Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vasopressin and oxytocin, which are hormones of the posterior pituitary, are synthesized in specialized neurons of the hypothalmus. These neurons, notable for their large size, are termed magnocellular neurons . In the hypothalamus, the magnocellular neurons are clustered in the paired paraventricular and supraoptic nuclei ( Fig. 206-1 ). Vasopressin and oxytocin are also synthesized in parvocellular (i.e., small cell) neurons of the paraventricular nuclei, and vasopressin (but not oxytocin) is also synthesized in the suprachiasmatic nucleus.
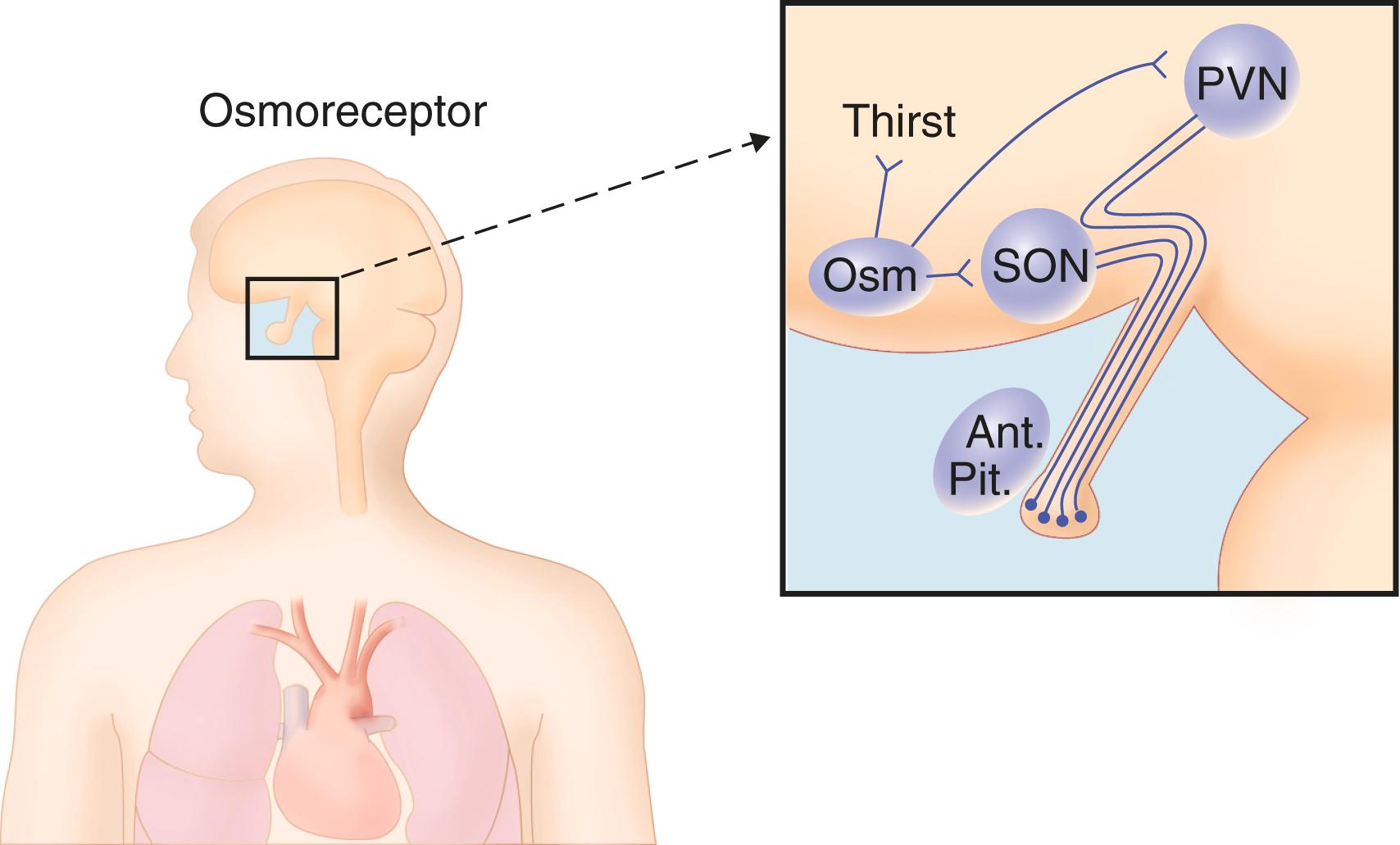
Transcription of vasopressin and oxytocin messenger RNA and translation of the vasopressin and oxytocin prohormones occur entirely in the cell bodies of the neurohypophyseal neurons. The prohormones, provasopressin and pro-oxytocin, are packaged along with processing enzymes into neurosecretory granules that are transported out of the perikaryon of the neurohypophyseal neurons via microtubules and down the long axons that form the supraopticohypophysial tract, which terminates in the posterior pituitary. During transport, the processing enzymes cleave provasopressin into vasopressin (9 amino acids), vasopressin-neurophysin (95 amino acids), and vasopressin glycopeptide, or copeptin (39 amino acids). Pro-oxytocin is similarly cleaved to oxytocin (which differs from vasopressin by only two of nine amino acids) and oxytocin-neurophysin. The neurophysins form neurophysin-hormone complexes that stabilize the hormones. Stimulatory (e.g., glutamatergic, cholinergic, and angiotensin) neurotransmitter terminals and inhibitory (e.g., γ-aminobutyric acid and noradrenergic) neurotransmitter terminals control the release of vasopressin through the activity of synaptic contacts on the neurohypophyseal cell bodies. Physiologic release of vasopressin or oxytocin into the general circulation occurs at the level of the posterior pituitary. Although each of the other prohormone fragments are released into the circulation, vasopressin and oxytocin are the only known biologically active components of the prohormones. Factors that stimulate the release of neurohypophyseal hormones also