Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pulmonary disease is common in patients with end-stage liver disease, and as many as 50% to 70% of patients with end-stage liver disease complain of shortness of breath. The differential diagnosis includes obstructive airway disease, infection, fluid retention with hydrothorax, and decreased lung capacities secondary to ascites. Other conditions such as α 1 -antitrypsin deficiency and cystic fibrosis can affect both the lung and liver. This chapter focuses on the pulmonary vascular complications of liver disease. There are two clinical and pathophysiological pulmonary abnormalities usually associated with portal hypertension, and often liver cirrhosis. These are hepatopulmonary syndrome (HPS) and portopulmonary hypertension (POPH). These syndromes appear to be paradoxical responses by the pulmonary vascular endothelium to the adverse effects of portal hypertension often associated with liver disease. Both entities can have a significant effect on survival and liver transplantation (LT) candidacy. It is likely that these entities are underdiagnosed and undertreated worldwide. This chapter will describe the different presentations, pathophysiology, distinct treatment, and unique responses of these conditions to LT.
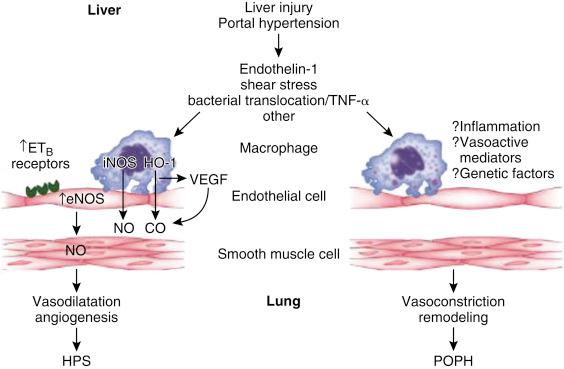
The pulmonary vascular endothelium is a dynamic organ that provides the interface between the blood in the vessel lumen and the vascular wall. The integrity of the endothelium is vital for vasoregulation, antithrombosis, laminar blood flow, selective permeability to hematopoietic cells and nutrients, and the regulation of growth of the surrounding smooth muscle tissue. The intact vascular endothelium produces nitric oxide (NO), which is vital for the regulation of vasomotor tone, vascular permeability, and protection from inflammatory mediators and provides a powerful antioxidant capacity. In patients with portal hypertension, exposure to inflammatory cytokines and stress forces caused by high cardiac output render the pulmonary vascular endothelium dysfunctional. As a result, several responses may occur. Vasodilatation, with the formation of aneurysms and shunts, results in HPS. Vasoconstriction, with proliferation of smooth muscle and microthrombosis, causes an increase in pulmonary vascular resistance (PVR) and POPH ( Fig. 39-1 ). These two pathological changes may be seen in the same lung, but one entity usually predominates.
The relationship between the lung and liver was first noted in 1884 when Flückiger described a female patient with cyanosis, clubbing, and cirrhosis. In 1935 Snell reported a series of three patients with “hypoxemia of cirrhosis.” The term hepatopulmonary syndrome was first used in 1977 by Kennedy and Knudson, who accurately suggested that intrapulmonary vascular dilatations were responsible for the hypoxemia. These early reports described the association of hypoxemia with cirrhosis. However, the significant prevalence and the prognostic importance of HPS are only now being appreciated. The remarkable therapeutic impact of LT has led to intense interest in the role of transplantation in the management of HPS.
HPS is defined as the triad of liver disease and increased alveolar-arterial (A-a) oxygen gradient in the presence of intrapulmonary vascular dilatations (IPVDs). The European Respiratory Society HPS diagnostic criteria are similar except for the addition of positive contrast-enhanced echocardiography ( Table 39-1 ). Early definitions emphasized the need to exclude other causes of A-a gradient; however, now it is recognized that HPS can coexist with other conditions that cause hypoxemia. Age correction of the normal A-a gradient criteria to less than 20 mm Hg in patients over 64 years of age, instead of less than 15 mm Hg in younger adults, will avoid overdiagnosis of HPS.
