Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pleural biopsy, pleural drainage, pleural debridement
Spontaneous pneumothorax surgery, parietal pleurectomy
Decortication of empyema
Pleurectomy/decortication (extended) for mesothelioma
Excision of solitary pleural tumour
Thoracic sympathectomy, facial flushing, axillary and palmar hyperhidrosis, angina, splanchnicectomy
The surface marking of the lung represents the markings of the visceral pleura. The apex of the lung extends convexly upwards to a distance of approximately 2.5 cm above the junction of the medial and intermediate thirds of the clavicle. The anterior border of the right lung descends from the posterior aspect of the sternoclavicular joint, behind the sternal angle, to the level of the xiphisternal joint. The left lung has a similar course until it reaches the level of the fourth or fifth intercostal space, where it curves laterally beyond the lateral margin of the sternum to accommodate the cardiac notch. After this, the anterior border of the left lung turns sharply to the level of the xiphisternal joint. The lower lung border extends in mid-inspiration to the sixth rib in the mid-clavicular line, the eighth rib in the mid-axillary line and the tenth rib adjacent to the vertebra posteriorly.
The surface marking of the parietal pleura closely follows that of the lung. The apical extension of the parietal pleura is almost identical to that of the visceral pleura because only a thin film of fluid separates them. The anteromedial border of the parietal pleura on the right is similar to that of the visceral pleura. The left parietal pleura takes a course similar to the visceral pleura, except at the level of the fourth costal cartilage, where the deviation of the parietal pleura extends up to, but not beyond, the lateral sternal edge and then turns sharply to the xiphisternal joint. The lower parietal pleural border is similar on both sides, crossing the eighth rib at the mid-clavicular line, the tenth rib at the mid-axillary line and the twelfth rib just adjacent to the vertebra posteriorly.
To avoid iatrogenic injuries during percutaneous entry into the pleural cavity, an understanding of the anatomy of an intercostal space, the position of the subcostal neurovascular bundle and the position of the hemidiaphragm (which may be elevated in the presence of adhesions) is essential. The correct point of chest wall penetration for drainage of an uncomplicated pleural collection is usually between the mid-axillary and posterior axillary line at the level of the sixth intercostal space. For simple thoracocentesis, the needle is advanced on to the rib and ‘walked’ to the superior border of the rib. For tube thoracostomy, a small 1–2 cm skin incision is made parallel to the intercostal space, and a channel is created through the subcutaneous tissue and intercostal muscle layer on to the upper border of the lower rib: this reduces the risk of injury to the neurovascular bundle.
The pleurae consist of two serous membranes: the visceral and parietal pleurae. The visceral pleura is adherent to the lung surface and extends into the interlobar fissures. The parietal pleura lines the corresponding thoracic wall and extends over the diaphragm and the lateral aspect of the mediastinum in what are often described as costal, diaphragmatic and mediastinal parietal pleural segments. The transition between each of the parietal pleural segments is referred to as a pleural sinus and includes the mediastinophrenic sinus, the costophrenic sinus and the costomediastinal sinuses (anterior and posterior). The surfaces of the parietal pleura at the level of the sinuses are in contact at the end of expiration.
The parietal pleura is continuous with the visceral pleura at the hilum. In a radical pleurectomy for malignant tumour, the tissue plane between the underlying lung parenchyma and the overlying visceral pleura may be entered at this pleural reflection over the rigid structure of the left or right main bronchus. Once this plane has been developed, the entire visceral pleural layer can be dissected off the lung surface. At the inferior hilum, the reflection of the pleura extends towards the diaphragm as the inferior pulmonary ligament. The endothoracic fascia between the parietal pleura and the chest wall forms the dissection plane for parietal pleurectomy.
