Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Antenatal steroids (ANS) are one of the oldest and most effective therapies in perinatal medicine.
The randomized controlled trials (RCTs) are dated and do not necessarily apply for current clinical populations in high-resource environments where treatments reduce mortality.
In low-resource environments, there is potential for risk and unclear benefit of ANS.
There are minimal RCT data to support ANS use for deliveries before 28 weeks’ gestational age.
The risk to benefit ratio for gestations after 34 weeks is unclear.
The dose and drug choice for ANS has not been optimized to decrease potential risks.
Postnatal steroids (PNS) can effectively decrease bronchopulmonary dysplasia (BPD), but there are risks.
The trend is to use lower doses, for shorter periods of treatment, and later, without randomized data to support.
Four new trials of PNS begun shortly after birth are proof of principle that PNS decrease BPD.
PNS should only be used for infants at high risk of severe BPD.
This chapter reviews knowledge gaps and new information about the two primary uses of corticosteroids in the perinatal period: antenatal (maternal) treatments (antenatal steroids [ANS]) to decrease respiratory distress syndrome (RDS) and infant mortality, and post-delivery corticosteroid treatments (postnatal steroids [PNS]) to prevent or treat bronchopulmonary dysplasia (BPD). Clinical and experimental information for both treatments is not optimal for current clinical practice as a result of the histories for the development and testing of corticosteroids for both antenatal and postnatal indications. Both therapies were used by clinicians in the early period of modern perinatal medicine without formal drug development and licensure. Thus, optimal drug selection, dose, and patient selection remain poorly defined today. Much of the clinical information is quite old, and patient populations and clinical management have changed strikingly. Further, the therapies were evaluated and adopted before modern molecular techniques were available, leaving large gaps in knowledge about the mechanisms of action and the potential risks of treatment. Corticosteroids are potent drugs with pleotropic effects on multiple organ systems that play essential roles in basal physiology, stress responses, and when used in higher doses, therapeutic effects. The major focus of this chapter is on the fetal and newborn lung, but cardiovascular and mortality outcomes cannot be separated from lung responses.
While evaluating the role of fetal exposures to corticosteroids on labor in sheep, Liggins reported in 1969 that fetal dexamethasone infusions caused preterm delivery of lambs that had unanticipatedly good lung aeration. That result was consistent with other early reports that corticosteroids could mature developing organ systems in other animal models. His observation in sheep was quickly evaluated in a clinical trial reported in 1972 that randomly assigned 282 women to a maternal intramuscular treatment with the drug betamethasone (Celestone) that is used to suppress inflammation. Celestone Soluspan is a mixture of two prodrug forms of the fluorinated corticosteroid betamethasone: betamethasone phosphate and a micronized suspension of betamethasone acetate. When injected intramuscularly, the betamethasone phosphate is rapidly dephosphorylated while the betamethasone acetate is slowly deacylated over many hours and days. Both compounds yield free betamethasone, which crosses the placenta to achieve a prolonged fetal exposure when given as a two-dose treatment of 12 mg at a 24-hour interval. This level of detail is important to understand today’s approach for ANS dosing (see the following text).
The Liggins and Howie trial demonstrated decreased RDS and mortality in infants of an average gestational age of 35 weeks at delivery. More than 20 trials were completed by 1993 that, in aggregate, demonstrated comparable effects without clear risks. However, ANS were not widely used in the United States until recommended by a National Institutes of Health Consensus Conference in 1994. The majority of trials tested Celestone against placebo, but dexamethasone phosphate, a similar drug given as four doses of 6 mg every 12 hours, was used in some trials as summarized by the definitive meta-analysis of Roberts et al. in 2006 that was last updated in 2020. , The primary outcomes of most of the trials were decreased RDS and mortality, although ANS also had benefits for decreased intraventricular hemorrhage (IVH) and necrotizing enterocolitis. ANS became the standard of care for all women at risk of preterm delivery before 34 weeks and ANS were recognized as a major advancement in perinatal medicine. There was minimal information developed for drug selection or dose in animal models or humans, and ANS remain unapproved by the US Food and Drug Administration.