stimulate their synthesis. Because synthesis is delayed, maintenance of a large store of hormone in the posterior pituitary is essential to enable the instantaneous release of each hormone that is necessary following acute hemorrhage (vasopressin) or during parturition (oxytocin). Sufficient vasopressin is stored in the posterior pituitary to support maximal antidiuresis for several days and to maintain baseline levels of antidiuresis for weeks.
The primary physiologic action of vasopressin is its function as a water-retaining hormone. The central sensing system (osmostat) for controlling the release of vasopressin is anatomically discrete, located in a small area of the hypothalamus just anterior to the third ventricle (see Fig. 206-1 ). The osmostat controls the release of vasopressin to allow water retention and also stimulates thirst to cause water repletion.
Osmotic regulation of vasopressin release and osmotic regulation of thirst are usually tightly coupled, but they can be dissociated under pathologic conditions. The primary extracellular osmolyte to which the osmoreceptor responds is sodium. Although basal osmolality in normal individuals ranges between 280 and 295 mOsm/kg H 2 O, extracellular fluid osmolality for each individual is maintained within narrow ranges. Increases in plasma osmolality as small as 1 to 2% are sufficient to stimulate vasopressin release. Basal plasma levels of vasopressin are generally 0.5 to 2 pg/mL, a level that maintains urine osmolality above plasma osmolality and urine volume in the range of 1 to 3 L/day. When vasopressin levels are suppressed below 0.5 pg/mL, urine osmolality decreases to below 100 mOsm/kg H 2 O, and a free water diuresis (or “aquaresis”) ensues at levels that approach 800 to 1000 mL/hour (18 to 24 L/day). Increases in plasma osmolality cause a linear increase in plasma vasopressin and a corresponding linear increase in urine osmolality. At a plasma osmolality of approximately 295 mOsm/kg H 2 O, urine osmolality is maximally concentrated to 1000 to 1200 mOsm/kg H 2 O. As a result, the entire physiologic range of urine osmolality is accomplished by relatively small changes in plasma vasopressin levels of 0 to 5 pg/mL ( E-Fig. 206-1 ).