| Portal hypertension |
| Arterial hypoxemia |
| Intrapulmonary vascular dilatations |
| Positive contrast-enhanced echocardiography |
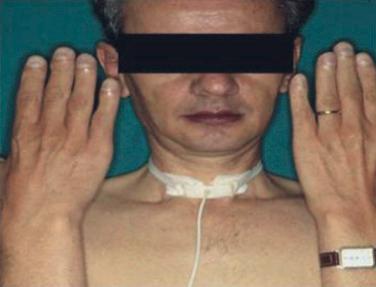
Thus the hallmark of HPS is impairment in oxygenation, with or without dyspnea. Orthodeoxia, a decrease in the partial pressure of arterial oxygen (Pa o 2 ) of 5% or more or 4 mm Hg or more upon standing, is a unique feature of HPS. This is caused by preferential perfusion of the lung bases in a standing patient. Platypnea, shortness of breath worsened by sitting, results from the same mechanism. Orthodeoxia can occur in other settings, such as after pneumonectomy, pulmonary thromboemboli, and patent foramen ovale, but it is highly specific for HPS in the presence of liver disease. Spider angiomas occur commonly in HPS but are not specific, because they are seen in patients with cirrhosis without HPS. Clubbing and cyanosis should raise suspicion for HPS ( Fig. 39-2 ).
HPS is the most common pulmonary vascular abnormality, with 15% to 20% of patients undergoing evaluation for liver transplantation. The prevalence of HPS depends on the predictive cutoff used to define the condition. In a study of 33 HPS patients diagnosed by contrast-enhanced echocardiography, a Pa o 2 of less than 70 mm Hg had a higher positive predictive value for HPS than an A-a gradient of more than 15 mm Hg (positive predictive value of 93% and 34%, respectively). HPS appears to be as prevalent in pediatric patients as adults, although less data are available.
The natural history of HPS patients is progressive worsening over time, with a Pa o 2 decline of approximately 5 mm Hg per year. Improvement in the absence of LT is rare but has been reported. As recently as 2010, severe hypoxemia was considered a contraindication to LT.
However, over time an increasing number of reports described a reversal of hypoxemia after successful LT. The reversibility of hypoxemia in HPS patients has been compared to the improvement of hepatorenal syndrome that occurs with recovery of liver function. Currently LT is the treatment of choice for HPS, and in the United States patients with Pa o 2 of less than 60 mm Hg are given priority for organ allocation. Interestingly, HPS can coexist with POPH in the same patient.
Intrapulmonary arterial oxygenation can be impaired by alveolar ventilation-perfusion imbalance, increased intrapulmonary shunt, and diffusion defects, and all of these mechanisms may be present in HPS. However, the response to the administration of 100% oxygen is typically preserved; shunts appear to be functional rather than anatomical. Diffusion limitation and ventilation-perfusion mismatch appear to be predominant factors. The common finding of a low diffusing capacity of lung for carbon monoxide (DLCO) supports diffusion impairment. This most likely results from the increased distance between the alveoli and the red blood cells in the central stream of the dilated pulmonary capillary ( Fig. 39-3 ). The hyperdynamic circulation (high rate of blood flow) found in cirrhotic patients decreases the exposure time of the red blood cell to the alveolus, further worsening the diffusion impairment.