Widespread adoption of ANS for fetal lung maturation took place without careful consideration of pharmacokinetics of the different drugs available. Moreover, regimens have been modified according to local availability and some were adopted with minimal or no information of clinical efficacy. The regimen initially trialed by Liggins is a mixture of two prodrugs: (1) betamethasone phosphate, which is rapidly dephosphorylated; and (2) betamethasone acetate, which is slowly deacylated. In nonpregnant reproductive-age women, this mixture reaches peak concentration at 3 hours and has a half-life of 59 hours, with detectable levels up to 11 days after an initial 6 mg dose, which was just 25% of the clinically used dose. The other commonly used drug is intramuscular dexamethasone, which is usually given as four doses of 6 mg 12 hours apart. In nonpregnant reproductive age women, this dose reaches peak concentration at 3 hours but has a much shorter half-life of 5.2 hours. Large animal models of preterm pregnancy have provided information on the transfer and pharmacokinetics of these drugs in the fetus. In order to achieve fetal lung maturation in preterm sheep and rhesus macaques, a low continuous fetal exposure with a concentration of 1 to 3 ng/mL of plasma betamethasone for at least 48 hours is needed for a persistent fetal lung maturation response that lasts 5 to 7 days after initiation of treatment ( Fig. 11.1 ).
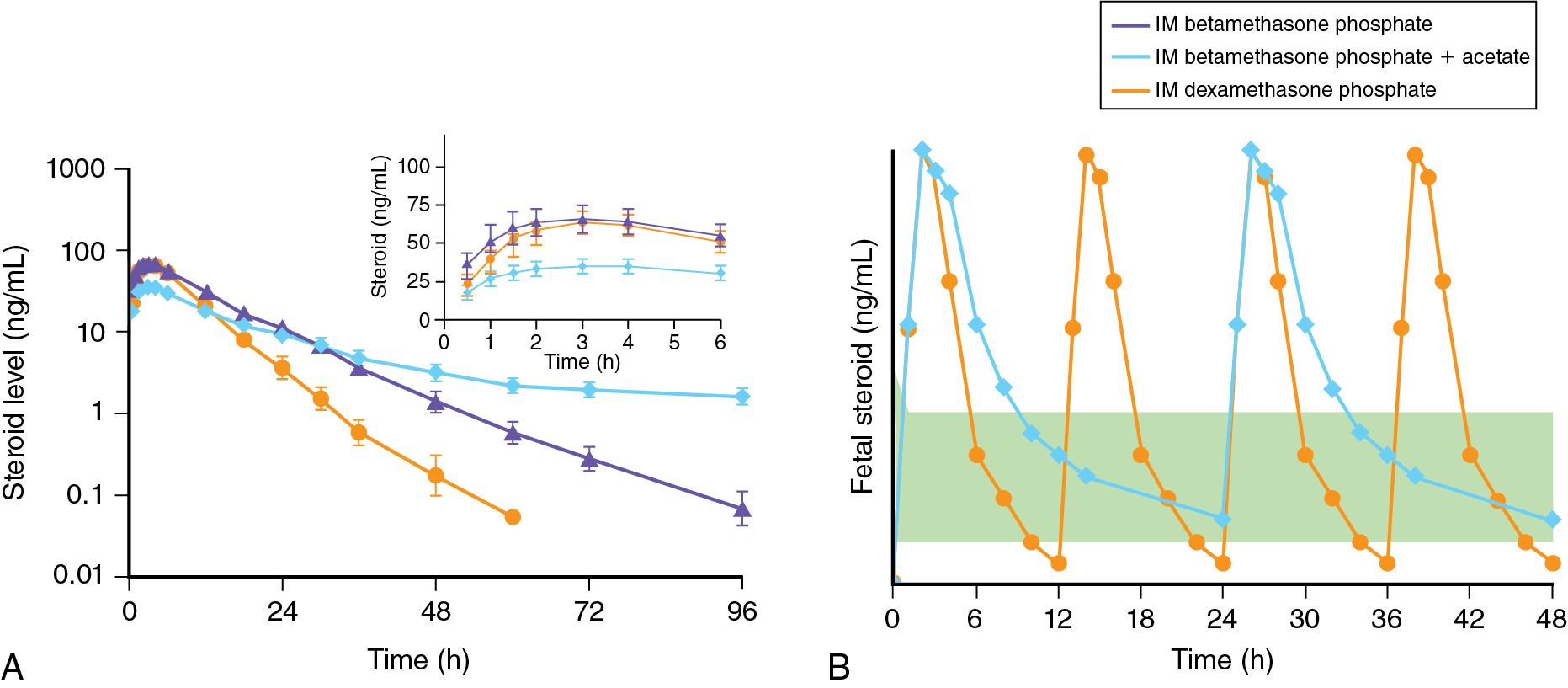
The current treatment regimens used expose the fetus and the mother to an excess peak concentration of corticosteroids that does not contribute to fetal lung maturation. While a long-acting formulation provides the continuous and prolonged exposure necessary for fetal lung maturation, they may result in prolonged and unnecessary maternal and fetal exposure. Clinicians cannot accurately predict timing of preterm birth in about 50% of the cases, with women often delivering before 24 hours or after 7 days of administration of ANS, when they are no longer effective. In women that deliver soon after administration of Celestone, they will have continued exposure to betamethasone for several days with no benefit and potential side effects of hyperglycemia and hypertension which may aggravate pregnancies complicated by diabetes and hypertension.
