Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
| Adjusted odds ratio | aOR |
| American College of Obstetricians and Gynecologists | ACOG |
| Anticardiolipin antibodies | aCL |
| Antiphospholipid antibodies | aPL |
| Antiphospholipid syndrome | APS |
| Assisted reproductive technology | ART |
| Bacterial vaginosis | BV |
| β-human chorionic gonadotropin | β-hCG |
| Chorionic villus sampling | CVS |
| Chromosomal microarray | CMA |
| Comparative genome hybridization | CGH |
| Confidence interval | CI |
| European Society of Human Reproduction and Embryology | ESHRE |
| Fluorescence in situ hybridization | FISH |
| Immunoglobulin | Ig |
| Intrauterine adhesions | IUAs |
| In vitro fertilization | IVF |
| Lupus anticoagulant | LAC |
| Luteal phase defect | LPD |
| Odds ratio | OR |
| Oxygen | O 2 |
| Preimplantation genetic screening | PGS |
| Randomized controlled trial | RCT |
| Recurrent early pregnancy loss | REPL |
| Relative risk | RR |
| Royal College of Obstetricians and Gynecologists | RCOG |
| Standard deviation | SD |
| Three-dimensional | 3D |
| Thyroid-stimulating hormone | TSH |
Not all conceptions result in a live-born infant, and human reproduction is extremely inefficient compared with that of other mammal species. About 50% to 70% of spontaneous conceptions are lost before completion of the first trimester, most before implantation or during the first month after the last menstrual period. These losses are often not recognized as conceptions. Of clinically recognized pregnancies, 10% to 15% are lost. Although epidemiologic data on animals living in the wild, such as monkeys, are limited, laboratory rodents are known to have postimplantation pregnancy loss rates of around 10%. The US Department of Health and Human Services reported that among married women between 1965 and 1982, 4% experienced two fetal losses, and 3% experienced three or more. A subset of women manifest repetitive spontaneous miscarriages as opposed to randomly having repeated untoward events. This chapter considers the frequency and timing of pregnancy losses, the causes of fetal loss, and the management of couples experiencing repetitive losses.
Embryos implant 6 days after conception. Physical signs are not generally appreciated until 5 to 6 weeks after the last menstrual period. Fewer than half of preimplantation embryos persist , as witnessed by assisted reproductive technology (ART) success rates rarely exceeding 40% to 50% of in vitro fertilization (IVF) cycles initiated. Even immediately after implantation, determined chemically by the presence of β-human chorionic gonadotropin (β-hCG) in maternal serum, about 30% of pregnancies are lost. After clinical recognition, 10% to 12% are lost. Most clinical pregnancy losses occur before 8 weeks.
Before widespread availability of ultrasound imaging, early fetal demise was often not appreciated until 9 to 12 weeks’ gestation, when bleeding and passage of tissue (products of conception) occurred. With the widespread availability of ultrasound, it has been shown that fetal demise often occurs weeks before overt clinical signs are manifested. This conclusion was reached on the basis of cohort studies showing that only 3% of viable pregnancies are lost after 8 weeks’ gestation. Fetal viability (heart rate activity) may thus cease weeks before maternal symptoms of pregnancy loss. That almost all losses are retained in utero for an interval before clinical recognition means that virtually all losses could be considered “missed abortion or miscarriage.” After the first trimester, pregnancy losses occur at a slower rate. Loss rates are only 1% in women confirmed by ultrasound to have viable pregnancies at 16 weeks.
Two factors influencing clinical pregnancy loss rates are clinically relevant. Firstly, maternal age is positively correlated with pregnancy loss rates ; a 40-year-old woman has twice the risk of a 20-year-old woman. This occurs in euploid as well as aneuploid pregnancies, as discussed later. Secondly, prior pregnancy loss also increases loss rates, but far less than once believed . Among nulliparous women who have never experienced a loss, the likelihood of pregnancy loss is low: 5% in primiparas and 4% in multiparas ( Table 33.1 ). After one loss, the risk of another is increased but does not exceed 30% to 40% even for women with three or more losses. These risks apply not only to those women whose losses were recognized at 9 to 12 weeks’ gestation but also to those whose pregnancies were ascertained in the fifth week of gestation. Of clinical relevance, there is no scientific evidence that women with three losses are etiologically distinct from those with two losses or even one loss. The situation may be different if, rarely, four or more losses have occurred; different etiologic factors may exist in this uncommon subgroup.
| Prior Abortions | Risk (%) | |
|---|---|---|
| Women with live-born infant | 0 | 5–10 |
| 1 | 20–25 | |
| 2 | 25 | |
| 3 | 30 | |
| 4 | 30 | |
| Women without live-born infant | 3 | 30–40 |
The clinical consequence of the above information is that in order to be judged efficacious in preventing recurrent early pregnancy loss (REPL), therapeutic regimens must show success rates substantially greater than 70%. Essentially no therapeutic regimen proposed so far can make this claim.
