/610 | 1/658 | 1/776 |
| 32 | 1/461 | 1/518 | 1/559 | 1/659 |
| 33 | 1/383 | 1/430 | 1/464 | 1/547 |
| 34 | 1/312 | 1/350 | 1/378 | 1/446 |
| 35 | 1/249 | 1/280 | 1/302 | 1/356 |
| 36 | 1/196 | 1/220 | 1/238 | 1/280 |
| 37 | 1/152 | 1/171 | 1/185 | 1/218 |
| 38 | 1/117 | 1/131 | 1/142 | 1/167 |
| 39 | 1/89 | 1/100 | 1/108 | 1/128 |
| 40 | 1/68 | 1/76 | 1/82 | 1/97 |
| 42 | 1/38 | 1/43 | 1/46 | 1/55 |
| 44 | 1/21 | 1/24 | 1/26 | 1/30 |
| 45 | 1/16 | 1/18 | 1/19 | 1/23 |
In 2007 (reaffirmed in 2016 and 2020), the American College of Obstetricians and Gynecologists (ACOG) recommended that both screening and diagnostic testing should be available to all women who present for prenatal care before 20 weeks of gestation regardless of maternal age. The decision for a woman older than 35 to have invasive testing is based on many factors, including the risk that the fetus will have an abnormality, the risk for pregnancy loss from an invasive procedure, and the patient’s perceived consequences of having an affected child. Studies of women’s preferences have shown that they weigh these potential outcomes differently. ,
Two maternal serum analytes are useful for first-trimester aneuploidy risk modification. Pregnancy-associated plasma protein A (PAPP-A) has been demonstrated to have a mean value of 0.4 multiples of the median (MoM) in trisomy 21 pregnancies. The free β subunit of human chorionic gonadotropin (hCG) is elevated in Down syndrome pregnancies, with a mean value of 1.8 MoM. Screening using PAPP-A alone identifies about 40% to 45% of trisomy 21 pregnancies, and free β-hCG identifies about 23%, both with a screen-positive rate of 5%. Combining both free β-hCG and PAPP-A can identify 60% to 65% of trisomy 21 pregnancies, for a similar 5% screen-positive rate.
The total hCG molecule can also be used for first-trimester screening but has slightly less discrimination than the free β subunit, especially at less than 11 weeks’ gestation. Free β-hCG begins to perform as a Down syndrome marker by 9 weeks’ gestation, reaching values almost twice those in unaffected pregnancies by 13 weeks. Levels of total hCG begin to increase above levels in unaffected gestations at 11 weeks. , The impact of substituting total hCG for the free β subunit on overall Down syndrome screening remains uncertain. A meta-analysis showed that in younger patients (<35 years old), detection of Down syndrome increased by 4, 5, 6, and 7 percentage points at 9, 10, 11, and 12 weeks when free β subunit was added to PAPP-A and nuchal translucency (NT), compared with 0, 0, 2, and 4 percentage points when intact hCG was added. In patients with advanced maternal age (>35 years old), inclusion of free β-hCG reduced the false-positive rate by 2.5, 3.1, 3.8, and 4.4 percentage points compared with 0.1, 0.3, 1.0, and 2.2 percentage points for intact hCG at 9, 10, 11, and 12 weeks. Other authors have found less impact. Using samples from the First and Second Trimester Evaluation of Risk (FASTER) study, Canick and coworkers showed that at 12 weeks’ gestation, the addition of free β-hCG to NT and PAPP-A increased detection only 0.9% (−3.3 to 6.3). However, at earlier gestational ages, the impact of free β-hCG would be greater.
In his initial description of the syndrome that bears his name, Langdon Down described skin so deficient in elasticity that it appeared to be too large for the body. This was particularly noticeable in the neck area. The skin and underlying lymphatic fluid in the fetal neck can now be seen with ultrasound at 10 to 12 weeks’ gestation. NT is defined as the collection of fluid under the skin behind the neck in fetuses between 11 and 14 weeks’ gestation. It can be successfully measured by transabdominal ultrasound examination in approximately 95% of cases, and quantification of NT is used for first-trimester Down syndrome screening.
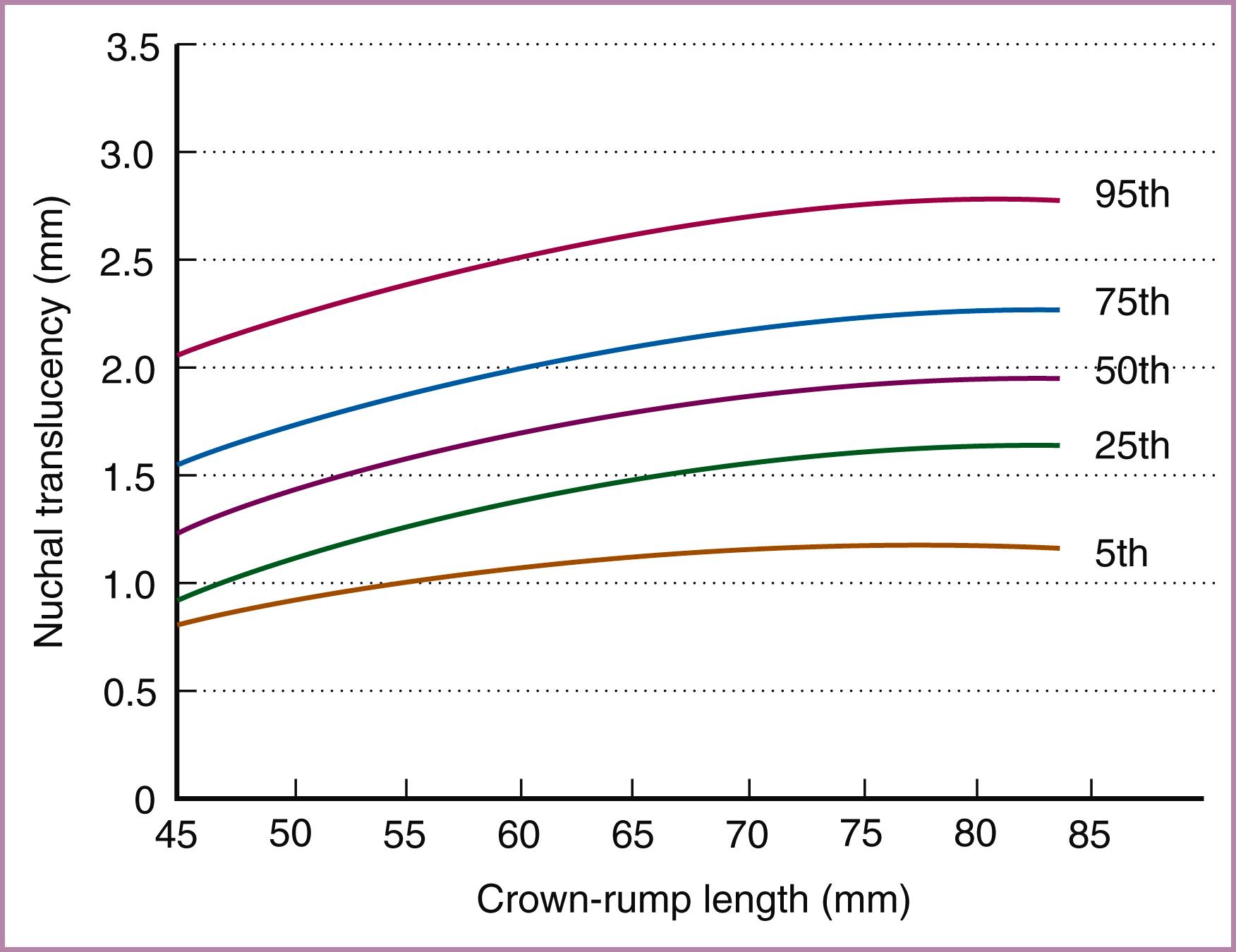
An association between increased NT and fetal chromosomal defects is now well known. This association allows the NT measurement to be converted into a likelihood ratio. Because the median NT increases with gestational age and thus crown-rump length, the actual measurement must first be converted into gestational age–specific MoM or a measurement of the deviation from the expected mean (delta NT) before conversion into a likelihood ratio. Fig. 30.2 illustrates the NT measurement between 11 and 14 weeks’ gestation.