Multiple experimental studies with animals, human fetal lung explants, and isolated lung cells followed the initial Liggins observation in fetal sheep. Exposure to steroids induced the enzymes that contribute to surfactant synthesis and increased surfactant lipid and protein components in tissue and air spaces that became the explanation for the effects of ANS on fetal lungs. However, in large animal models such as sheep and primates, improved lung function as assessed by increased gas volumes and improved lung mechanics occur within 15 to 24 hours, prior to increase in lung surfactant which is only detectable at 3 to 5 days after treatment ( Fig. 11.2 ). , ANS decrease lung mesenchyme tissue volume, and the barrier function of the air space epithelium improves rapidly after ANS in animal models, which contributed to improved lung function soon after ANS treatment. At the cellular level, ANS promote the differentiation of proliferative mesenchymal progenitors into matrix fibroblasts as well as differentiation of alveolar type 1 and type 2 epithelial cells. , At the molecular level, ANS upregulate and downregulate the expression of thousands of genes within a few hours, with the net effects of improved lung function after preterm delivery. These multiple effects certainly cause improved lung function, but the ANS response is not simply an acceleration of normal lung development. In animal models, fetuses exposed to ANS have a transient decrease in saccular/alveolar septation and microvascular arborization that is similar to the phenotype of delayed lung development associated with BPD with suppression of angiogenesis and lung morphogenesis pathways. , Alveolarization and microvascular development “catches up” in fetal sheep if they remain undelivered. In a 30-year follow-up, ANS do not seem to alter subsequent lung function after preterm delivery. Empirically very preterm infants exposed to ANS who do not have BPD seem to fare well, although their lung function is not equivalent to the lung function of term infants. However, term-born infants exposed to ANS have decreased forced vital capacity and forced expiratory volume compared to nonexposed term-born infants. Further work is needed to understand the effects of ANS on the fetal lung and on long-term lung function.
![Fig. 11.2, Timeline of changes in static lung compliance (V40) , gas exchange (ventilation efficiency index [VEI] ), and saturated phosphatidylcholine (satPC) in the bronchoalveolar lavage fluid based on data from preterm lambs treated with ANS. Lung structural changes can be seen as early as 15 hours after treatment with improvement in gas exchange seen at 24 to 48 hours, and increases in surfactant only seen at 5 days and peaking at 7 days when there is already a decreasing effect on lung compliance and gas exchange. ANS , Antenatal steroids; d , days; h , hours. Fig. 11.2, Timeline of changes in static lung compliance (V40) , gas exchange (ventilation efficiency index [VEI] ), and saturated phosphatidylcholine (satPC) in the bronchoalveolar lavage fluid based on data from preterm lambs treated with ANS. Lung structural changes can be seen as early as 15 hours after treatment with improvement in gas exchange seen at 24 to 48 hours, and increases in surfactant only seen at 5 days and peaking at 7 days when there is already a decreasing effect on lung compliance and gas exchange. ANS , Antenatal steroids; d , days; h , hours.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Prenatalandpostnatalsteroidsandpulmonaryoutcomes/1_3s20B9780323878746000205.jpg)
When ANS were initially used, RDS was a lethal disease for most infants and very preterm infants with or without RDS had very high mortality. The single ANS treatment trials versus placebo reported more than 25 years ago have RDS outcomes for just 102 randomized patients that delivered before 28 weeks’ gestational age ( Table 11.1 ). The most recent updates of the Roberts et al. meta-analysis have not provided updated information by gestational age ranges. More recently, the WHO ACTION-I trial in low-resource countries reported 226 infants between 26 and 28 weeks randomized to ANS or placebo with 6% decrease in mortality in the ANS group (relative risk [RR] 0.89, confidence interval [CI] 0.70–1.14, number needed to treat [NNT] 16), but the rates of RDS were not reported. In contrast, with current practices, RDS in infants more than 28 weeks’ gestation is generally easily managed with surfactant and standard techniques for respiratory support. The recommendation at a 1994 Consensus Conference was to administer ANS for women at risk of preterm delivery between 24 and 34 weeks’ gestational age, even though there were minimal data to support that recommendation. We lack the randomized controlled trial (RCT) evidence that use of ANS benefits the pulmonary outcomes of the population of infants most targeted to receive ANS today.