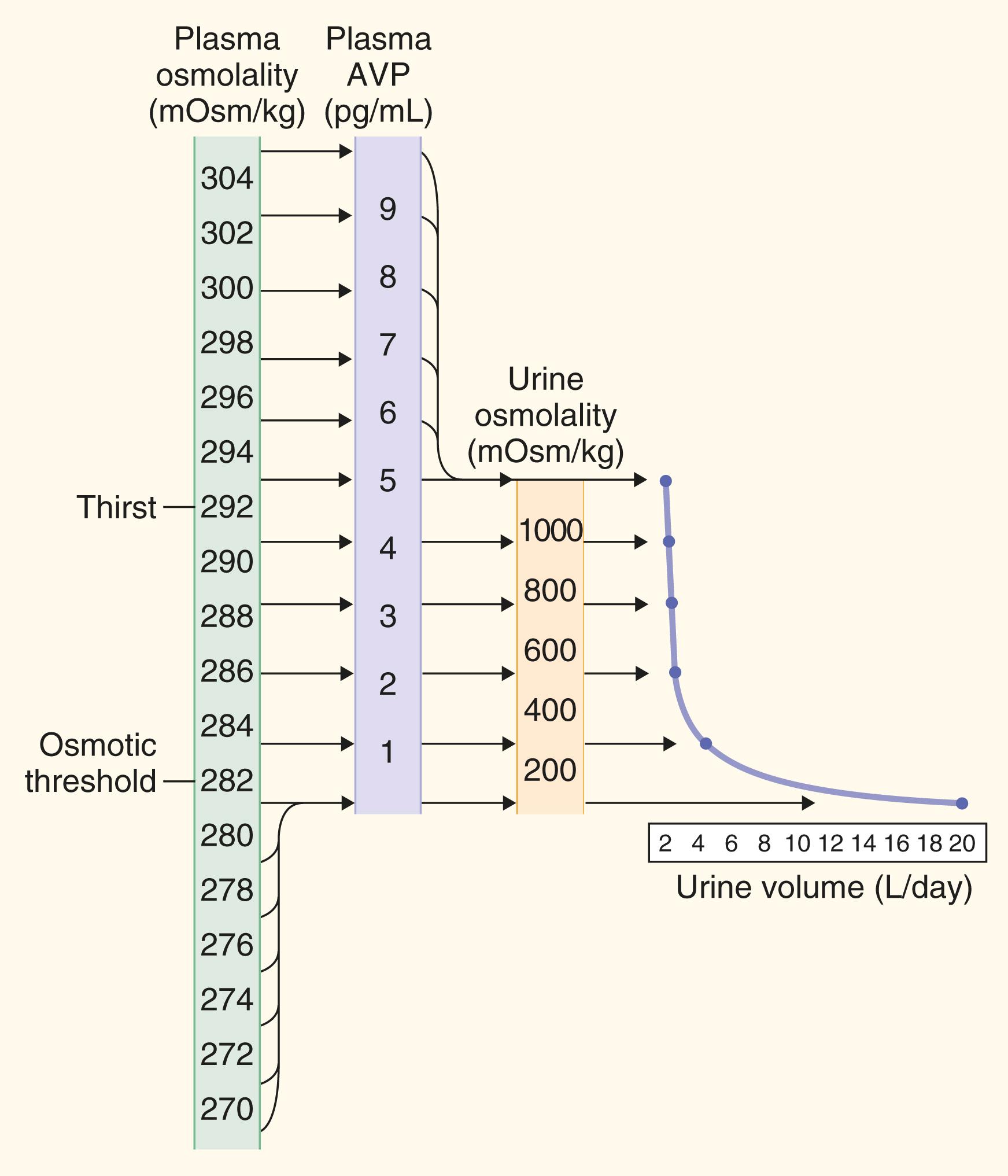
Thirst is not stimulated until a somewhat higher plasma osmolality (5 to 10 mOsm/kg H 2 O) above the threshold for release of vasopressin. Most humans derive sufficient water from habitual fluid intake and catabolism of food to maintain plasma osmolality below the threshold that activates thirst. Therefore, under normal physiologic conditions, water balance (and hence plasma osmolality) is regulated more by secretion of vasopressin than by thirst. However, with severe degrees of dehydration, thirst is essential to restore body water deficits.