The performance of the NT test combined with maternal and gestational age to assess the risk for Down syndrome was studied in a series of more than 100,000 pregnancies. The risk for Down syndrome was calculated by the maternal age–specific and gestational age–specific prevalence multiplied by the NT likelihood ratio, with a cutoff of greater than 1:300 used as screen positive. The sample included 326 fetuses with trisomy 21; 82% of trisomy 21 fetuses were identified, for a screen-positive rate of 8.3%. When a screen-positive rate of 5% was selected, the sensitivity was 77% (95% confidence interval [CI], 72%–82%). Subsequent studies have demonstrated similar Down syndrome detection rates, between 70% and 75% ( Table 30.4 ).
| Study | Gestation (weeks) | N | Successful Measurement (%) | NT Cutoff (mm) | FPR (%) | Detection Rate of Trisomy 21 |
|---|---|---|---|---|---|---|
| Pandya et al., 1995 | 10–14 | 1763 | 100 | >2.5 | 3.6 | 3 of 4 (75%) |
| Szabo et al., 1995 | 9–12 | 3380 | 100 | >3.0 | 1.6 | 28 of 31 (90%) |
| Roberts et al., 1995 | 8–13 | 1704 | 66 | >3.0 | 6.0 | 1 of 3 (33%) |
| Bower et al., 1995 | 8–14 | 1481 | 97 | >3.0 | 6.3 | 4 of 8 (50%) |
| Kornman et al., 1996 | 8–13 | 923 | 58 | >3.0 | 6.3 | 2 of 4 (50%) |
| Zimmermann et al., 1996 | 10–13 | 1131 | 100 | >3.0 | 1.9 | 2 of 3 (67%) |
| Taipale et al., 1997 | 10–16 | 10,010 | 99 | >3.0 | 0.8 | 7 of 13 (54%) |
| Hafner et al., 1998 | 10–14 | 4371 | 100 | >2.5 | 1.7 | 4 of 7 (57%) |
| Pajkrt et al., 1998 | 10–14 | 1547 | 96 | >3.0 | 2.2 | 6 of 9 (67%) |
A screening paradigm using an ultrasound measurement to determine a likelihood ratio is reliable only if NT is measured in a standard fashion. Standards for NT measurements include the following:
The minimal crown-rump length should be 45 mm, and the maximal length should be 84 mm. The success rate for accomplishing a measurement for these gestational ages is between 98% and 100%. The success rate decreases to 90% at 14 weeks and onward.
Either transabdominal or transvaginal scanning can be used; about 95% of cases can be imaged by the transabdominal route.
A true midline sagittal view of the fetal spine must be seen in the cervical and thoracic region with the tip of the nose seen in the face area and the third and fourth ventricle seen in the fetal central nervous system.
The magnification must be such that the fetal head, neck, and upper thorax occupy greater than 50% of the image.
Care must be taken to clearly distinguish between the fetal skin and the amnion. At this gestational age, both structures appear as thin membranes. The structures can be distinguished by either waiting for spontaneous fetal movement away from the amniotic membrane or by bouncing the fetus off the amnion by asking the mother to cough or by tapping on her abdomen ( Fig. 30.3 ).
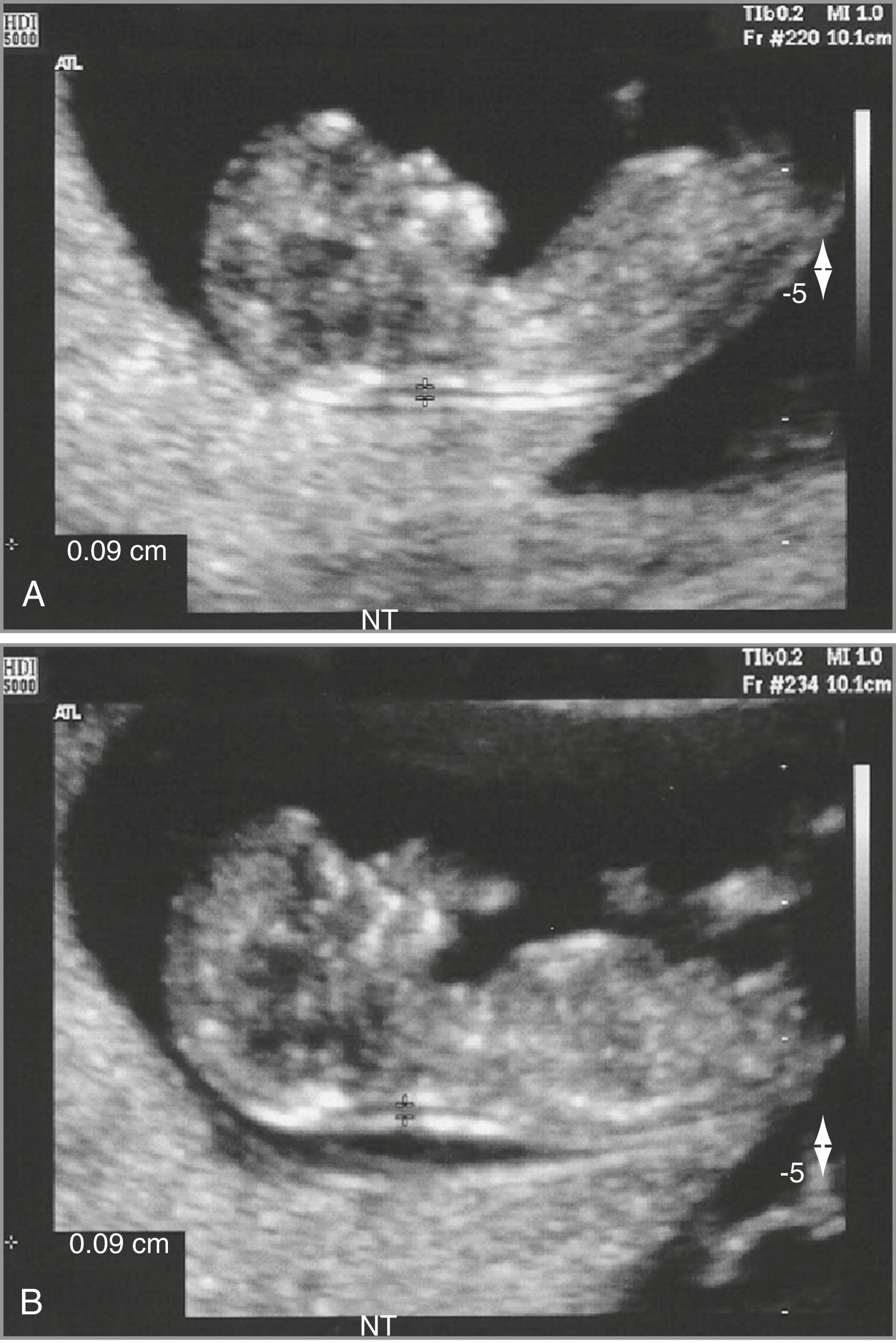
The maximal thickness of the subcutaneous translucency between the skin and the soft tissue overlying the cervical spine should be measured by placing the “+” calipers on the inner borders of the nuchal space with none of the horizontal crossbar protruding into the space, as illustrated in Fig. 30.4 .
The maximum measurement is recorded and used for Down syndrome risk calculation.
The NT should be measured with the fetal head in the neutral position. When the fetal neck is hyperextended, the measurement can be increased by 0.6 mm, and when the neck is flexed, the measurement can be decreased by 0.4 mm.
The umbilical cord is found around the fetal neck in approximately 5% to 10% of cases, which may produce a falsely increased NT, adding approximately 0.8 mm to the measurement. In such cases, the measurements of NT above and below the cord differ, and the smaller measurement is the more appropriate one.
Even with these criteria, standardization of NT measurements is difficult. The ability to achieve a reliable measurement has been linked to the motivation of the sonographer. One study compared results obtained from hospitals where NT measurement was used for clinical purposes with results from hospitals where NT was merely measured but not acted on; in the interventional groups, successful measurement was achieved in 100% of cases, whereas the noninterventional groups achieved successful measurement in only 85%. In a prospective study, NT was measured by two to four operators in 200 pregnant women; after an initial measurement, a second one made by the same operator or another operator varied from the initial measurement by less than 0.5 or 0.6 mm, respectively, in 95% of cases. It is suggested that placement of the calipers rather than generation of the appropriate image accounts for a large part of the variation between operators. Subsequent studies continue to report small interoperator differences.
Because NT values are incorporated into a standardized algorithm along with biochemical analyte values, it is critical that these ultrasound measurements be performed and monitored appropriately. To accomplish this, certification and quality review programs have been developed to ensure that accurate and precise NT measurements are obtained. The Fetal Medicine Foundation of London was the first to offer formalized NT training and quality review. In the United States, the Nuchal Translucency Quality Review program was initiated in 2005. Both programs teach the mechanics of obtaining an NT measurement, have an image review process to ensure that the standard technique is used correctly, and perform ongoing epidemiologic monitoring of sonographer and sonologist performance. Two studies have evaluated the techniques used to ensure consistent NT results. Both confirmed that ongoing expert review of images is an inefficient and impractical approach. Epidemiologic monitoring in which an individual operator’s performance is compared with expected standards is preferable.
Combining NT with serum analytes improves Down syndrome detection rates in the first trimester. Table 30.5 summarizes the large international experience with first-trimester Down syndrome screening using free β-hCG, PAPP-A, and NT measurements. Overall, for a 5% screen-positive rate, combined first-trimester risk assessment provides a Down syndrome detection rate of approximately 88% (95% CI, 84.0%–89.4%). In women older than age 35, 90% to 92% of trisomy 21 pregnancies can be identified with a 16% to 22% false-positive rate. , First-trimester screening can also identify trisomy 18 pregnancies. Greater than 90% of such pregnancies are screen positive when combined biochemical and NT screening is used.
| Study | Pregnancies Screened | Down Syndrome Cases (Screen-Positive/Total) | Detection Rate (%) |
|---|---|---|---|
| Wapner et al., 2003 (BUN study) | 8216 | 48/61 | 79 |
| Malone et al., 2005 (FASTER Consortium) | 38,033 | 100/117 | 86 |
| Wald et al., 2003 (SURUSS study) | 47,053 | 84/101 | 83 |
| Nicolaides et al., 2005 | 75,821 | 321/325 | 93 |
| Total | 167,210 | 533/604 | 88.2 |
a Screening tests were for free β-subunit of human chorionic gonadotropin, pregnancy-associated plasma protein A, and nuchal translucency (with a 5% false-positive rate).