The pleural cavity is the space between the two pleural layers. It contains a thin film of between 0.1 and 0.2 mL/kg of glycoprotein-rich lubricating fluid which eases gliding of the two pleural surfaces during the various phases of respiration by facilitating the synchronous movements of thoracic wall and lung expansion. Fluid volume is maintained by a balance between fluid filtration and absorption, which in turn are controlled by a balance between lymphatic drainage and the hydrostatic and oncotic pressures of pleura and plasma. Any imbalance in this mechanism may lead to development of pleural effusion. The outward pull of the chest wall and inward elastic recoil of the lung generate a negative intrapleural pressure. Changes in the elasticity of these structures or the accumulation of fluid or air within the pleural cavity will therefore alter respiratory activity. Pleural effusion, the abnormal collection of fluid in the pleural cavity, may result from an imbalance in filtration or absorption, or from a change in the composition of the pleural fluid. Pleurodesis is carried out to obliterate the pleural space and prevent recurrent fluid accumulation. Failure of lung expansion following drainage of pleural effusion may be due to an underlying malignant process causing excessive visceral pleural thickening or due to the formation of an inflammatory cortex covering the visceral pleura.
The arterial supply and venous drainage of the visceral pleura are provided by the bronchial vessels. The bronchial arteries at the hilum form a circle surrounding the principal bronchus, and branches from this ring supply the mediastinal interlobar and apical visceral pleura and part of the diaphragmatic visceral pleura. Pulmonary veins drain the visceral pleura, apart from an area around the hilum which drains into bronchial veins. The lymphatic drainage of the visceral pleura is to the deep pulmonary plexus within the interlobar and peribronchial spaces. Visceral afferents from the visceral pleura travel along the bronchial vessels with the autonomic fibres.
Solitary fibrous tumours usually arise as a solitary pedunculated mass from the submesothelial layer of the visceral pleura. Multiple tumour sites have been reported.
The costovertebral parietal pleura is supplied by the intercostal and internal thoracic arteries; the mediastinal pleura by branches from the bronchial, upper diaphragmatic, internal thoracic and mediastinal arteries; the cervical pleura by branches from the subclavian artery; and the diaphragmatic pleura by the superficial part of the diaphragmatic microcirculation. The veins join the thoracic wall veins, eventually draining into the superior vena cava. Lymph from the costovertebral pleura drains into the internal thoracic chain anteriorly and intercostal chains posteriorly, while that from the diaphragmatic pleura drains into the mediastinal, retrosternal and coeliac axis nodes. The importance of the difference in lymphatic drainage of the pleurae and the lungs has been recognized in the latest (eighth) TNM revision of malignant mesothelioma: there is no demarcation between hilar (N1) and mediastinal (N2) lymph node metastases as per lung cancer, just N1 for all ipsilateral nodal metastasis. Less than a quarter of all solitary fibrous tumours originate from the diaphragmatic or mediastinal surface of the parietal pleura.
The costal and peripheral diaphragmatic parietal pleurae are innervated by intercostal nerves; irritation results in pain that is referred to the appropriate part of the thoracic or abdominal wall. The mediastinal and central diaphragmatic parietal pleurae are innervated by the phrenic nerves; irritation of the central diaphragmatic pleura causes pain that is referred to the lower neck and shoulder tip, i.e. to the C3 and 4 dermatomes.
When a parietal pleurectomy is performed to achieve pleurodesis, the pleura is usually a thin membrane. Dissection is begun from a port site and extended into the extrapleural space. The parietal pleura is separated from the underlying endothoracic fascia from the parasternal internal thoracic artery anteriorly to the vertebral bodies posteriorly. The pleurectomy is then extended across the dome of the thoracic cavity. The subclavian artery and vein are subpleural apical structures at risk of injury during this procedure ( Fig. 46.1 ).