As judged by adult tissue criteria, the human fetus develops in a low oxygen (O 2 ) environment. Development of the human placenta is modulated heavily by the intrauterine environment. During the first trimester, development takes place in a low O 2 environment supported by histotrophic nutrition from the endometrial glands. Consequently, the rate of growth of the chorionic sac in normally developing pregnancies is almost invariable across this period and is remarkably uniform between individuals. Toward the end of the first trimester, the intrauterine environment undergoes radical transformation in association with the onset of the maternal arterial circulation and the switch to hemochorial nutrition ( Chapter 1 ). The accompanying rise in intraplacental O 2 concentration leads to extensive villous remodeling and in particular to the formation of the membranes of the definitive placenta.
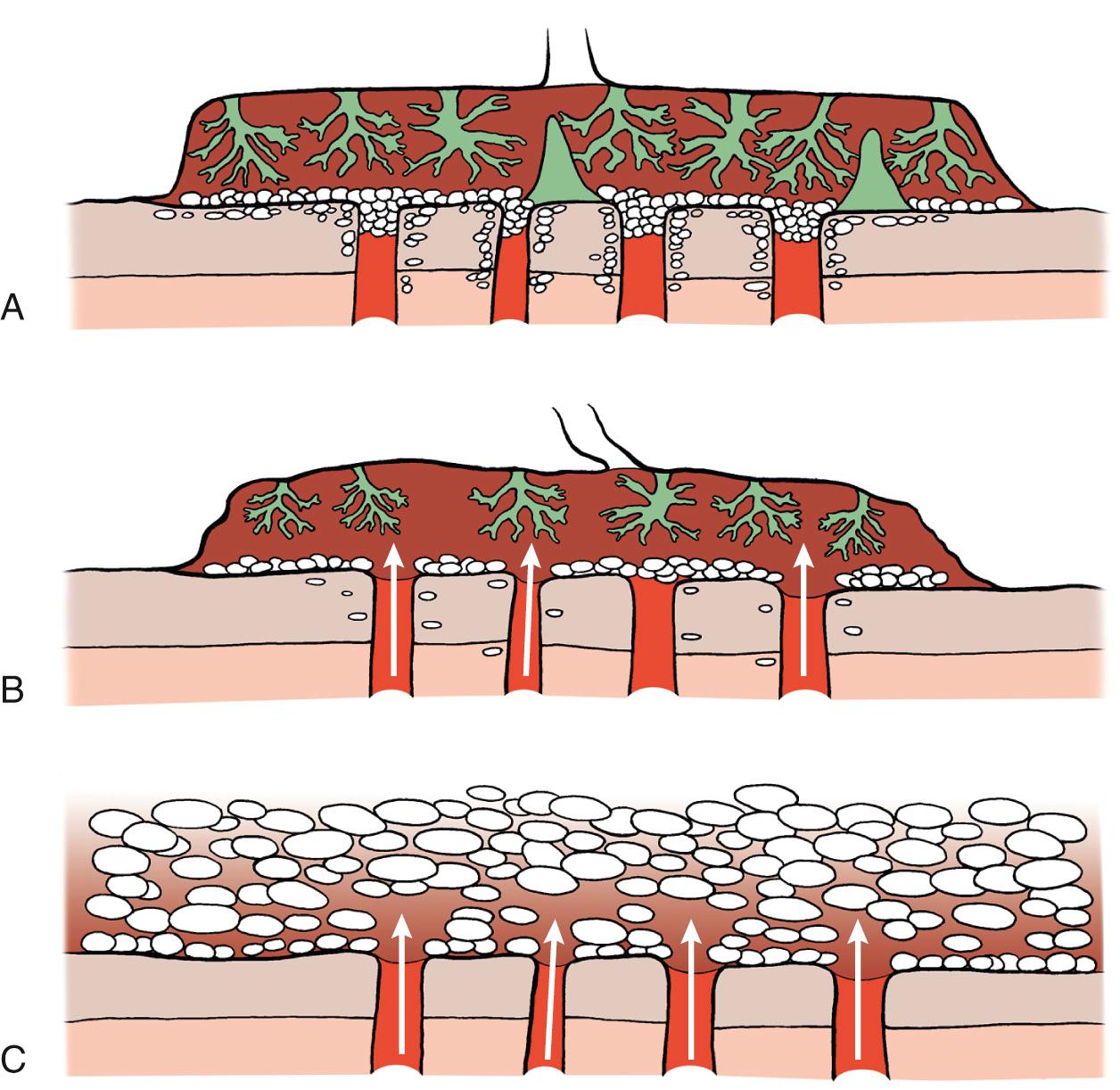
The early human gestational sac is designed to minimize the flux of O 2 from the maternal blood to fetal circulation. In particular, the extravillous trophoblast that migrates inside the uterine decidua and superficial myometrium creates a cellular shell with plugs inside the tip of the uteroplacental arteries. This additional barrier keeps most of the maternal circulation outside the placenta and thus reduces the chemical activity of free O 2 radicals inside the placenta during most of the first trimester of human pregnancy. In normal pregnancies, the onset of maternal circulation is a progressive phenomenon, starting at about 9 weeks at the periphery and gradually extending toward the center of the placenta. This process correlates closely with the pattern of trophoblast invasion across the placental bed ( Fig. 33.1 ).