Vasopressin acts on the V 2 subtype of vasopressin receptors in the collecting duct principal cells of the kidney. Vasopressin V 2 receptors activate adenylate cyclase, and the subsequent increased intracellular levels of cyclic adenosine monophosphate (cAMP) initiate the movement of aquaporin-2 (AQP2) water channels to the apical (luminal) membrane of the collecting duct cells. These channels allow facilitated rapid transport of water from the collecting duct lumen into the principal cell along osmotic gradients. The water then exits the cell through the basolateral membrane into the kidney medullary circulation through constitutively expressed aquaporin-3 and aquaporin-4 water channels. This entire process is termed antidiuresis . In the absence of vasopressin, the AQP2 channels are reinternalized from the apical membrane into subapical vesicles. This process prevents active reabsorption of water from the collecting duct lumen, thereby resulting in diuresis. In addition to this rapid shuttling of the AQP2 channels to regulate water reabsorption on a minute-to-minute basis, vasopressin acts through the V 2 receptors to regulate long-term stores of AQP2. The absence of vasopressin results in decreased synthesis of AQP2. The hypertonic medullary interstitium is the determinant of the maximal concentration of the urine, which is in equilibrium with the osmolality of the inner medulla of the kidney under conditions of maximal antidiuresis ( Chapter 101 ).
High-pressure baroreceptors are located in the aorta and carotid sinus, and low-pressure baroreceptors are located in the right and left atria. Decreases in blood pressure or intravascular volume stimulate the release of vasopressin, whereas situations that increase blood volume or left atrial pressure (e.g., negative-pressure breathing) decrease the secretion of vasopressin. The release of vasopressin in response to changes in volume or pressure is much less sensitive than the release in response to osmoreceptors; generally a 10 to 15% reduction in blood volume or pressure is needed to stimulate the release of vasopressin. However, once arterial pressure falls below this threshold, the stimulated response is exponential, thereby resulting in plasma levels of vasopressin that are markedly greater than those resulting from osmotic stimulation.
The pressor effects of vasopressin are mediated through a separate vasopressin receptor subtype, the V 1a receptors, located on vascular smooth muscle. However, the renin-angiotensin-aldosterone system ( Chapter 208 ) is more important for controlling extracellular and blood volume than is the regulation of water homeostasis. Nevertheless, the pressor effects of vasopressin to increase blood pressure can become prominent when other blood pressure regulatory systems are deficient (e.g., autonomic neuropathy [ Chapter 386 ] or blockade of the renin-angiotensin-aldosterone system with medication) or in states of pathologic vasodilation (e.g., liver cirrhosis [ Chapter 139 ], septic shock [ Chapter 94 ]).
Vasopressin stimulates secretion of adrenocorticotropic hormone (ACTH) via stimulation of the vasopressin V 1b receptor subtype that is located on anterior pituitary corticotroph cells. Although the major regulator of ACTH secretion is corticotropin-releasing hormone ( Chapter 205 ), vasopressin activates a different signal transduction system in the corticotrophs, so these hormones have synergistic effects on the secretion of ACTH.
The vasopressin system optimizes water homeostasis. Water is consumed as available in the absence of stimulated thirst, and vasopressin secretion then regulates water excretion to maintain plasma osmolality. Thirst serves as a reserve mechanism if dehydration becomes excessive. Because pressure-volume regulation of vasopressin is less sensitive, modest changes in pressure or volume, which are exacerbated by upright posture, do not interfere with the regulation of osmolality. Yet the pressor effect of high vasopressin levels serves to maintain blood pressure if volume depletion or hypotension becomes excessive. Usually, the physiologic regulation of osmolality and pressure-volume is synergistic. Dehydration causes an increase in plasma osmolality and a decrease in blood volume, both of which stimulate the release of vasopressin. Conversely, excess fluid administration causes a decrease in plasma osmolality and an expansion of blood volume, both of which inhibit vasopressin secretion.
With volume expansion, the secretion of vasopressin decreases. During pregnancy, plasma osmolality decreases by approximately 10 mOsm/kg H 2 O as a result of a resetting of the osmostat for vasopressin secretion, and the osmostat for thirst is reset downward in parallel. These effects appear to be mediated by the placental hormone relaxin.