Gestational age–specific variation in the performance of individual analytes can affect screening performance. , At all gestational ages between 9 and 12 weeks, NT and PAPP-A are the most efficient markers. In combination, they are most efficient at 11 weeks—a gestational age when free and total hCG are less efficient. In practice, screening is performed between 11 and 13 weeks of gestation.
Assessment of the fetal nasal bone (NB) can be used in the first trimester to predict trisomy 21. This ultrasound marker is based on the flat nasal bridge area, which is a well-described component of the Down syndrome phenotype, and is supported by histopathologic and radiographic studies demonstrating differences in NBs of fetuses with Down syndrome. Stempfle and colleagues found that NB ossification was absent in one-quarter of fetuses with Down syndrome investigated between 15 and 40 weeks’ gestation compared with none of the control fetuses. Similarly, Tuxen and colleagues evaluated fetuses with Down syndrome between 14 and 25 weeks of gestational age by radiographic and pathologic studies and found that the NB was absent in one-third.
Sonek and coworkers published the first large prospective trial of aneuploid risk evaluation using first-trimester ultrasound assessment of the fetal NB. They determined that the fetal NB could be imaged routinely and that its absence was associated with trisomy 21 ( Fig. 30.5 ). The NB was absent in 73% of trisomy 21 fetuses compared with only 0.5% of euploid fetuses. They estimated that if NB assessment were combined with maternal age and NT measurement, 93% of Down syndrome pregnancies would be detected with a false-positive rate of 5%, and 85% would be detected with a false-positive rate of 1%.
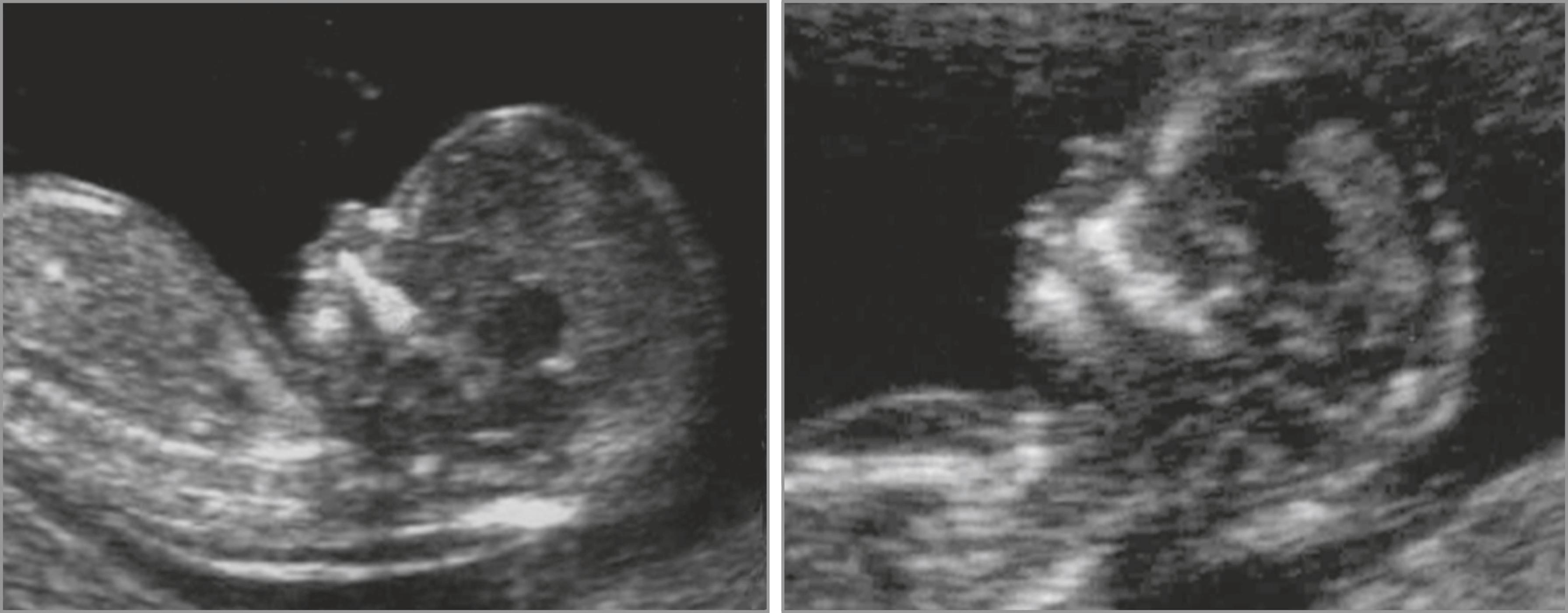
A review of the literature by Rosen and D’Alton evaluated 35,312 women having first-trimester ultrasound assessment for NB. In 33,314 cases (94.3%), the NB was successfully imaged. The sensitivity of NB alone for detecting trisomy 21 was 65%, with a false-positive rate of 0.8%. The positive predictive value (PPV) of the screen was 54%, meaning that approximately one in two fetuses with an absent NB had trisomy 21. If the NB was absent, the likelihood that a fetus had trisomy 21 was increased 87-fold. The negative likelihood ratio with a normal NB was 0.35 (95% CI, 0.32–0.39).
As experience with NB has increased, relationships between absent NB, fetal crown-rump length (i.e., gestational age), NT, and ethnicity have been established. Current data demonstrate that in euploid pregnancies, NB absence occurs more frequently with increasing NT. In a series of 5,851 high-risk patients with 333 trisomy 21 fetuses, absence of the NB had a likelihood ratio of 37.1 when the NT was less than the 95th percentile, and this was reduced to 13.4 when the NT was 4 mm or greater. The same study showed that the NB was more likely to be absent at earlier gestational ages. For example, in euploid fetuses with a crown-rump length between 45 and 54 mm, the NB was absent in 4.7% of cases. At a crown-rump length between 75 and 84 mm, the NB was absent in only 1.0% of cases. Prefumo and colleagues found that NB hypoplasia was more common in the euploid fetuses of women of African descent than in either Asian or White populations (odds ratio = 2.3). Cicero and colleagues also found an increased incidence of absent fetal NB in the first trimester in women of Afro-Caribbean and southern Asian descent. The NB was absent in 2.5%, 9.0%, and 5.0% of White, Afro-Caribbean, and southern Asian populations. Likelihood ratios for trisomy 21 with absent NB were 31.3, 8.8, and 14.2 in these three populations.
NB status is independent of serum biochemistry, allowing NB assessment to be combined with measurements of NT and maternal serum markers to increase first-trimester screening performance. In a retrospective case-control study of a high-risk population with a median maternal age of more than 38 years assessed by NT, NB, and biochemistry, it was estimated that 97% of Down syndrome cases would be detected, with a false-positive rate of 5%. For a false-positive rate of 0.5%, the detection rate would be 90.5%. Although these data are promising, performance using this combined screen would be expected to be significantly lower in an unselected population.
NB assessment is technically more difficult to perform than NT, which may limit its usefulness as a component of first-tier screening. To address this, Nicolaides and associates proposed a two-stage screen, reserving NB assessment for patients at intermediate risk after the combined first-trimester screen is complete. In this model, patients evaluated by NT and serum markers with a risk of 1:100 or greater would be offered chorionic villus sampling (CVS), and patients with a risk of less than 1:1000 would be deemed to have such a low risk that no further testing would be offered. Patients with a risk between 1:101 and 1:1000 would have NB evaluation. In initial studies, performance of this two-stage approach was similar to using NB assessment as part of the initial screen. The two-stage approach would have a significant advantage, as only about 15% of pregnancies would require NB evaluation, which could be performed in centers that have developed special expertise in this technique.
Another potential ultrasound marker is tricuspid regurgitation determined by pulsed wave Doppler ultrasonography. This finding is present in approximately 8% of normal fetuses and 65% of fetuses with trisomy 21. Combining tricuspid regurgitation with NT and PAPP-A would be expected to achieve a detection rate of 95% with a 5% false-positive rate or 90% with a 2% false-positive rate.
A fourth potential marker is abnormal blood flow through the ductus venosus. Studies have shown that pulsation of the ductus venosus gives detection rates of 65% to 75% with a 4% to 5% false-positive rate, and the rate increased to 75% to 80% when NT was added. When serum biochemical markers measured at 10 weeks were also added, the modeled detection rate increased to 92% with a 5% false-positive rate or 84% with a 1% false-positive rate.
A potential disadvantage of earlier screening is that chromosomally abnormal pregnancies that are destined to miscarry will be identified. Of trisomy 21 fetuses living in the first trimester, 69% will be born alive, and 76% of trisomy 21 fetuses identified in the second trimester will be born alive. Using this information, Dunstan and Nix calculated that a detection rate of 80% in the first trimester is approximately equivalent to a second-trimester sensitivity of 75%, suggesting that even when early spontaneous losses of trisomy 21 pregnancies are considered, first-trimester screening is superior to screening presently available in the second trimester.