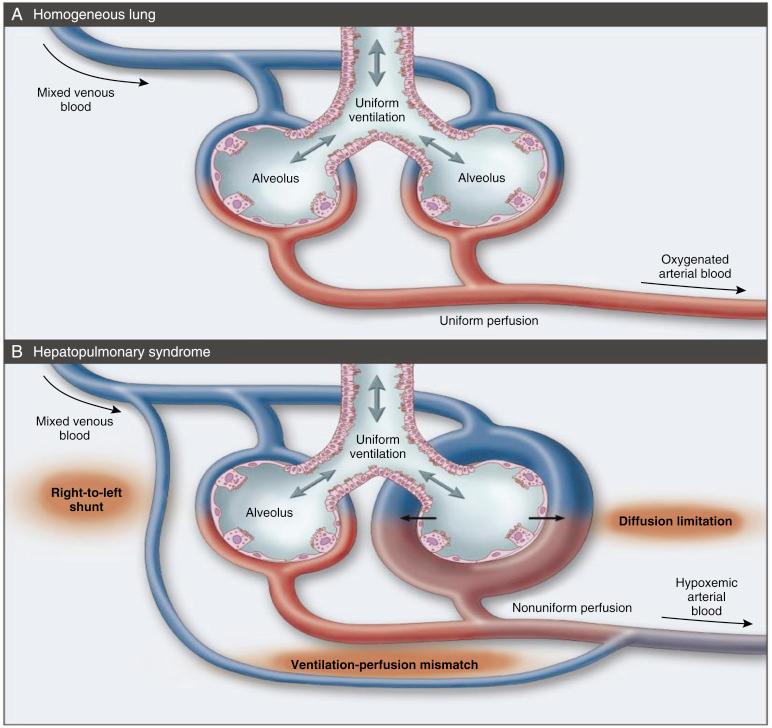
The pathophysiological hallmark of HPS is the presence of IPVDs. The diameter of pulmonary capillaries is normally between 8 and 15 μm. Red blood cells, which possess a similar diameter, travel through the capillaries in single file. This configuration minimizes the distance required for oxygen diffusion and facilitates oxygen transfer to red cells. In HPS pulmonary capillaries dilate to a diameter of 50 to 500 μm, impeding oxygenation. Occasionally a single large shunt may be found that bypasses the pulmonary vasculature; this may be amenable to coiling by the interventional radiologist.
Elevated levels of vascular vasodilators, including NO, are responsible for IPVD. This is supported by increased levels of exhaled NO in HPS patients, which normalize after LT in parallel with resolution of HPS. Further support for this mechanism is reports of transient improvement in HPS by inhibitors of NO production such as N-nitro-L-arginine methyl ester (L-NAME) or methylene blue, which inhibits cyclic guanosine monophosphate (cGMP). However, other mechanisms may be responsible, because inhaled L-NAME administration does not consistently improve HPS. The coexistence of HPS and POPH may be explained by differential effects of endothelin A and B receptors, the former being associated with vasoconstriction and the latter, after stimulation by endothelin-1, is associated with upregulation of endothelial NO synthase (eNOS) ( Fig. 39-4 ).
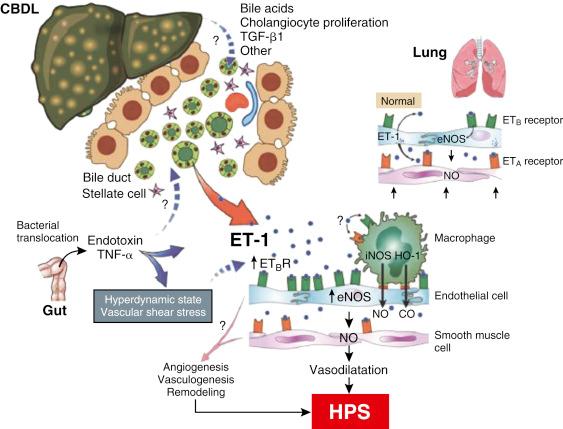
The only established experimental model capable of reproducing human HPS is chronic common bile duct ligation in the rat. Other rodent models of cirrhosis (thioacetamide induced) and portal hypertension (partial portal vein ligation) do not produce HPS. Bile duct ligation–produced HPS results in increased production of NO via eNOS. Endothelin-1 appears to be responsible for eNOS increases and overexpression of endothelin-B receptors. The association between elevated endothelin-1 levels and intrapulmonary vasodilatation has been shown in humans. In experimental HPS, macrophages accumulate and produce inducible NO synthetase (iNOS) and heme oxygenase-1 (HO-1). This enhances vasodilatation through the production of NO and HO-1 derived carbon monoxide. Angiogenesis, stimulated by increased levels of vascular endothelial growth factor, appears to play a role in experimental HPS. Norfloxacin inhibits both bacterial translocation and tumor necrosis factor-α (TNF-α), which decreases the iNOS response. This suggests that bacterial translocation, which stimulates TNF-α, is responsible for macrophage accumulation. Nonspecific phosphodiesterase inhibition with pentoxifylline increases intracellular cyclic adenosine monophosphate (cAMP) levels and inhibits TNF-α, resulting in prevention or attenuation of HPS. In humans, gene polymorphism that results in increased monocyte migration is associated with HPS.