Abnormalities in water and electrolyte balance are common in the elderly, in part owing to age-related changes in body volume (as much as a 50% decrease in total body water occurs in those older than 75 years) and renal function. The elderly also have a decreased sense of thirst. Despite a normal or even increased ability to secrete vasopressin with age, the ability to achieve either maximal urine concentration to retain water or maximal dilution of urine to excrete water is diminished. Consequently, the elderly are particularly prone to both hypernatremia and hyponatremia ( Chapter 102 ) when faced with diseases or medications that affect water balance.
Prolactin is the main hormone necessary for milk production, but oxytocin is essential for milk secretion. Suckling stimulates tactile receptors in the nipple, thereby producing an afferent signal to the hypothalamus that causes a synchronized release of oxytocin from the posterior pituitary. Oxytocin binds to oxytocin receptors in the breast and induces the alveoli and ductules to eject milk. Upregulation of uterine oxytocin receptors also dramatically increases uterine smooth muscle contractions at the end of pregnancy, when it induces uterine contraction to inhibit blood loss after delivery. No pathologic syndromes of either increased or decreased secretion of oxytocin have yet been defined, although oxytocin is clearly important for maternal and affiliative behaviors. However, because of their structural similarity, at high plasma levels oxytocin can activate vasopressin receptors, and vasopressin can activate oxytocin receptors.
Excess secretion of vasopressin can be caused by abnormally regulated secretion from the posterior pituitary or by ectopic synthesis and secretion of vasopressin by tumors. Osmotically inappropriate secretion of vasopressin causes renal water retention and volume expansion, with consequent dilutional hyponatremia. This disorder, called the syndrome of inappropriate antidiuretic hormone secretion (SIADH), is discussed in Chapter 102 .
Diabetes insipidus is the excretion of a large volume of hypotonic insipid (tasteless) urine, usually manifested by polyuria (increased urination) and polydipsia (increased thirst). The large urine volume, usually in excess of 50 to 60 mL/kg/day, must be distinguished from an increased frequency of small urine volumes and from large volumes of isotonic or hypertonic urine, both of which have different clinical significance.
Diabetes insipidus is a rare disease with a reported prevalence of 1:25,000. Less than 10% of diabetes insipidus can be attributed to hereditary forms such as X-linked nephrogenic diabetes insipidus, which represents 90% of cases of congenital nephrogenic diabetes insipidus and occurs with a frequency of 4 to 8 per 1 million male live births. No sex difference has been reported for the other forms.
Five pathophysiologic mechanisms must be considered in the differential diagnosis of diabetes insipidus.
Central diabetes insipidus is caused by the inability of the hypothalamus-posterior pituitary to secrete (and usually to synthesize) vasopressin in response to increased osmolality. Central diabetes insipidus can be inherited as an autosomal dominant disease that is typically characterized by an asymptomatic infancy and an onset later in childhood. Most genetic defects are either in the signal peptide of the pre-prohormone or in the neurophysin portion of the prohormone. Most cases are believed to result from disruption of cleavage from the signal peptide or abnormal folding of the neurophysin, which slows trafficking of the mutant prohormone through the endoplasmic reticulum, thereby leading to neuronal cell dysfunction or death. Because this is a cumulative process, this explains the later onset of central diabetes insipidus with these types of mutations.
As few as 10 to 15% of the normal number of vasopressinergic neurons in the hypothalamus is sufficient to maintain an asymptomatic urine volume, but the further loss of just a small number of these neurons produces a rapid increase in urine volume and symptomatic polyuria. The dilute glomerular filtrate is not concentrated in the renal collecting duct, and consequently, a large volume of hypotonic (i.e., dilute) urine is excreted. The result is a secondary increase in serum osmolality, with stimulation of thirst and secondary polydipsia. Levels of vasopressin in plasma are unmeasurable or inappropriately low for the plasma osmolality. Most cases are acquired, for example, from tumors, neurosarcoidosis of the hypothalamus ( Chapter 83 ), tuberculous meningitis ( Chapter 299 ), multiple sclerosis ( Chapter 380 ), histiocytosis ( Chapter 155 ), or brain trauma ( Chapter 368 ), particularly neurosurgical resection of pituitary adenomas, but some are idiopathic, and a small proportion are genetic.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here