First-trimester screening would be less desirable if screen-positive pregnancies or pregnancies with enlarged NTs were naturally aborted. In a study of 108 fetuses with trisomy 21 diagnosed in the first trimester because of increased NT, Hyett and colleagues found that six patients elected to continue the pregnancy. In five of the six fetuses, the translucency resolved, and at the second trimester scan the nuchal fold thickness was normal. All six of these trisomy 21 fetuses were born alive. Wapner and colleagues calculated that greater than 80% of screen-positive trisomy 21 pregnancies will be born alive.
Serum screening performed in the second trimester (approximately 16 to 18 weeks’ gestation) was the primary tool for Down syndrome risk assessment before the development of first-trimester screening. This approach may be still used for patients who present for prenatal care after the first trimester given that maternal serum alpha fetoprotein (MSAFP) levels reported in women carrying a Down syndrome fetus have median MSAFP values of 0.75 multiples of the unaffected median. Using this deviation to calculate a likelihood ratio, the age-related risk for Down syndrome could be modified. When the standard 1:270 cutoff was used, approximately 25% of Down syndrome pregnancies among women younger than 35 years of age were screen positive.
Elevated hCG (mean, 2.3 MoM) and reduced levels of unconjugated estriol (mean, 0.7 MoM) were subsequently linked to increased trisomy 21 risk and the term “triple screen” entered common usage in the , risk calculation with MSAFP. The sensitivity of the triple screen for Down syndrome detection in women younger than 35 years is between 57% and 67% if the false-positive rate is held constant at 5%. Overall, the odds of having an affected pregnancy with a positive screen are approximately 1:33 to 1:62, depending on the age range of the population studied, an improvement over the 1:100 odds when maternal age was the sole screening parameter. Because of the impact of maternal age on the risk analysis, screening women who are 35 years of age or more increases the sensitivity, using the same cutoffs, to approximately 87% but with a false-positive rate of nearly 25%. ,
Inhibin-A, a protein produced initially by the corpus luteum and later by the placenta, is routinely included in second-trimester Down syndrome screening, resulting in a quad screen. Inhibin-A levels are elevated in Down syndrome pregnancies (1.3 to 2.5 MoM) and do not vary with gestational age in the second trimester. There is, however, a small correlation with hCG levels, making the added sensitivity for Down syndrome detection less robust. The detection rate for Down syndrome using a quad screen consisting of AFP, hCG, unconjugated estriol, and inhibin-A is approximately 75% (screen-positive rate of 5%) in women younger than 35 years. For women older than 35 years, the detection rate is approximately 92%, with a screen-positive rate of 13%.
Other analytes or combinations of analytes have been tested to further increase sensitivity. Hyperglycosylated hCG excreted in maternal urine has been tested as a marker for Down syndrome. One study of nearly 1500 women (1448 control subjects and 39 Down syndrome pregnancies) reported a sensitivity of 96% of affected pregnancies with a 5% false-positive rate and 71% detection with a 1% false-positive rate when a combination of hyperglycosylated hCG, urine β-core hCG fragment, MSAFP, and maternal age was used. However, this detection rate has not been duplicated by others. Hyperglycosylated hCG can also be used as a serum marker for Down syndrome screening and is offered by some laboratories as part of a “penta-test.”
Screening performance may be improved by combining analytes assessed in the first and second trimesters. , These approaches include the following.
Wald and colleagues described a protocol for screening based on tests performed during both the first trimester (NT and PAPP-A) and the second trimester (quad screen). A single risk estimate is calculated in the second trimester using all six of the measured analytes. Integrated screening has a detection rate of approximately 95% with a 5% false-positive rate. , Approximately 85% of affected pregnancies would be detected with a false-positive rate of only 0.9%. Although this screening approach is quite sensitive and specific, withholding a risk estimate until the second trimester precludes earlier prenatal diagnosis by CVS and is not an acceptable approach for many women.
In an attempt to maximize screening performance by combining first- and second-trimester analytes and yet retain the benefit of first-trimester diagnosis, various methods of sequential screening have been proposed. In these approaches, first-trimester risk results are calculated and used for clinical management, with second-trimester testing performed in selected cases. The following three approaches to sequential risk assessment are available at the present time:
Independent sequential testing. A first-trimester combined risk is calculated with a 1:270 screen-positive cutoff. Decisions regarding invasive testing are made on the basis of these results. In the second trimester, a quad screen is performed and calculated independently of the first-trimester results. This approach provides detection rates greater than 95%, , but it has an unacceptably high false-positive rate (>10%) because the independent calculation of the quad screen risk does not take into consideration the reduced a priori second-trimester prevalence of Down syndrome after removing the Down syndrome cases identified in the first trimester.
Stepwise sequential testing. The high false-positive rate of independent sequential testing is reduced. This approach offers patients with the highest risk the option of first-trimester invasive testing by using a high first-trimester risk cutoff and calculating the second-trimester risk by integrating information from both trimesters. For example, using a 1:65 cutoff in the first trimester identifies 70% of affected pregnancies with only a 1% false-positive rate. If all screen-negative patients proceed to second-trimester screening, an overall detection rate of 95% can be obtained with a 5% false-positive rate. Although this approach has excellent performance, with a high proportion of affected pregnancies identified in the first trimester, it may be logistically challenging for some patients and care providers to coordinate the second-trimester blood draw.
Contingent sequential screening. This approach is similar to stepwise sequential screening, but patients with a very low first-trimester combined risk do not have second-trimester analysis performed, as rescreening this group would have a minimal yield because their risk of an affected pregnancy is low. Using an approach in which patients with a first-trimester risk of 1:1300 or less complete screening in the first trimester, only 15% to 20% of patients have to return for second-trimester analysis. , Contingent sequential screening has a detection rate of 92% to 94% with a 5% screen-positive rate. The primary advantages of this approach are the less complex logistics compared with rescreening all patients and the reduced cost.
If NT scanning is not available or if the NT is unobtainable at the time of first-trimester ultrasound evaluation, an integrated serum-only screen may be performed (PAPP-A in the first trimester and a quad screen in the second trimester). This approach has a detection rate of 86% to 90% with a 5% false-positive rate. , The patient does not receive a test result until after the quad screen has been drawn.
Numerous significant associations between abnormal maternal serum analytes and adverse obstetric outcomes have been reported and are reviewed in this section and summarized in Table 30.6 . The associations noted in Table 30.6 were derived from studies that included data on adjusted odds ratios. The likelihood of an adverse obstetric outcome is increased as the values of the markers become more extreme. In addition, the association with adverse outcomes strengthens as the number of abnormal markers increases. Although there are many significant associations between abnormal first-trimester and second-trimester maternal serum markers and adverse obstetric outcomes, the sensitivity and PPVs for the individual outcomes are too low for them to be clinically useful as screening tests.
| Fetal Death ≤24 Weeks | Fetal Death >24 Weeks | Birth Weight <10th Percentile | Preterm Birth | Preeclampsia | |
|---|---|---|---|---|---|
| PAPP-A (<0.42 MoM) | ++ | ++/+++ | ++/+++ | + (≤32 weeks) | +/++ |
| ++ (<34 weeks) | |||||
| Free β-hCG (<0.21 MoM) | +++ | − | −/++ | − | − |
| AFP (>2.0 MoM) | +++ | −/+++ | ++ | − (≤32 weeks) | − |
| + (<37 weeks) | |||||
| hCG a (>2.0 MoM) | − | − (>24 weeks) | − | − | − |
| +++ (>500 g) | |||||
| uE3 (<0.5 MoM) | +++ | − | +/++ | − | − |
| Inhibin-A (>2.0 MoM) | − | ++ | + | ++ | ++ |
a Although an isolated hCG was not significantly associated with adverse obstetric outcomes, hCG levels >2.0 MoM were significantly associated with early and late fetal loss, birth weight <10th percentile, preterm birth, and preeclampsia.
Decreased PAPP-A levels have been associated with increased frequency of obstetric complications including preeclampsia, early fetal loss, late fetal loss (stillbirth), preterm birth, and fetal growth restriction (FGR). , , In a study involving more than 45,000 women who underwent first-trimester aneuploidy screening in the United Kingdom, Spencer and coworkers reported that PAPP-A levels less than the 5th percentile (0.415 MoM) were associated with odds ratios of 3.7, 2.4, 3.7, 3.3, and 1.9 for birth weight less than the 3rd percentile, delivery before 34 weeks’ gestation, preeclampsia, fetal loss before 24 weeks’ gestation, and fetal loss at or after 24 weeks’ gestation ( P < .001 for all comparisons). The lowest PAPP-A levels were associated with the highest positive likelihood ratios for the adverse outcomes. For example, PAPP-A levels at or below 0.2 MoM were associated with a likelihood ratio of approximately 7.0 for birth weight less than the 3rd percentile, whereas PAPP-A levels at or below 0.4 MoM were associated with a likelihood ratio of approximately 4.0.