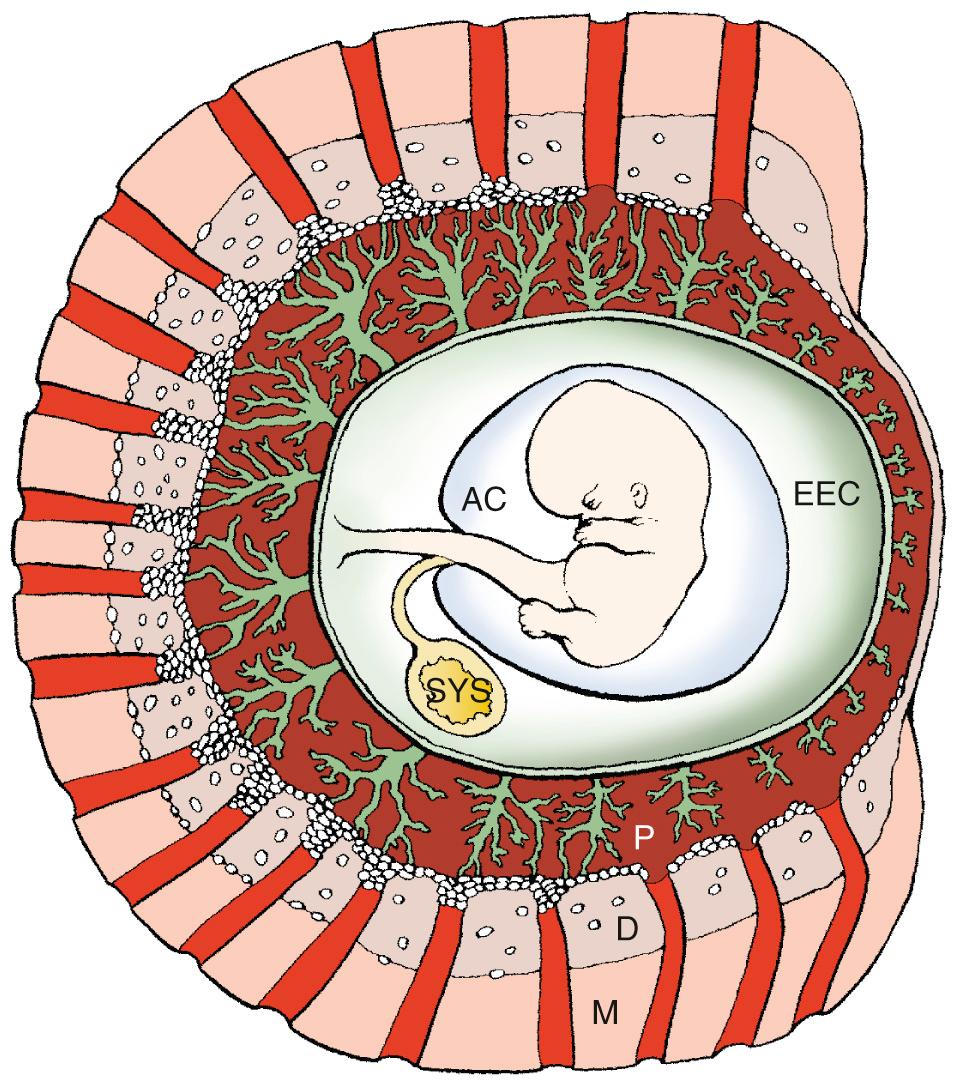
In about two-thirds of early pregnancy failures, there is anatomic evidence of defective placentation, which is mainly characterized by a thinner and fragmented trophoblast shell and reduced trophoblast infiltration of the lumen at the tips of the spiral arteries. This is associated with premature onset of maternal circulation throughout the placenta in most cases of miscarriages. These defects are similar in euploid and most aneuploid miscarriages but are more pronounced in complete hydatidiform moles ( Fig. 33.2 ). In vivo ultrasound imaging and histopathologic data indicate that in most early pregnancy losses, the onset of intervillous circulation is premature and widespread, owing to incomplete transformation and plugging of the uteroplacental arteries. In about 80% of missed miscarriages, the onset of maternal placental circulation is both precocious and generalized throughout the placenta. This occurs independently of the karyotype of the conceptus , leading to high O 2 concentrations too early in pregnancy with secondary widespread trophoblastic oxidative damage and placental degeneration. Although in vitro studies have demonstrated the ability of damaged syncytium to regenerate from the underlying cytotrophoblast, it is likely that in the face of extensive damage this ability will be overwhelmed, leading to complete pregnancy failure.