Interestingly, the severity of HPS, as determined by the Child-Turcotte-Pugh classification, does not mirror the degree of hepatic dysfunction. This, coupled with the fact that dyspnea is a common, nonspecific symptom in patients with end-stage liver disease, makes screening important.
Pulse oximetry is commonly used to diagnosis hypoxemia. However, the oxygen saturation as measured by pulse oximetry (Sp o 2 ) has been shown to overestimate arterial oxygen saturation (Sa o 2 ), both in patients with cirrhosis and controls. To reliably detect a Pa o 2 of less than 70 mm Hg, patients with an Sp o 2 of 98% or less warrant arterial blood gas (ABG) analysis. Obtaining ABG levels based upon an Sp o 2 of 98% or less is 100% sensitive for hypoxemia (defined as Pa o 2 <70 mm Hg) but has a low specificity (a significant number of ABG analyses will reveal a Pa o 2 >70 mm Hg). In a study of 200 patients who were LT candidates, approximately one third were referred for ABG analysis based upon an Sp o 2 of less than 97%, which preserved sensitivity (96%) and was moderately specific (75%). The cost effectiveness of ABG analysis in the presence of Sp o 2 of less than 97% compares favorably to screening using a validated dyspnea questionnaire. ABG analysis performed while the patient is supine and standing, while breathing room air, is helpful, although desaturation upon standing may be present in as many as one third of cirrhotic patients. The A-a gradient should be age corrected to avoid inappropriately diagnosing older patients. The normal A-a gradient is less than 10 + 0.43 (age − 20). In a study of 207 patients with HPS, 66% met standard A-a gradient criteria; however, after age correction 35% met criteria.
Chest x-ray examination and pulmonary function tests are frequently obtained to assess for other causes of cardiopulmonary disease. However, these tests have a low specificity for HPS and should not be used to exclude (or diagnosis) HPS. The DLCO is commonly impaired in HPS patients. One study found the predicted DLCO was less than 80% in 15 of 18 HPS patients. However, a number of other conditions, such as interstitial lung disease, vasoocclusive disease, and severe anemia, can lower diffusing capacity, so this finding is nonspecific.
Contrast-enhanced echocardiography (CEE) and perfusion lung scanning using technetium Tc 99m-labeled macroaggregated albumin are the most common approaches for IPVD assessment. During CEE agitated saline is injected intravenously. Echogenic shadows are normally visible only in the right heart, because they are filtered by the pulmonary microcirculation. Visualization in the left heart implies an intracardiac or intrapulmonary shunt. With an intracardiac shunt, microbubbles appear in the left heart within three heartbeats of their appearance in the right heart. In contrast, intrapulmonary shunts result in the delayed (four to six beats) appearance of microbubbles in the left heart ( Fig. 39-5 ). Transesophageal echocardiography with contrast enhancement may be more sensitive than transthoracic CEE but requires sedation and carries a theoretical risk to patients with esophageal varices.