Decreased PAPP-A levels were also associated with an increased risk of preeclampsia, early and late fetal loss, birth weight less than the 5th percentile, and preterm birth in the FASTER trial, a multicenter study that recruited greater than 34,000 women undergoing aneuploidy screening in the United States. A consistent pattern of increased risk of an adverse outcome was observed as the PAPP-A level became increasingly decreased.
In addition to its relationship with aneuploidy, an increased NT may also be a marker for fetal structural disorders, such as cardiac defects, diaphragmatic hernias, and fetal skeletal and neurologic abnormalities. Subchromosomal copy number variants and single-gene disorders are also an important cause of an increased NT in a euploid pregnancy.
The association of increased NT with fetal cardiac and great vessel defects is significant. Table 30.7 shows that the prevalence of major cardiovascular abnormalities increases with increasing NT. In a retrospective study of approximately 30,000 chromosomally normal singleton pregnancies, the prevalence of cardiac defects increased from 0.8:1000 in individuals with NT less than the 95th percentile to almost 64:1000 in individuals with NT greater than the 99th percentile.
| NT (mm) | n | Major Cardiac Defects | Prevalence (per 1000) |
|---|---|---|---|
| <95th percentile | 27,332 | 22 | 0.8 |
| ≥95th percentile to 3.4 | 1507 | 8 | 5.3 |
| 3.5 to 4.4 | 208 | 6 | 28.9 |
| 4.5 to 5.4 | 66 | 6 | 90.0 |
| ≥5.5 | 41 | 8 | 195.1 |
| Total | 29,154 | 50 | 1.7 |
This information strongly suggests that the presence of an NT greater than the 99th percentile, and perhaps above the 95th percentile, should be followed with a fetal echocardiogram. In this group of individuals, the frequency of major cardiac defects would be anticipated to be about 2%, which is higher than the threshold risk for pregnancies in which an echocardiogram is recommended based on historical or medical factors, such as a mother with diabetes mellitus or a family history of an affected offspring. If a cutoff at the 95th percentile is used, 5% of all pregnancies would be offered fetal echocardiography, in which case resources to accomplish this may be insufficient. However, if a 99th percentile NT measurement is used, only about 1% of patients would require echocardiograms, with an incidence of positive findings of approximately 6%.
Recent improvements in the resolution of ultrasound and increasing experience with first-trimester cardiac scanning suggest that major cardiac defects can often be identified by the end of the first trimester. This means that patients with an enlarged NT and normal karyotype who currently have echocardiograms performed at 20 weeks may have this evaluation four weeks earlier.
Noncardiac fetal anomalies occur frequently in conjunction with an elevated NT; thus follow-up scans are recommended. These anomalies include diaphragmatic hernias, severe skeletal defects, and omphaloceles. Box 30.1 lists anomalies that have a possible relationship with an increased NT. In approximately 90% of explored cases in which NT is greater than the 99th percentile but below 4.5 mm, a healthy infant can be expected. In cases with NT between 4.5 and 6.4 mm, about 80% of births will result in a healthy newborn. Measurements greater than 6.5 mm have only a 45% incidence of normal results. More than 50% of major structural anomalies can be identified by 14 weeks, and more than 90% can be identified if a second-trimester scan is also done.
Achondrogenesis type II
Achondroplasia
Asphyxiating thoracic dystrophy
Beckwith-Wiedemann syndrome
Blomstrand osteochondrodysplasia
Body stalk anomaly
Camptomelic dysplasia
Cardiac defects
Diaphragmatic hernia
Ectrodactyly–ectodermal dysplasia–cleft (EEC) syndrome
Exomphalos
Fetal akinesia deformation sequence
Fryn syndrome
GM 1 gangliosidosis
Hydrolethalus syndrome
Jarcho-Levin syndrome
Joubert syndrome
Meckel-Gruber syndrome
Nance-Sweeny syndrome
Noonan syndrome
Opitz trigonocephaly (C syndrome)
Osteogenesis imperfecta type II
Perlman syndrome
Roberts syndrome
Short rib–polydactyly syndrome
Smith-Lemli-Opitz syndrome
Spinal muscular atrophy type I
Thanatophoric dysplasia
VACTERL association ( v ertebral abnormality, a nal atresia, c ardiac defect, t racheo e sophageal fistula, r enal agenesis, and radial l imb abnormality)
Zellweger syndrome
A meta-analysis of 17 studies demonstrated a yield of 4.0% for the detection of pathogenic copy number variants in cases of isolated elevated NT and a normal karyotype and 7.0% when other malformations were present. Based on this, microarray analysis should be offered to patients having a diagnostic procedure for an elevated NT. The most common pathogenic CNVs reported are 22q11.2 deletion, 22q11.2 duplication, 10q26.12q26.3 deletion, and 12q21q22 deletion. Although having lower sensitivity and specificity than diagnostic testing, cell-free fetal DNA (cffDNA) testing for 22q11.2 deletion is an option for patients declining an invasive procedure.
More than 150 genetic syndromes such as Noonan syndrome, achondrogenesis type II, and Smith-Lemli-Opitz syndrome have been reported to be associated with an elevated NT. For example, using a sequencing panel that identifies common mutations associated with Noonan syndrome in 483 pregnancies with elevated NT, pathogenic mutations were seen in 5.2% of cases. Although the population frequency of Noonan syndrome is approximately 1:1000, in cases in which NT was greater than 4 mm, the incidence was approximately 1:90. Other studies have found similar results, with the frequency of Noonan syndrome being reported as high as 16.8% in some series.
Patients with an elevated NT >3.5 mm and a normal microarray should be offered molecular testing. Currently, sequencing for variants related to Noonan syndrome and other RASopathies is recommended because of their clear association with an elevated NT. This can be efficiently performed using panels developed for this diagnosis but will not identify other genomic causes. If additional anomalies are detected on scanning, either at the time of the identification of the NT or later in gestation, whole exome sequencing should be considered. Because first-trimester ultrasound screening for anomalies may be incomplete, a portion of the DNA collected from the CVS should be saved in case additional anomalies are identified later.
Evaluation of patients with elevated NT >3.5 mm requires complete evaluation of all possible etiologies. Ideally, a detailed anatomic scan and echocardiogram should be performed between 13 and 14 weeks’ gestation. Although a normal result is reassuring, a repeat scan and echocardiogram later in the second trimester are required to optimize detection of a fetal structural anomaly.
If CVS or amniocentesis is obtained, karyotype and chromosomal microarray should be performed. Since this testing will resolve more than half of cases, additional testing including a RASopathy panel may be delayed until these results are received. In approximately 5% of the cases with a normal microarray, genetic syndromes not identifiable by ultrasound may be present (see Box 30.1 ), so at the time of the procedure the laboratory should be requested to retain specimen for subsequent molecular testing.
The most difficult counseling issue involves patients with an elevated NT and normal testing. At the present time there is insufficient information to counsel patients about long-term fetal development, but the available data are reassuring. Sotiriadis and coworkers performed a systematic review of neurodevelopmental outcome in such pregnancies and found that the rate of neurodevelopmental delay was not higher than that reported for the general population. In a single-center study of 171 singleton fetuses with increased NT but no other findings that evaluated the children at 2 years of age, Mula and associates found that long-term neurodevelopmental delay was not increased. Despite these reassuring reports, more large-scale, prospective case-control studies are needed to enhance the robustness of the results. An increased NT in a monochorionic twin may be associated with later development of twin-twin transfusion symdrome (see Chapter 36 ).
When elevated MSAFP is reported in a structurally normal pregnancy in which the gestational age is correctly assigned and the amniotic fluid alpha fetoprotein (AFAFP) is normal, the biologic explanation is almost always a breach in the maternal-fetal interface, although rare cases of families with hereditary persistence of elevated AFP have been reported. Women with unexplained elevated AFP levels have increased risk for perinatal complications, including fetal growth restriction, fetal death, prematurity, oligohydramnios, abruptio placentae, and preeclampsia. , , The higher the MSAFP level, the greater the risk. Crandall and colleagues studied 1002 women with MSAFP values greater than 2.5 MoM and stratified them by the degree of elevation. In women with a normal ultrasound and amniocentesis, the risk for adverse outcome was 27% overall but varied with the degree of elevation. Adverse outcome occurred in 16% when the MSAFP was 2.5 to 2.9 MoM, 29% when it was 3.0 to 5.0 MoM, and 70% when it was greater than 5.0 MoM. Waller and coworkers investigated 51,008 women screened for MSAFP in California to evaluate the predictive value of high MSAFP compared with low levels. The risk for delivery before 28 weeks was 0.4% for women with low values (<0.81 MoM) and 3.2% for women with high values (>2.5 MoM), an eightfold difference. The rates for delivery before 37 weeks were 2.6% for the low MSAFP group and 24.3% for the high MSAFP group. Women with MSAFP values greater than 2.5 MoM had a 10.5-fold increase in preeclampsia and a 10-fold increased risk for placental complications.