Chromosomal abnormalities are the main cause of both preimplantation and clinically-recognized pregnancy loss. The frequency of losses in human preimplantation embryos is very high. Of morphologically normal embryos, 25% to 50% show chromosomal abnormalities (aneuploidy or polyploidy), depending on maternal age. The frequency of chromosomal abnormalities in morphologically abnormal embryos is even higher. Moreover, these data are based on studies using fluorescence in situ hybridization (FISH) with chromosome-specific probes for only seven to nine chromosomes; rates would be higher using technologies that can assess all chromosomes, such as 24-chromosome FISH, single nucleotide polymorphism, or array comparative genome hybridization (CGH) analysis. The high aneuploidy rate in morphologically normal embryos is consistent with a rate of aneuploidy of 5% to 10% in sperm of ostensibly normal men and of 20% in oocytes (deduced from polar bodies) of women undergoing ART. Aneuploidy rates in oocytes and embryos predictably increase as maternal age increases.
At least 50% of clinically recognized pregnancy losses result from a chromosomal abnormality. The frequency is probably higher because if one analyzes chorionic villi recovered by chorionic villus sampling (CVS) immediately after ultrasound diagnosis of fetal demise or during surgical curettage (rather than culturing spontaneously expelled products, which has a lower success rate), the chromosomal abnormalities are detected in 65% to 90% of the products. In addition, microarray-based comparative genomic hybridization analysis can detect subtle abnormalities not evident by karyotype.
Approximately 25% of second-trimester losses have been attributed to genetic etiologies. The most common cytogenetic abnormalities are similar to those seen in live-born infants and include trisomy 21, trisomy 18, and trisomy 13, monosomy X and sex chromosomal polysomy. Cytogenetic abnormalities are more common when fetal structural anomalies are identified. Overall, the frequency of aneuploidies is less than that observed in first-trimester miscarriages but much higher than that found among live-born infants (0.6%).
Autosomal trisomies represent the largest (about 50%) single class of chromosomal complements in cytogenetically early pregnancy failure . That is, 25% of all miscarriages are trisomic, given half of all abortuses have a chromosomal abnormality. Frequencies of various trisomies are listed in Table 33.2 . Trisomy for every chromosome has been observed. The most common trisomy in first-trimester losses is trisomy 16. Most trisomies show a maternal age effect, but the effect varies markedly among chromosomes. The increased maternal age effect is especially impressive for double trisomies.
| Chromosomal Complement | Frequency | Percent |
|---|---|---|
| Normal 46,XX or 46,XY | 54.1 | |
| Triploidy | 7.7 | |
| 69,XXX | 2.7 | |
| 69,XYX | 0.2 | |
| 69,XXY | 4.0 | |
| Other | 0.8 | |
| Tetraploidy | 2.6 | |
| 92,XXX | 1.5 | |
| 92,XXYY | 0.55 | |
| Not stated | 0.55 | |
| Monosomy X | 18.6 | |
| Structural abnormalities | 1.5 | |
| Sex chromosomal polysomy | 0.2 | |
| 47,XXX | 0.05 | |
| 47,XXY | 0.15 | |
| Autosomal monosomy (G) | 0.1 | |
| Autosomal trisomy for chromosomes | 22.3 | |
| 1 | 0 | |
| 2 | 1.11 | |
| 3 | 0.25 | |
| 4 | 0.64 | |
| 5 | 0.04 | |
| 6 | 0.14 | |
| 7 | 0.89 | |
| 8 | 0.79 | |
| 9 | 0.72 | |
| 10 | 0.36 | |
| 11 | 0.04 | |
| 12 | 0.18 | |
| 13 | 1.07 | |
| 14 | 0.82 | |
| 15 | 1.68 | |
| 16 | 7.27 | |
| 17 | 0.18 | |
| 18 | 1.15 | |
| 19 | 0.01 | |
| 20 | 0.61 | |
| 21 | 2.11 | |
| 22 | 2.26 | |
| Double trisomy | 0.7 | |
| Mosaic trisomy | 1.3 | |
| Other abnormalities or not specified | 0.9 | |
| 100.0 |
Trisomies incompatible with life predictably show slower growth than trisomies compatible with life (e.g., trisomies 13, 18, 21), but otherwise there are usually no features distinguishing the two groups. Fetuses from the former group may show anomalies consistent with those found in full-term live-born trisomic neonates. Malformations present have been said to be more severe than those observed in induced abortuses following prenatal diagnosis.
Aneuploidy usually results from errors at meiosis I, specifically maternal meiosis I. Errors of maternal meiosis I are associated with advanced maternal age. Once thought to involve mostly mis-segregation of whole chromosomes, it is now clear that chromatid errors are an equally prevalent cause of maternal meiotic errors. Irrespective, the cytologic mechanism involves decreased or absent meiotic recombination. In trisomy 13 and trisomy 21, 90% of these maternal cases arise at meiosis I. Almost all trisomy 16 cases arise in maternal meiosis I. An exception is trisomy 18, in which two thirds of the 90% of maternal meiotic cases arise at meiosis II.