| Result | Trials | Treated Patients | Controls | Relative Risk (95% CI) |
|---|---|---|---|---|
| Respiratory distress syndrome | 4 | 48 | 54 | 0.79 (0.53–1.18) |
| Death | 2 | 45 | 44 | 0.79 (0.56–1.12) |
| Intraventricular hemorrhage | 1 | 34 | 28 | 0.34 (0.14–0.86) |
Other information is available to support ANS use at early gestational ages. Very immature animal models and in vitro explants of human fetal lungs have maturational responses to steroids, indicating that receptors and response pathways are present from early gestational ages. Very large databases from neonatal networks are being extensively mined for outcomes of very low-birth-weight (VLBW) infants who were and were not exposed to ANS. These analyses focus on mortality and outcomes such as IVH but not respiratory outcomes soon after birth, presumably because most infants at less than 28 weeks’ gestation age receive some respiratory support and are imprecisely coded as having RDS. A weakness of the studies is a lack of information about the severity of the early respiratory distress and any accurate categorization as to whether early deaths are caused by RDS.
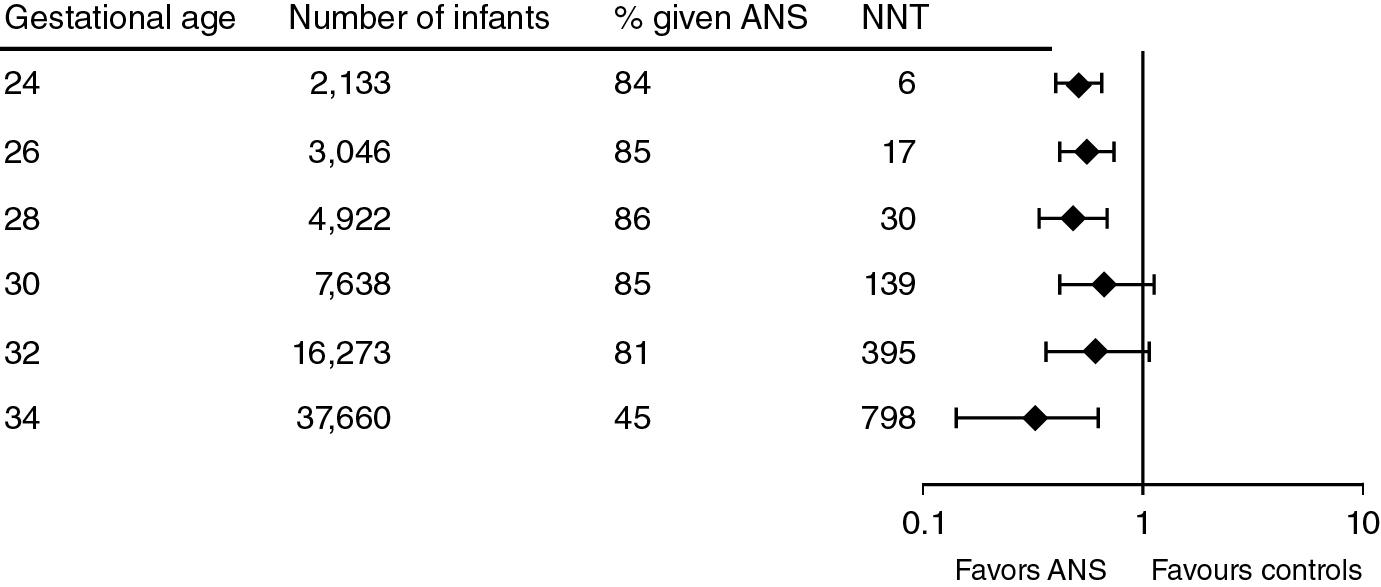
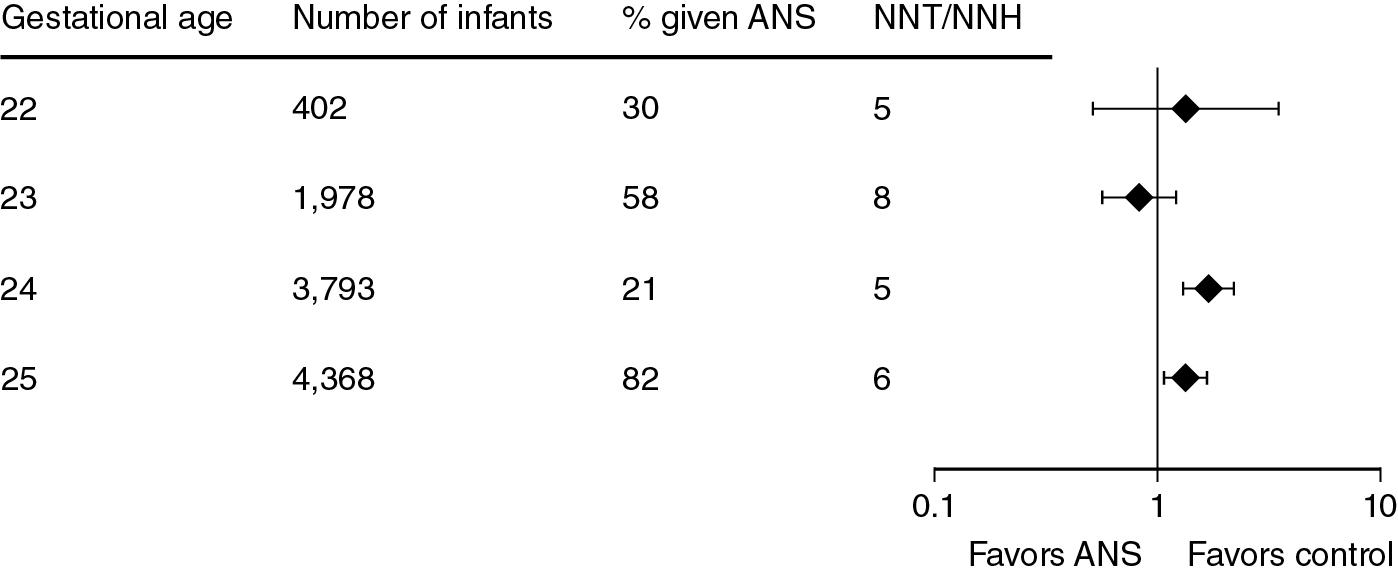
Travers et al. reported that ANS decreased mortality for all gestational ages from 24 to 34 weeks. The data are for the period from 2009 to 2013 (n = 61,571 infants) with about 84% of infants exposed to ANS, except at 34 weeks when the percent of exposure was 45% ( Fig. 11.3 ). ANS exposure was associated with a remarkably consistent decreased mortality even at 34 weeks’ gestation. A similar report from Carlo et al. documented decreased mortality and IVH for more than 10,000 infants for the gestational age range from 22 to 25 weeks with 74% of the population exposed to ANS. No respiratory outcomes are reported other than BPD, which is increased with the use of ANS ( Fig. 11.4 ). The association of increased BPD with ANS is common across the RCTs and epidemiology studies. The generally accepted explanation is that ANS improve survival in the most marginal infants who are at increased risk of developing BPD. However, the arrest of septation and microvascular