Rarely, maternal or placental disease can result in elevated MSAFP. For example, some maternal liver or ovarian tumors are known to excrete AFP. These include endodermal sinus tumors (yolk sac carcinoma), hepatoblastoma, and hepatocellular carcinoma. Increased serum levels are also seen in acute hepatitis, colitis, and ataxia telangiectasia. , Additionally, a breech in the fetal-maternal barrier resulting in leakage of fetal blood into the maternal circulation can result in an elevated MSAFP. In these cases, Kleihauer-Betke testing may be informative. An elevated AFP can also be a normal finding in rare families with hereditary persistence of elevated AFP as an autosomal dominant condition.
Data are conflicting as to whether the risk for adverse pregnancy outcome with elevated hCG levels is independent of the risks associated with elevated AFP. Although some studies have shown that unexplained elevated hCG (>2.0 MoM) is associated with an increased risk for preeclampsia, preterm birth, low birth weight, fetal demise, and possibly hypertension, a FASTER trial analysis, which included data from greater than 33,000 women with quad screen results, did not detect an association between isolated elevated hCG levels greater than 2.0 MoM and any of these adverse obstetric outcomes. Elevated hCG levels were associated with all the adverse outcomes in an analysis that did not control for the presence of elevated MSAFP and inhibin-A. Similarly, Spencer and coworkers did not detect an association between isolated free β-hCG levels (>2.0 MoM) and low birth weight, preterm birth, FGR, and stillbirth. The early studies evaluating elevated hCG reported that the higher the hCG, the greater the risk.
Elevated inhibin-A levels at 15 weeks of gestation have been associated with later development of preeclampsia, FGR, preterm birth, and stillbirth. The association between elevated inhibin-A levels greater than 2.0 MoM and these adverse obstetric outcomes is reported to be independent of the other analyte deviations.
The combination of elevated MSAFP and hCG levels occurs rarely but may have an overall pregnancy complication rate exceeding 50%. A study of 66 singleton and 33 multiple pregnancies with MSAFP of greater than 2 MoM and hCG of greater than 3.0 MoM found that 60% of singletons and 81% of twins had at least one of several obstetric complications, including preeclampsia, preterm birth, growth restriction, placental abnormalities, and fetal death. Confined placental mosaicism for trisomy 16 has been reported to be associated with extremely high levels of both analytes as well as with similarly poor outcomes.
Low maternal serum unconjugated estriol levels have been linked to adverse pregnancy outcomes. Very low or absent estriol levels of 0.0 to 0.15 MoM suggest biochemical abnormalities of the fetus or placenta, including placental steroid sulfatase deficiency, , Smith-Lemli-Opitz syndrome, congenital adrenal hypoplasia, adrenocorticotropin deficiency, hypothalamic corticotropin deficiency, and anencephaly. Levels of unconjugated estriol less than 0.5 MoM have been associated with an increased incidence of fetal growth restriction and fetal loss at 24 weeks’ gestation or sooner.
Smith-Lemli-Opitz syndrome occurs in approximately 1:60,000 pregnancies as an autosomal recessive disorder resulting from a defect in 3β-hydroxysteroid-Δ7-reductase, altering cholesterol synthesis and resulting in low cholesterol levels and accumulation of the cholesterol precursor 7-dehydrocholesterol in blood and amniotic fluid. Because cholesterol is a precursor of estriol, the defect results in reduced or undetectable levels of estriol in maternal serum and amniotic fluid. Smith-Lemli-Opitz syndrome is characterized by low birth weight, failure to thrive, and moderate to severe intellectual disability. It is associated with multiple structural anomalies including syndactyly of the second and third toes, microcephaly, ptosis, and a typical-appearing facies. Undermasculinization of the genitalia, including complete sex reversal, can be seen in male fetuses.
Palomaki and colleagues summarized findings in 33 women who delivered infants with Smith-Lemli-Opitz syndrome. Of 26 women whose second-trimester estriol values were obtained, 24 had levels less than the 5th percentile (<0.5 MoM). The median level in this group was 0.23 MoM (below the 1st percentile). A risk assessment based on low maternal serum unconjugated estriol levels, usually less than 0.2 MoM, has been suggested but is not routinely used. Prenatal ultrasound should be performed in women with unconjugated estriol levels less than 0.25 MoM to evaluate for findings that may be associated with Smith-Lemli-Opitz syndrome, which may include fetal growth restriction, nuchal edema, ambiguous genitalia, and major structural malformations involving the fetal brain, heart, kidneys, or limbs. Historically prenatal diagnostic testing for Smith-Lemli-Opitz syndrome was based on amniotic fluid cholesterol or 7-dehydrocholesterol levels. Currently, prenatal diagnosis for Smith-Lemli-Opitz is performed on chorionic villi or amniocytes using sequencing of the DHCR7 gene.
Placental steroid sulfatase (STS) deficiency is an X-linked recessive disorder that occurs in 1:1500 male fetuses. This condition most frequently results from an STS gene deletion on the short arm of the X chromosome, (Xp22.3), although some cases may result from a point mutation. STS enzyme deficiency prevents removal of the sulfate molecule from fetal estrogen precursors, preventing conversion to estriol. The fetal phenotype depends on the extent of the deletion, with greater than 90% of cases manifesting as X-linked ichthyosis. However, in about 5% of cases, there can be a deletion of contiguous genes causing intellectual disability. The deletion can occasionally extend to cause Kallmann syndrome or chondrodysplasia punctata. The lack of estrogen biosynthesis may result in delayed onset of labor, prolonged labor, or stillbirth.
Prenatal diagnosis of the deletion leading to placental sulfatase deficiency and congenital ichthyosis can be made by identifying the gene deletion by chromosomal microarray or fluorescence in situ hybridization. Although very low estriol levels, usually below the level of detection, can identify males at risk for this disorder, testing in these cases is not routinely offered because the phenotype is usually mild. However, rarer, more serious cases of extensive deletions will be missed.
To date, no management protocol has been demonstrated to improve outcome in these cases. Although increased fetal surveillance with ultrasound assessment of fetal growth and antenatal testing has been proposed, the optimal type and frequency of testing, if any, remain undetermined and require further study.
As noninvasive screening for trisomy 21, 18, and 13 has a sensitivity of over 90%, initial identification of cases by second-trimester ultrasound has become infrequent. For patients presenting late to prenatal care and patients not desiring earlier testing, ultrasound may still have value in Down syndrome risk assessment.
The diagnosis of Down syndrome is suspected when certain associated anomalies or physical features are noted on an ultrasound examination. Certain structural anomalies, such as atrioventricular canal defect or duodenal atresia, have a strong association with Down syndrome. Although these anomalies are highly specific for trisomy 21, they have low sensitivity and are not useful for population screening. For example, 40% of pregnancies with duodenal atresia have trisomy 21, but it is seen in only 8% of affected fetuses.
Physical characteristics that are not structural anomalies but that occur more commonly in fetuses with Down syndrome are called soft markers. The ratio of the prevalence of these markers in fetuses with Down syndrome to their prevalence in the normal population will result in a likelihood ratio that can be used to modify the a priori age or screening risk. Available markers and their likelihood ratios are listed in Box 30.2 and Table 30.8 , respectively.
Brachycephaly
Increased nuchal thickness
Congenital heart defects
Hyperechoic bowel
Shortened femur
Shortened humerus
Renal pyelectasis
Duodenal atresia
Hypoplasia of midphalanx of the fifth digit
Echogenic intracardiac focus
Sandal gap foot
Widened ischial spine angle
Short foot length
Short or absent nasal bone
| Sonographic Marker | AAURA, LR a ( N = 1042) | Nyberg et al., LR (95% CI )b ( N = 8830) | Smith-Bindman et al., LR (95% CI) c (Meta-analysis, N = >131,000) |
|---|---|---|---|
| Nuchal thickening | 18.6 | 11.0 (5.2–22) | 17.0 (8.0–38) |
| Hyperechoic bowel | 5.5 | 6.7 (2.7–16.8) | 6.1 (3.0–12.6) |
| Short humerus | 2.5 | 5.1 (1.6–16.5) | 7.5 (4.7–12) |
| Short femur | 2.2 | 1.5 (0.8–2.8) | 2.7 (1.2–6) |
| Echogenic intracardiac focus | 2.0 | 1.8 (1.0–3) | 2.8 (1.5–5.5) |
| Pyelectasis | 1.5 | 1.5 (0.6–3.6) | 1.9 (0.7–5.1) |
a LR assumed by original AAURA model by Nyberg DA, Luthy DA, Resta RG et al. Age-adjusted ultrasound risk assessment for fetal Down’s syndrome during the second trimester: description of the method and analysis of 142 cases. Ultrasound Obstet Gynecol . 1998;12:8.
b Nyberg DA, Souter VL, El-Bastawissi A et al. Isolated sonographic markers for detection of fetal Down syndrome in the second trimester of pregnancy. J Ultrasound Med . 2001;20:1053.
c LR of meta-analysis by Smith-Bindman R, Hosmer W, Feldstein VA et al. Second-trimester ultrasound to detect fetuses with Down syndrome: a meta-analysis. JAMA 2001;285:1044.