A practical consequence of these data is that deducing chromosomal status of oocytes by analysis of polar bodies can detect 95% of more of chromosomally abnormal embryos. This is relevant because polar body analysis is a more robust predictor of embryo status than analysis of blastomere from a 3-day-old embryo. In the latter, mitotic nondisjunction can lead to spurious and unrepresentative results. Errors in paternal meiosis account for 10% of acrocentric (13, 14, 15, 21, and 22) trisomies . In trisomy 21, paternal meiotic errors are equally likely to arise in meiosis I or II, a circumstance that contrasts with the situation in maternal meiotic errors. Among nonacrocentric chromosomes, paternal contribution is uncommon. A surprising exception involves trisomy 2.
In polyploidy, more than two haploid chromosomal complements are present. Nonmosaic triploidy (3 n = 69) and tetraploidy (4 n = 92) are common in miscarriage products . This phenomenon is presumably distinct from the diploid or triploid mosaicism that is found in about 30% of blastocysts. Triploid miscarriages are usually 69,XXY or 69,XXX, resulting from dispermy (paternally inherited). An association exists between these diandric triploidy and the development of partial hydatidiform moles associated with persistent trophoblastic disease; thus, in these cases, appropriate hCG follow-up should be recommended. The more common “complete” (classic) hydatidiform mole is 46,XX, androgenetic in origin, and composed exclusively of villous tissue. Pathologic findings in diandric triploid and tetraploid placentas include a disproportionately large gestational sac, focal (partial) hydropic degeneration of placental villi, and trophoblast hyperplasia. Placental hydropic changes are progressive and may be difficult to identify in early pregnancy. By contrast, placental villi often undergo hydropic degeneration after fetal demise. This can occur in all types of miscarriage and thus, histologic and cytogenetic investigations are essential to differentiate between true mole and pseudo-mole because only a true mole can be associated with persistent trophoblastic disease. Fetal malformations associated with triploid miscarriage include neural tube defects and omphaloceles, anomalies reminiscent of those observed in triploid conceptuses surviving to term. Facial dysmorphia and limb abnormalities have also been reported. Tetraploidy is uncommon, rarely progressing beyond 2 to 3 weeks of embryonic life. This chromosomal abnormality can also be associated with persistent trophoblastic disease and thus needs to be identified in order to offer hCG follow-up.
The complements 47,XXY and 47,XYY each occur in about 1 per 800 live-born male births; 47,XXX occurs in 1 per 800 female births . X or Y polysomy are only slightly more common in miscarriages than in live-born infants. In pregnancies conceived by intracytoplasmic sperm injection, the frequency of 47,XXX and 47,XXY embryos and fetuses appears to be increased.
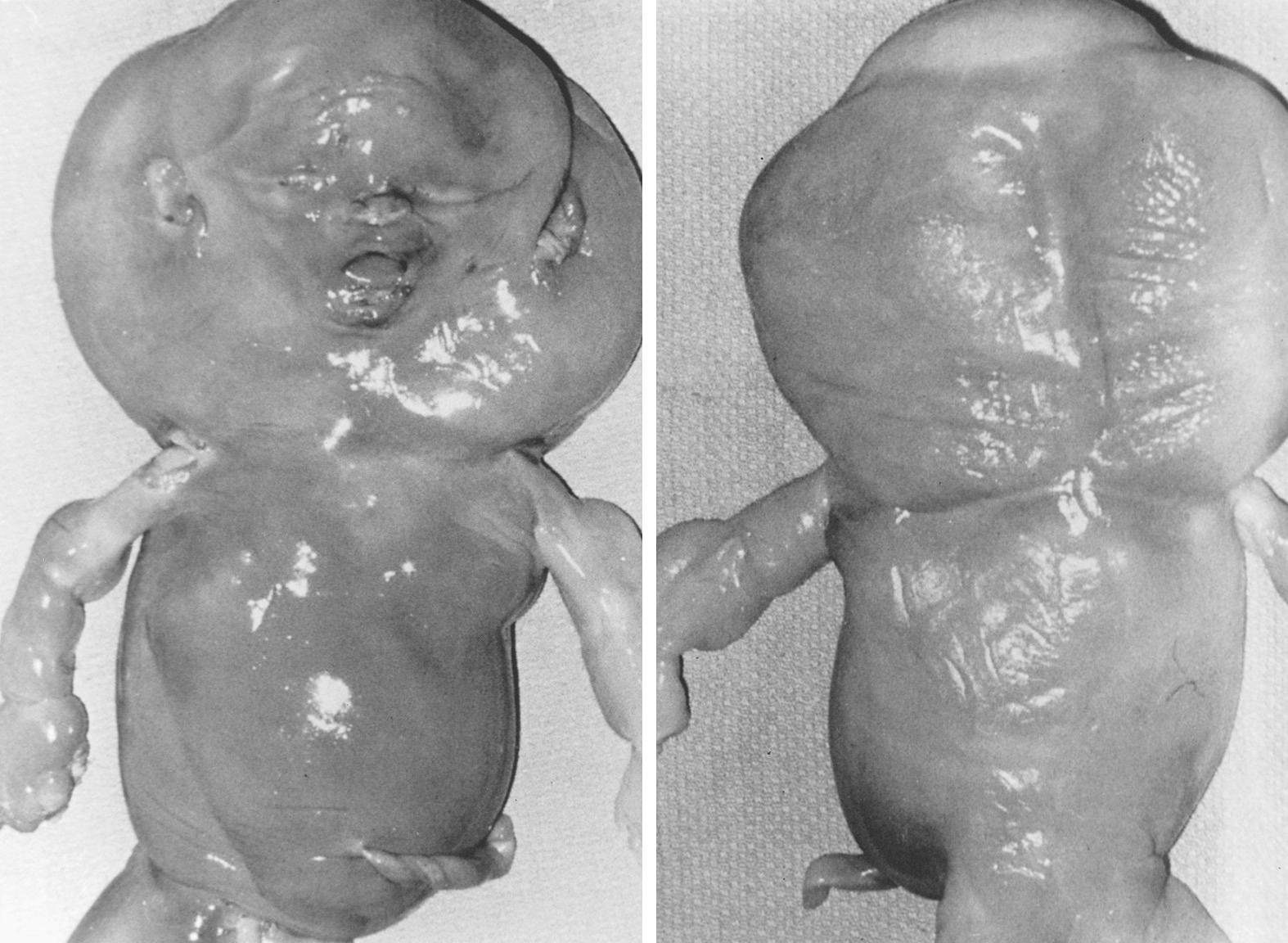