development with ANS may contribute to this adverse lung outcome. , A more recent cohort of 29,932 infants born at 22 to 25 weeks’ gestation, of which 87% received ANS, showed similar positive effects in survival with no association with BPD. The severity of BPD relative to ANS has not been analyzed and is worthy of evaluation.
The optimal interval from ANS treatment to delivery for maximal benefit based on the RCTs is 1 to 7 days. However, the timing of maximal benefit to the lung is unclear and effects on other organs follow different timelines. A new analysis of a large European dataset indicates that maximal benefit for decreasing death may be an exposure interval as short as 18 to 36 hours, with benefit after 3 hours of administration. Such rapid effects would be more consistent with acute steroid effects on cardiovascular function rather than for lung maturation. In preterm lambs, improved cardiovascular function is observed at 24 hours after ANS and disappears by 2 days (our unpublished observation). However, at 24 hours post steroids, there is increased lung injury and pulmonary interstitial emphysema from mechanical ventilation, and maximal improvement in pulmonary function is seen at 5 days with residual effect up to 10 days. , ,
A concern with the observational associations between ANS and the clinical outcomes is that the great majority of women are treated with ANS. The women not given ANS differ in ways that cannot be reliably adjusted for in the analyses. The women not exposed to ANS have different causes and severities of problems related to prematurity. Many likely deliver before ANS can be given. The epidemiologic analyses may overestimate the benefits of ANS at early gestational ages. Another concern is treatment creep. The RCT data to support use of ANS at early gestational ages are minimal, and RCTs for the use of ANS at “previable” gestational ages are nonexistent. Again, the epidemiology supports improved outcomes at gestations of 22 and 23 weeks, but that information likely reflects highly selected use of ANS in pregnancies being actively managed to achieve infant survival.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here