Ultrasound markers modifying the risk for Down syndrome include the following:
Increased nuchal fold (>6 mm) in the second trimester is the most distinctive marker. The fetal head is imaged in the transverse plane used to measure the biparietal diameter. The thalami and the upper portion of the cerebellum should be in the image. The distance between the external surface of the occipital bone and the external surface of the skin is then measured. About 35% of fetuses with Down syndrome, but only 0.7% of normal fetuses, have a nuchal skin fold measurement greater than 5 mm. This ratio yields a likelihood ratio of 50 but includes fetuses with more than one marker. When an increased nuchal fold is an isolated finding, the likelihood ratio is still strong at 20-fold. This high likelihood ratio is obtained because of the rarity of an increased nuchal fold in an unaffected population (i.e., high specificity).
The fetal NB is hypoplastic or absent in up to 60% of Down syndrome pregnancies imaged in the second trimester but in only about 1% to 2% of unaffected pregnancies. Complete absence occurs in about 37% of affected cases, and hypoplasia occurs in about 50%. In normal pregnancies, absence of NB occurs in 0.9% of cases and hypoplasia occurs in 2.4%. NB length can be converted to a likelihood ratio and used for Down syndrome risk assessment. When performed by experienced operators, NB evaluation may be the best single ultrasound marker for second-trimester risk assessment; however, as discussed in the earlier section on first-trimester NB screening, ethnic variation can occur.
Fetuses with Down syndrome may have short proximal extremities (humerus and femur) relative to the expected length for their biparietal diameter in the second trimester. This can be used to identify at-risk pregnancies by calculating a ratio of observed-to-expected femur length based on the biparietal diameter of the fetus. An observed-to-expected ratio of less than 0.91 or a biparietal diameter-to-femur ratio of more than 1.5 has a reported likelihood ratio of 1.5 to 2.7 when present as an isolated finding. A short humerus is more strongly related to Down syndrome, with reported likelihood ratios of 2.5 to 7.5. Bahado-Singh and coworkers combined humerus length with nuchal skin fold to estimate Down syndrome risk and calculated the likelihood ratios for various measurements to adjust estimated Down syndrome risk for each patient.
Echogenic intracardiac foci is observed in up to 5% of normal pregnancies and in approximately 13% to 18% of Down syndrome pregnancies. The likelihood ratio for Down syndrome when an echogenic focus is present as an isolated marker is 1.8 to 2.8 but may be lower in an Asian population, where the frequency in unaffected pregnancies may be higher. The risk does not seem to vary if the focus is in the right or left ventricle or if it is unilateral or bilateral.
Increased echogenicity of the fetal bowel, when brighter than the surrounding bone, has a likelihood ratio for Down syndrome of 5.5 to 6.7. This finding can also be seen with fetal cystic fibrosis (CF), congenital cytomegalovirus infection, swallowed bloody amniotic fluid, and severe FGR or placental insufficiency. Therefore, if amniocentesis is performed for this finding, testing for the other potential etiologies should be considered.
Mild fetal pyelectasis (renal pelvis anterior-posterior diameter >4 mm) has been suggested as a marker for Down syndrome. As an isolated marker, the likelihood ratio is 1.5 to 1.9 (see Table 30.8 ). However, Snijders and coworkers found that mild renal pyelectasis is not significantly more frequent in Down syndrome pregnancies than in normal pregnancies.
Other markers described include a hypoplastic fifth middle phalanx of the hand, short ears, a sandal gap between the first and second toes, an abnormal iliac wing angle, an altered foot-to-femur ratio, an altered frontomaxillary angle, , and increased prenasal thickness. , These markers are inconsistently used because of the time and expertise required to obtain them.
As biochemical and DNA screening tests have improved, the value of ultrasound soft markers in Down syndrome screening has become less significant. Soft markers should not independently be used to calculate Down syndrome risk but should be part of a total risk analysis. Many centers do not inform patients of a single soft marker unless the overall risk for trisomy 21 exceeds that at which they routinely offer invasive testing. This cutoff is frequently between 1: 150 and 1:300. With the use of cell-free DNA screening, which has a positive likelihood ratio of 1720 and a negative likelihood ratio of 0.006, it is recommended that ultrasound should not be used to modify a positive cell-free DNA result.
Fetal aneuploidy other than Down syndrome can be suspected on the basis of ultrasound findings ( Table 30.9 ). Choroid plexus cysts occur in 1% of fetuses between 16 and 24 weeks’ gestation and have been associated with trisomy 18. Among fetuses with trisomy 18, 30% to 35% have choroid plexus cysts. Among fetuses with a choroid plexus cyst, about 3% have trisomy 18, and most (65% to 90%) of these have other ultrasound findings ( Table 30.10 ). Although an isolated choroid plexus cyst was estimated to yield a probability of trisomy 18 of 1:150, many series contain a high proportion of older women, which would overstate the risk. Snijders and coworkers calculated that an isolated choroid plexus cyst has a likelihood ratio of 1.5 for trisomy 18, and thus it can be used to calculate an individual’s risk for trisomy 18 when combined with other screening modalities. The size, location, or persistence of the cyst does not alter this risk. In women who screen negative for trisomy 18 (either first-trimester or second-trimester screening or cell-free DNA) and in whom no other fetal structural abnormalities are visualized, the finding of an isolated choroid plexus cyst does not require additional genetic testing.
| Ultrasound Finding | Isolated a (%) | Multiple b (%) | Trisomy 13 | Trisomy 18 | Trisomy 21 | Other | 45,X |
|---|---|---|---|---|---|---|---|
| Holoprosencephaly ( n = 132) | 4 | 39 | 30 | 7 | — | 7 | — |
| Choroid plexus cysts ( n = 1806) | 1 | 46 | 11 | 121 | 18 | 11 | — |
| Facial cleft ( n = 118) | 0 | 51 | 25 | 16 | — | 6 | — |
| Cystic hygroma ( n = 276) | 52 | 71 | — | 13 | 26 | 11 | 163 |
| Nuchal skin fold | 19 | 45 | — | 9 | 85 | 19 | 10 |
| Diaphragmatic hernia ( n = 173) | 2 | 34 | — | 18 | — | 14 | — |
| Ventriculomegaly ( n = 690) | 2 | 17 | 10 | 23 | 13 | 14 | — |
| Posterior fossa cyst ( n = 101) | 0 | 52 | 10 | 22 | — | 8 | — |
| Major heart defects ( n = 829) | 16 | 66 | 30 | 82 | 68 | 31 | 30 |
| Duodenal atresia ( n = 44) | 38 | 64 | — | — | 21 | 2 | — |
| Hyperechoic bowel ( n = 196) | 7 | 42 | — | — | 22 | 17 | — |
| Omphalocele ( n = 475) | 13 | 46 | 28 | 108 | — | 31 | — |
| Renal anomalies ( n = 1825) | 3 | 24 | 40 | 52 | 48 | 62 | — |
| Mild hydronephrosis ( n = 631) | 2 | 33 | 8 | 6 | 27 | 9 | — |
| Fetal growth restriction (early) ( n = 621) | 4 | 38 | 11 | 47 | — | 18 | 36 (triploidy) |
| Talipes ( n = 127) | 0 | 33 | — | — | — | — | — |
| Finding | Frequency (%) |
|---|---|
| Growth restriction | 46 |
| Hand or foot abnormalities a | 39 |
| Cardiac abnormality | 31 |
| CNS abnormality | 29 |
| Diaphragmatic hernia | 13 |
| Ventral wall defect | 10 |
| Facial abnormality | 7 |
| At least one abnormality | 90 |
Table 30.9 displays the magnitude of the associations between various ultrasound findings and aneuploid conditions as estimated from a referral population. The rates noted may overestimate the strength of the association when such findings are noted on a screening examination.
Approximately 3% to 15% of the cell-free DNA circulating in maternal plasma comes from the placenta. Analysis of this fetal DNA has led to the development of techniques for noninvasive prenatal screening. Since it became clinically available in 2011, cell-free DNA testing has been increasingly used to screen pregnant women for fetal aneuploidy. While prenatal screening for fetal aneuploidy was initially focused on the detection of trisomy 21, cell-free DNA screening has the potential to screen for additional aneuploidies including trisomy 18 and 13 and sex chromosome aneuploidies. Screening with cell-free DNA may be performed from 9 weeks’ gestation until delivery.
Circulating fetal DNA is predominantly a product of placental apoptosis as opposed to degradation of fetal erythroblasts. This cell-free DNA consists of small fragments (<450 base pairs), undergoes a rapid turnover, and may appear in apoptotic bodies or nucleosomes. Fetal SRY gene sequences are present in the circulation 18 days after embryo transfer, before the definitive fetoplacental circulation, which is not established until 28 days after conception. Fetal DNA is continuously liberated into the maternal circulation with a mean half-life estimated to be 16 minutes at term. Levels increase until about 10 weeks’ gestation, remain level between 10 and 21 weeks, and then continue to increase until the third trimester. Cell-free fetal DNA is undetectable about 2 hours after birth.