Monosomy X is the single most common chromosomal abnormality among miscarriages, accounting for 15% to 20% of abnormal specimens. Monosomy X embryos usually consist of only an umbilical cord stump. Later in gestation, anomalies characteristic of Turner syndrome may be seen, such as cystic hygromas and generalized edema ( Fig. 33.3 ). Unlike live-born 45,X individuals, 45,X abortuses show germ cells; however, germ cells rarely develop beyond the primordial germ cell stage. The pathogenesis of 45,X germ cell failure thus involves not so much failure of germ cell development as more rapid attrition in 45,X compared with 46,XX embryos. Monosomy X usually (80%) occurs as result of paternal sex chromosome loss. This observation is consistent with the lack of a maternal age effect in 45,X.
In both preimplantation and first-trimester early pregnancy failure, recurrent aneuploidy occurs more often than expected by chance. Recurrent aneuploidy is a frequent explanation, at least until the number of losses reaches or exceeds four. In a given family, successive miscarriages are likely to be either recurrently normal or recurrently abnormal. Table 33.3 shows that if the complement of the first abortus is abnormal, recurrence usually involves aneuploidy, although not necessarily of the same chromosome. Further supporting recurrent aneuploidy as a genuine phenomenon is the occurrence of trisomic preimplantation embryos in successive ART cycles. In couples with repeated REPL, there is an increased rate of aneuploid embryos compared with couples undergoing preimplantation genetic screening (PGS) for mendelian indications (71% vs. 45%). This rate increases with maternal age and is 37% versus 21% in women younger than 35 years, and 34% versus 31.5% in women older than 35 years.
| Complement of First Miscarriage | COMPLEMENT OF SECOND MISCARRIAGE | |||||
|---|---|---|---|---|---|---|
| Normal | Trisomy | Monosomy | Triploidy | Tetraploid | De Novo Rearrangement | |
| Normal | 142 | 18 | 5 | 7 | 3 | 2 |
| Trisomy | 31 | 30 | 1 | 4 | 3 | 1 |
| Monosomy X | 7 | 5 | 3 | 3 | 0 | 0 |
| Triploidy | 7 | 4 | 1 | 4 | 0 | 0 |
| Tetraploidy | 3 | 1 | 0 | 2 | 0 | 0 |
| De novo rearrangement | 1 | 3 | 0 | 0 | 0 | 0 |
The concept of recurrent aneuploidy implies certain corollaries, one of which has often been the subject of controversy. Recurrent aneuploidy stratifies into recurrent losses in which couples experience chromosomally abnormal miscarriages repeatedly versus in which couples experience chromosomally normal recurrent miscarriages. Given that at least 50% of all miscarriages are abnormal cytogenetically, aneuploidy should be as likely to be detected in randomly karyotyped miscarriages as in sporadic miscarriages. This concept is supported by the following data: a 57% prevalence of chromosomal abnormalities was found among abortuses of women with REPL, a frequency identical among abortuses of women with sporadic miscarriages. In a study of 420 specimens of products of conception obtained from women with REPL, 46% were found to be cytogenetically abnormal; 31% of the original sample was trisomic. The frequency of euploid miscarriages was significantly higher in women less than 36 years of age with recurrent miscarriage compared with controls. The distribution of cytogenetic abnormalities in the recurrent miscarriage group was not significantly different from controls when stratified by maternal age.