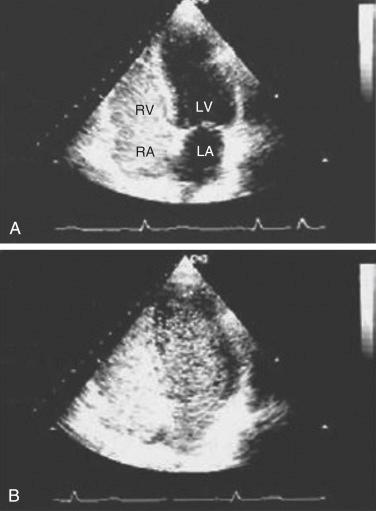
Technetium 99m labeled macroaggregated albumin, approximately 20 μm in diameter, is filtered in the pulmonary capillary bed. In the presence of intrapulmonary vasodilatation, albumin aggregates pass through to the systemic circulation. Shunting of more than 6% reflects an intracardiac or intrapulmonary shunt. However, CEE is generally the screening test of choice, because it has been shown to be more sensitive than lung perfusion scan, and CEE can differentiate intracardiac from intrapulmonary shunts. CEE in the standing position has been shown to increase the number and size of shunts compared with supine injection and may detect early HPS before changes are apparent via ABG analysis. Because echocardiography is routinely used to screen for POPH, some centers elect to perform CEE at the same time. Radionuclide perfusion scanning is useful to quantify the magnitude of the shunt, which may help distinguish the contribution of HPS to hypoxemia in patients with HPS and coexisting respiratory conditions.
Other diagnostic tests, such as high-resolution computed tomography scanning and angiography, may provide useful information about the appearance of pulmonary vessels. However, these tests have not been studied sufficiently to warrant their use to detect IPVD. Computed tomography is helpful when pulmonary arteriovenous malformation is suspected. The combination of hypoxemia, liver disease, and IPVD is sufficiently unique that it is diagnostic for HPS even in the presence of other conditions (such as chronic lung disease) that might worsen hypoxemia. Cirrhosis is the most common liver disease associated with HPS, although HPS has occurred in the setting of acute and noncirrhotic liver diseases such as Budd-Chiari syndrome, hepatitis A, hypoxic hepatitis, and chronic hepatitis without cirrhosis.
The severity of HPS is not easily quantified via CCE or lung perfusion scans. Instead, Pa o 2 on room air is useful for this purpose, with very severe HPS defined by the European Respiratory Society Task Force as a resting, room air Pa o 2 of less than 50 mm Hg (<300 mm Hg on 100% O 2 ), whereas a Pa o 2 greater than or equal to 50 mm Hg and less than 60 mm Hg defines severe HPS. The severity of HPS is an important prognostic tool for predicting survival and determining the timing of LT. All patients presenting for LT should be screened with pulse oximetry while breathing room air in both the sitting and supine positions.
LT is indicated for patients with HPS. LT offers a survival advantage compared to HPS patients who do not undergo transplantation. The American Association for the Study of Liver Diseases recommends that patients with HPS, regardless of the degree of hepatic dysfunction, undergo evaluation for LT.
Although a number of medical therapies have been tried in an attempt to improve oxygenation in HPS, no therapies have been proven to be effective for long-term treatment. Mixed results or temporary improvement has been achieved with methylene blue, garlic, pentoxifylline, L-NAME, norfloxacin, and other antimicrobial agents. A single case report describes complete resolution of HPS after methadone withdrawal and postulates NO signaling by opiates as a contributing factor.
Intraoperative management focuses on the provision of adequate oxygenation. Given that HPS is typically responsive to supplemental oxygen, this goal is usually attainable. The prevention of embolic material, especially air bubbles, from entering the venous circulation is imperative because these may traverse through the shunts to the systemic circulation. This may be a factor when considering the use of venovenous bypass, because the risk for air emboli is a recognized complication. However, unique postoperative complications have been described, including pulmonary hypertension, cerebral embolic hemorrhage, and postoperative desaturation requiring prolonged mechanical ventilation.
There are case reports of improvement in hypoxemia after transjugular intrahepatic portosystemic shunt (TIPS) placement in patients with HPS and some case reports without convincing evidence for improvement. However, in a report that described improved oxygenation in an HPS patient after TIPS, intrapulmonary shunting persisted. Thus the mechanism of improvement may not be related to reversal of intrapulmonary vasodilatation but perhaps changes in cardiac output. In other cases HPS developed in the presence of a functional TIPS. Because of the uncertainty surrounding the response to TIPS, it should be reserved for experimental trials or as a bridge to LT.
Few data exist in children, although single-center reports of small series of pediatric patients with HPS suggest that treatment of the underlying cause of liver failure, such as splenectomy for splenic vein thrombosis or antiviral therapy for hepatitis C, may result in resolution of HPS.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here