Three approaches to cell-free DNA screening have been validated by different laboratories. The first technique reported was massively parallel sequencing, in which all of the DNA that is extracted from a maternal sample is sequenced. In 2008 two groups identified Down syndrome pregnancies , by analyzing the cell-free fetal DNA in the maternal plasma using massively parallel shotgun sequencing. The first 36 bases of all circulating DNA fragments (both maternal and fetal) were sequenced and identified to determine their specific chromosomal origin ( Fig. 30.6A ). If the fetus has a third chromosome 21, the percentage of chromosome 21 fragments compared with disomic chromosomes will be slightly higher than expected. In a pregnancy in which 10% of the cell-free DNA is fetal, a woman carrying a fetus with trisomy 21 should have approximately 1.05 times more DNA from chromosome 21 fragments than a woman carrying a fetus with disomy 21. Prediction of trisomy 21 is based on the ability to distinguish this difference by sequencing millions of fragments, identifying their chromosome origin, and then quantifying their relative proportion ( Fig. 30.6B ).
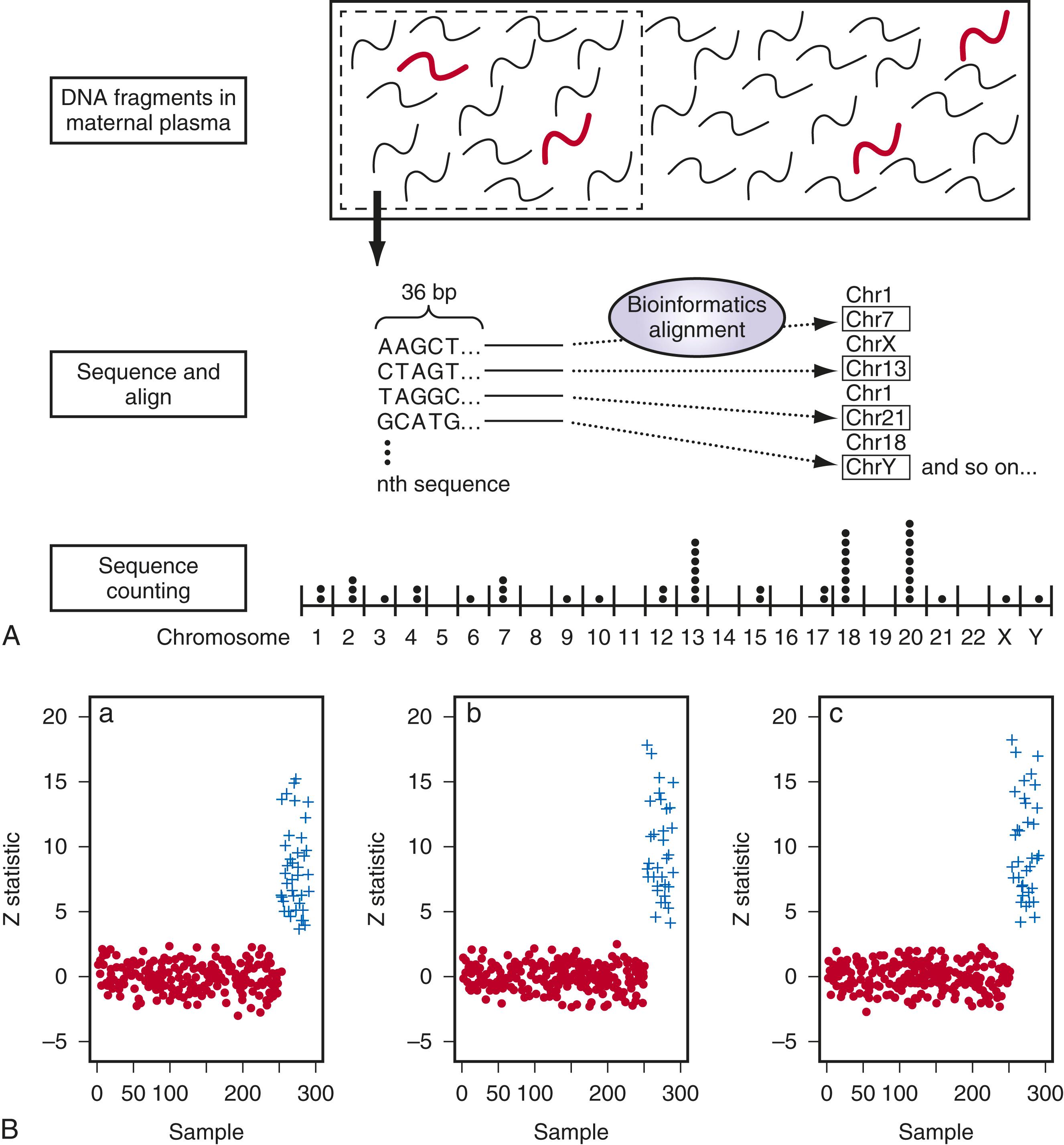
Targeted massive parallel sequencing, also known as chromosome-specific sequencing, selects the genomic regions of interest, such as nonpolymorphic loci on chromosomes 21, 18, and 13, and then reads and counts only those. The ability to sequence specific regions of the genome in cell-free DNA allows for a focused analysis of clinically important chromosomes such as 13, 18, 21, X, and Y. This strategy significantly reduces the total number of reads analyzed with a concomitant improvement in efficiency and a 10-fold reduction in overall costs. Also, the reduced read requirement enables the use of small bench-top sequencers that affords both financial and practical benefits to the laboratory.
Microarray-based technology is used by some labs to quantify the relative contributions from specific chromosomes and does not require next-generation sequencing. In this approach DNA fragments from the chromosomes of interest are initially selected and hybridized to a specially designed array and analyzed by the degree of fluorescence. Juneau and associates showed that microarray-based targeted analysis resulted in high sensitivity and specificity comparable to next-generation sequencing. The proposed advantages of microarray-based noninvasive prenatal screening are decreased variability that will allow testing samples with lower fetal fraction and improved turnaround time and reduced cost owing to individual hybridization instead of sample multiplexing. ,
A third approach focuses on evaluating single nucleotide polymorphisms. By measuring these polymorphic loci, the number and identity of each allele from each sequence read are determined. The allelic information from the mother (and from the father, if available) is used to model a set of hypotheses corresponding to different genetic inheritance patterns and crossover locations for every possible copy number count. Bayesian statistics then assign a probability to each hypothesis (whether the findings are most consistent with monosomy, disomy, or trisomy), and a maximum likelihood analysis is performed to calculate that the probability of that hypothesis is correct. Because the single nucleotide polymorphism–based method is dependent on analysis of maternal genotype information, it cannot be used in pregnancies resulting from egg donors. The SNP-based approach can identify triploidy. This approach may prove to be useful in the detection of vanishing twins and determination of zygosity.
Cell-free DNA screening has the highest sensitivity and the lowest false-positive rate of all the Down syndrome screening tests currently available. The detection rates based on samples for which a result is returned are approximately 99.7% for trisomy 21, 97.9% for trisomy 18, and 99.0% for trisomy 13 at a combined false-positive rate of 0.13% ( Table 30.11 ). Although Down syndrome screening using cell-free fetal DNA is better than biochemistry, with or without NT assessment, it is still not diagnostic. All screen-positive cases should be confirmed by invasive testing, and false-negative results do occur.
| Condition | Number of Cases | Detection (%) (95% CI) |
False Positive (%) (95% CI) |
Positive LR | Negative LR |
|---|---|---|---|---|---|
| Trisomy 21 | 1963 | 99.7 (99.1%–99.9%) | 0.04 (0.02%–0.07%) | 2506 | 0.003 |
| Trisomy 18 | 563 | 97.9 (94.9%–99.1%) | 0.0 (0.03%–0.07%) | 2122 | 0.018 |
| Trisomy 13 | 119 | 99.0 (65.8%–100%) | 0.04 (0.02%–0.07%) | 2819 | 0.010 |
| Monosomy X | 36 | 95.8 (70.3%–99.5%) | 0.14 (0.05%–0.38%) | 694 | 0.042 |
| 47,XXX; 47XYY; 47XXY | 17 | 100 (83.6%–100%) | 0.004 (0.0%–0.08%) | NA | NA |
Cell-free DNA screening was initially studied in high-risk populations consisting of women at an increased risk for having a fetus with trisomy 21 based on advanced maternal age (≥35 years old at delivery), a positive conventional aneuploidy screen, a history of a prior pregnancy with a trisomy, a parental balanced robertsonian translocation with increased risk of fetal trisomy 13 or trisomy 21, or an ultrasound abnormality associated with fetal aneuploidy. , Subsequent studies demonstrated that the detection rates and false-positive rates are similar in low-risk and high-risk populations. , , The detection rates appear to be similar using all three techniques.
Although the sensitivity and false-positive rates associated with cell-free DNA screening are similar in low-risk and high-risk populations, the PPV for trisomy 21, trisomy 13, and trisomy 18 is higher in older women owing to the increased prevalence of these conditions. It is helpful to provide a PPV when counseling women who have positive cell-free DNA test results. Although not all laboratory reports provide this information, patient-specific PPVs may be obtained using an online PPV calculator ( https://www.perinatalquality.org/Vendors/NSGC/NIPT/ ). The PPV associated with cell-free DNA screening is considerably higher in women of all ages compared with standard aneuploidy screening with biochemistry with or without NT. In a study of 1914 women with a mean age of 29.6 years who had standard aneuploidy screening during the first or second trimester with or without NT and cell-free DNA screening, the PPVs for standard screening versus cell-free DNA testing were 4.2% versus 45.5% for trisomy 21 and 8.3% versus 40.0% for trisomy 18.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here