In contrast to these data, fetal losses—recurrent or not—are much more likely to be cytogenetically normal (85%) when occurring after the first trimester. In one study among women having three or more REPL, the likelihood that the abortus would have an abnormal karyotype was only 29%. In that series, inclusion criteria extended to 20 weeks’ gestation, a time at which there is less reason to expect recurrent aneuploidy than recurrence of other etiologies.
Couples predisposed to recurrent aneuploidy are at increased risk not only for aneuploid miscarriage but also for aneuploid live-born neonates. The autosome trisomic in a subsequent pregnancy might be compatible with life (e.g., trisomy 21). Indeed, the risk for live-born trisomy 21 following an aneuploid early pregnancy loss has been stated to be about 1% (see Chapter 10 ). The risk is considered similar following other aneuploidies. An increased risk of karyotypic abnormality identified at the time of prenatal diagnosis has been shown in women with an increasing number of early pregnancy losses. When controlling for maternal age, parity, ethnicity, and mode of prenatal diagnosis, in comparison to women with no prior miscarriage, women with one prior miscarriage (adjusted odds ratio [aOR], 1.21; 95% confidence interval [CI], 1.01 to 1.47) or 3 or more prior spontaneous abortions (aOR, 1.51; 95% CI, 1.02 to 2.25) had a statistically significant increase in aneuploidy in a subsequent pregnancy. The findings of this study provided information for a counseling algorithm applicable following a prior miscarriage of unknown karyotype. If early pregnancy losses are recurrent, but no information is available on the chromosomal status, the odds ratio can be used to derive a patient-specific risk. For example, if the prior Down syndrome risk is 1 in 300 and the OR is 1.5, a woman's calculated risk after three abortions would be 1/300 × 1.5, or 1 in 200.
If no information is available concerning the chromosomal status of prior early pregnancy losses, paraffin blocks of conception products can be used to detect aneuploidy using FISH or array CGH. If the test shows a trisomy, the likelihood of a live-born trisomy is increased in subsequent pregnancies. If no information can be obtained, it is unclear whether prenatal genetic diagnosis is appropriate. The small but definite risk of postprocedure pregnancy loss for amniocentesis or CVS can be troublesome to couples who have had difficulty maintaining a pregnancy. Thus, noninvasive approaches such as cell-free DNA screening is often a chosen option (see Chapter 10 ). Some couples may be also troubled by the decision of terminating an ongoing pregnancy. Within this context, PGT (see Chapter 10 ) is another option. Selective transfer of euploid embryos has been shown to decrease the rate of clinical early pregnancy losses in couples with REPL because information on all chromosomes exists.
Structural chromosomal abnormalities are an unequivocal explanation for repetitive abortions. The most common structural rearrangement encountered is a translocation, found in about 2% to 3% of couples experiencing REPL. Individuals with balanced translocations are phenotypically normal, but their offspring (abortuses or abnormal live-born infants) often show chromosomal duplications or deficiencies as a result of normal meiotic segregation . Among couples with repetitive abortions, about 60% of translocations are reciprocal and 40% as a result of centric fusion (robertsonian). Women are about twice as likely as men to show a balanced translocation.
The clinical consequences of a balanced translocation are illustrated in Fig. 33.3 . If a child has a Down syndrome phenotype due to a chromosome 21 Robertsonian translocation, the rearrangement will have originated de novo in 50% to 75% of cases. That is, a balanced translocation will not exist in either parent. The likelihood of Down syndrome recurring in subsequent offspring of those parents is minimal. On the other hand, the risk is much higher (25% to 50%) in families in which children have Down syndrome as result of a balanced parental translocation [e.g., parental complement 45,XX,-14,-21,+t(14q;21q)]. In this circumstance, the theoretical risk for having a child with Down syndrome is 33%, but empirical risks are considerably less. The risk is only 2% if the father carries the translocation; the risk is 10% if the mother carries the translocation. If Robertsonian translocations involve other chromosomes, empirical risks are even lower. If the translocation is t(13q;14q), the risk for live-born trisomy 13 is 1% or less.
Reciprocal translocations do not involve centromeric fusion but rather, interchanges between two or more chromosomes. Empirical data for specific translocations is usually not available, but generalizations can be made on the basis of pooled data derived from many different translocations. Again, theoretical risks for abnormal offspring (unbalanced reciprocal translocations) are far greater than empirical risks. Overall, the risk is 12% for offspring of either female heterozygotes or male heterozygotes. Detecting a chromosomal rearrangement thus profoundly affects subsequent pregnancy management. Antenatal cytogenetic studies should be offered. The frequency of unbalanced fetuses is lower if parental balanced translocations are ascertained through repetitive abortions (3%) than through anomalous live-born infants (nearly 20%). Presumably more unbalanced products are lethal.
Analysis of preimplantation embryos using PGT reveals that most embryos from parents with translocations are unbalanced —58% from parents with Robertsonian translocations and 76% from parents with reciprocal translocations. This means that most conceptuses of these parents would be lost, usually preclinically. When a balanced translocation is detected in a couple experiencing recurrent abortions, cumulative prognosis for a live-born infant is little different than if a translocation had not been detected ; however, the length of time to achieve pregnancy is greatly increased (on average, 4 to 6 years). Thus, a more realistic strategy is to use PGT to identify and transfer only the few balanced embryos, increasing the statistical likelihood of conception. Overall, there are insufficient data indicating that PGT improves the live birth rate in couples with REPL carrying a structural chromosome abnormality.