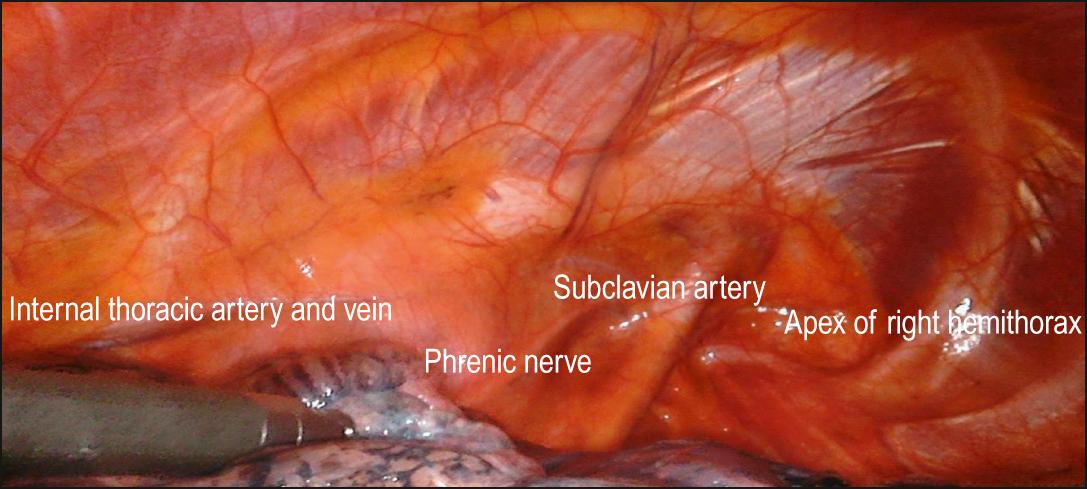
During decortication for empyema, a plane of dissection is developed between the fibrotic, thickened parietal pleura and the endothoracic fascia. With a combination of sharp and blunt dissection, the parietal pleura is mobilized off the aorta, left subclavian artery, left common carotid artery, phrenic and vagus nerves on the left and subclavian vessels, azygos vein, superior vena cava and phrenic nerve on the right.
Malignant solitary fibrous tumours arising from the parietal pleura tend to be sessile tumours with a broad base. Their complete removal requires a wide surgical excision margin: resection of the underlying chest wall is necessary if tumour invasion beyond the endothoracic fascia is suspected or apparent. In resection of diffuse primary malignant disease of the pleura it is possible to dissect the potential plane between the parietal pleura and the diaphragmatic muscle fibres and between the pericardium and mediastinal pleura. If there is direct invasion, then an en bloc resection is required.
Pleural fissures divide the lung into lobes. A fissure is defined as the space between two layers of adjacent lobar visceral pleura.
The right lung is partitioned into upper, middle and lower lobes by the oblique and the transverse (or horizontal) fissures. A single fissure, the oblique fissure, divides the left lung into upper and lower lobes. The surface anatomy of the fissures can vary significantly depending on pulmonary pathology, such as emphysema, fibrosis, malignancy or inflammation, as well as scarring or previous surgery.
The right oblique fissure courses from the level of the transverse process of the fourth thoracic vertebra posteriorly (at the height of the spinous process of the fifth thoracic vertebra). From here, the fissure can be followed across the posterior and lateral chest wall, along the course of the fifth rib. Its projection then crosses the anterior axillary line, descending further across the sixth rib to reach its end point just behind the costochondral junction of the sixth rib. This anterior segment of the oblique fissure separates the middle and lower lobes; the posterior and lateral segments separate the upper and lower lobes. The transverse fissure divides the upper and middle lobes and runs laterally from the fourth sternochondral junction on the anterior chest wall to meet the oblique fissure at the level of the anterior axillary line in the fifth intercostal space.
On the left, the surface projection of the oblique fissure starts at the height of the third or fourth vertebra posteriorly, and then courses across the curvature of the chest wall obliquely in a downward direction, crossing the fifth rib in the mid-axillary line and reaching the sixth or seventh sternocostal junction anteriorly.
Fissural development varies greatly, from complete (lung surface to lung hilum) to total absence. High-resolution CT scan series have reported a prevalence of incomplete or total absence of the oblique fissure as 64–87% (right) and 50–70% (left).
Areas of incomplete fissures show fusion of the pulmonary parenchyma of the two adjacent lobes. Incompleteness of fissures may complicate anatomical lung resection, such as lobectomy, because a neofissure must be developed and carefully sealed to avoid postoperative air leak with its risk of associated complications. Lobar fusion can have therapeutic significance. Indirect alveolar interlobar connections, enabling so-called collateral ventilation through the pores of Kohn or canals of Lambert, significantly limit the effectiveness of lung volume reduction therapy by preventing lobar collapse from endobronchial obstruction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here