Rarely, a translocation precludes the production of chromosomally-normal live-born infants. This occurs when a translocation involves homologous, acrocentric chromosomes (e.g., t[13q13q] or t[21q21q]). If the father carries such a structural rearrangement, artificial insemination may be appropriate. If the mother carries the rearrangement, donor oocytes or donor embryos and ART should be considered.
Inversions are uncommon parental chromosomal rearrangements but are responsible for repetitive pregnancy losses analogous to translocations. In inversions, the order of genes is reversed. Individuals heterozygous for an inversion should be normal if their genes are merely rearranged. However, individuals with inversions suffer untoward reproductive consequences as a result of normal meiotic phenomena. Crossing-over that involves the inverted segment yields unbalanced gametes. Pericentric inversions are present in perhaps 0.1% of women and 0.1% of men experiencing REPL. Paracentric inversions are even rarer.
Women with a pericentric inversion have a 7% risk of abnormal live-born infants whereas men carry a 5% risk. Pericentric inversions ascertained through phenotypically normal probands are less likely to result in abnormal live-born infants.
Inversions involving only a small portion of the total chromosomal length are less significant clinically because large duplications or deficiencies arise following crossing over, usually conferring lethality. By contrast, inversions involving only 30% to 60% of the total chromosomal length are relatively more likely to be characterized by duplications or deficiencies compatible with survival. Prenatal cytogenetic studies should be offered in these cases.
Paracentric inversions should carry less risk for unbalanced products than pericentric inversions because nearly all paracentric recombinants should, in theory, be lethal. However, abortions and abnormal live-born infants have been observed within the same kindred and the risk for unbalanced viable offspring has been tabulated at 4%. Thus prenatal cytogenetic studies should still be offered.
Even in the 30% to 50% of first-trimester pregnancy losses that show no chromosomal abnormalities, genetic causation is not excluded. Fetal demise may have occurred as a result of other genetic etiologies. Specifically, neither mendelian nor polygenic/multifactorial disorders show chromosomal abnormalities, and yet these etiologies explain far more congenital anomalies in live-borns than do chromosomal abnormalities. Mendelian and polygenic/multifactorial factors must therefore play a pivotal role in embryonic mortality and there are innumerable candidate genes. Especially likely to be mendelian or polygenic in etiology are early pregnancy losses that demonstrate isolated structural anomalies. Cytogenetic data are often lacking in these specimens, making it nearly impossible to determine the relative role of cytogenetic versus mendelian or polygenic mechanisms in early embryonic maldevelopment. Transcervical embryoscopy prior to curettage correlated with cytogenetic analysis of chorionic villous tissue in missed miscarriages has shown that embryos with chromosomal abnormalities usually showed one or more external anomalies but also that 18% had a morphologic defect with a normal karyotype.
In addition to traditional single-gene perturbations (mendelian etiology), novel nonmendelian forms of inheritance play a greater role in embryonic loss. Mosaicism may be restricted to the placenta, the embryo being normal per se. This phenomenon is termed confined placental mosaicism . Losses caused by this mechanism may already be subsumed in extant data because most studies involved analysis only of villous material. A corollary of confined placental mosaicism is uniparental disomy, in which both homologues for a given chromosome are derived from a single parent. This presumably occurs as result of expulsion of a chromosome from a trisomic zygote (“trisomic rescue”). Although the karyotype would appear normal (46,XX or 46,XY), the product would lack a contribution from one parent.
Implantation in an inhospitable endometrium is a plausible explanation for pregnancy loss. Progesterone deficiency in particular could result in the estrogen-primed endometrium being unable to sustain implantation. Luteal phase deficiency (LPD) has long been hypothesized, specifically caused by inadequate progesterone secreted by the corpus luteum. Yet, endometrial histology identical to that observed with luteal phase “defects” is observed in fertile women and there is no convincing evidence that LPD is associated with infertility and REPL.
A recent multicenter, double-blind, placebo-controlled, randomized trial investigated whether treatment with progesterone would increase the rates of live births among women with unexplained recurrent miscarriages. A group of 836 women who conceived naturally were randomly assigned to receive either progesterone (404 women) or placebo (432 women). There were no significant between-group differences in the rates of live births or of adverse events.
Luteal phase abnormalities arising during ovulation stimulation and oocyte retrieval as necessitated during ART could be a different phenomenon. Progesterone supplementation during the luteal phase is associated with higher rates of live birth or ongoing pregnancy than placebo in this setting. It is considered standard to administer progesterone until about 9 weeks’ gestation. In this circumstance, the cells surrounding the oocyte, which would ordinarily contribute to the corpus luteum, may have been removed when the oocyte was aspirated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here