Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Dual-antiplatelet therapy (DAPT) with aspirin and a P2Y 12 receptor inhibitor is the cornerstone of therapy after percutaneous coronary intervention (PCI) with stents.
The thienopyridines—ticlopidine, clopidogrel, and prasugrel—are P2Y 12 inhibitors that are prodrugs and therefore require conversion into an active metabolite to exert their antiplatelet effect. This active metabolite irreversibly binds and antagonizes the P2Y 12 receptor for the lifespan of the platelet.
Compared with clopidogrel, prasugrel reduces ischemic events in thienopyridine-naïve patients undergoing PCI, but is associated with a higher risk of bleeding. Net clinical benefit is greatest in patients without a history of stroke or transient ischemic attack (TIA) who are younger than 75 years of age and weigh over 60 kg. Compared with prasugrel administered after coronary angiography, pretreatment with prasugrel in invasively managed patients with non–ST-elevation acute coronary syndrome (NSTE-ACS) increases major bleeding, does not reduce ischemia, and should be avoided.
Ticagrelor is a direct, reversibly acting nonthienopyridine P2Y 12 antagonist. In patients with acute coronary syndrome (ACS), including those treated with an invasive strategy, ticagrelor reduced ischemic events and cardiovascular mortality compared with clopidogrel. Although overall bleeding was not increased with ticagrelor, the risk of non-coronary artery bypass grafting (CABG)-related bleeding was increased.
Substantial interindividual variability is evident in the antiplatelet effect of clopidogrel. High on-treatment platelet reactivity despite clopidogrel therapy can identify patients at risk for ischemic events after PCI. The clinical benefit of individualized antiplatelet strategies based on platelet function testing has yet to be demonstrated in randomized clinical trials.
Several genetic polymorphisms reduce the enzymatic activity of CYP2C19, which is critical for the conversion of clopidogrel to its active metabolite. When treated with clopidogrel, carriers of these alleles with reduced function, especially those with two copies (poor metabolizers), are at higher risk of thrombotic events after PCI compared with patients with normal alleles. The CYP2C19 genotype does not influence the clinical efficacy of prasugrel or ticagrelor.
Cangrelor is an intravenous P2Y 12 antagonist with a rapid onset and offset of effect that reduces ischemic events compared with conventional clopidogrel therapy in thienopyridine-naïve patients undergoing PCI, driven by reductions in myocardial infarction (MI) according to the universal definition and in stent thrombosis, with major bleeding rates similar to placebo.
The effects of thrombin, the most potent platelet activator, are mediated primarily through the protease-activated receptor 1 (PAR-1) receptor. The net clinical benefit of the PAR-1 antagonist, vorapaxar, is favorable in the setting of secondary prevention in patients with prior MI in combination with standard antiplatelet therapy but is not approved for use in the setting of PCI because of a lack of ischemic efficacy and increased bleeding.
In the modern era of pretreatment with P2Y 12 inhibitors, the benefit of glycoprotein (GP) IIb/IIIa inhibition appears to be restricted to high-risk patients with ACS who have elevated cardiac biomarkers.
Platelets play a critical role in normal hemostasis and in the pathogenesis of atherothrombotic disease processes. Platelets provide an initial hemostatic plug at the site of vascular injury and promote pathophysiologic thrombosis, which in turn precipitates MI, stroke, and peripheral vascular occlusion; therefore antiplatelet agents are key in cardiovascular disease management. In particular, the main goal of antiplatelet treatment strategies is to reduce the risk of recurrent atherothrombotic events without excessive bleeding complications. However, because both pathologic and physiologic functions of platelets are due to the same mechanism, it is difficult to separate therapeutic benefits from potential harmful effects.
Platelet plug formation at sites of vascular injury occurs in three stages: (1) the initiation phase involves platelet adhesion; (2) the extension phase includes activation, additional recruitment, and aggregation; and (3) the perpetuation phase is characterized by platelet stimulation and stabilization of clot. Circulating platelets are quiescent under normal circumstances and do not bind to the intact endothelium. However, endothelial damage leads to the exposure of circulating platelets to the subendothelial extracellular matrix (ECM) and triggers platelet recruitment and adhesion ( Fig. 10.1 ). In the initial phase of primary hemostasis, the tethering of platelets at sites of vascular injury is mediated by the glycoprotein (GP) Ib-IX-V receptor complex, which binds von Willebrand factor (vWF). Subendothelial collagen exposed by damaged vessels engages platelets via GP VI and GP Ia/IIa receptors. These interactions allow the arrest and activation of adherent platelets. In the extension phase, additional platelets are recruited and activated via soluble agonists. These platelet-activating factors include adenosine diphosphate (ADP), thromboxane A2 (TXA 2 ), epinephrine, serotonin, collagen, and thrombin. Signaling via ADP receptors contributes to platelet activation during both protective hemostasis and pathologic thrombosis. Two ADP receptors are expressed by platelets: P2Y 1 couples to Gαq and contributes to initial aggregation, and P2Y 12 couples to Gα 12 and decreases cyclic adenosine monophosphate (cAMP), stabilizing the platelet aggregate. P2Y 12 receptor signaling also stimulates surface expression of P-selectin and secretion of TXA 2 , which is produced de novo and, like ADP, is released from adherent platelets. Generated from arachidonic acid through conversion by cyclooxygenase 1 (COX-1) and thromboxane synthase, TXA 2 binds platelet receptors TPα and TPβ; however, its effects in platelets are mediated primarily through TPα. ADP and TXA 2 are secreted from adherent platelets, contribute to the recruitment of circulating platelets, and promote alterations in platelet shape and granule secretion; thus platelet activation is amplified and sustained during the extension phase. Thrombin, generated at the site of vascular injury, represents the most potent platelet activator and contributes to the formation of the hemostatic plug and platelet thrombus growth. Thrombin also directly activates platelets through stimulation of the protease-activated receptors (PARs). Human platelets express two PARs for thrombin, PAR-1 and PAR-4. Thrombin facilitates the production of fibrin from fibrinogen and thus contributes to the formation and stabilization of the hemostatic plug. The final common pathway is activation of the integrin GP IIb/IIIa, which allows platelets to bind fibrinogen with high affinity, leading to platelet aggregates. In the perpetuation phase, the platelet-rich thrombus and coagulation cascades reinforce one another and culminate in the generation of a stable platelet-fibrin–rich plug at the sites of injury.
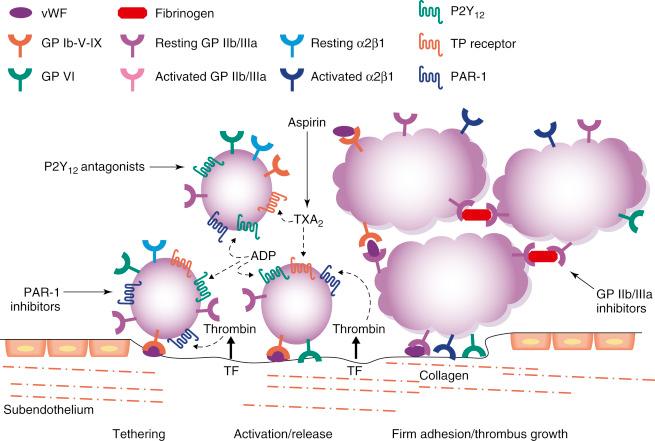
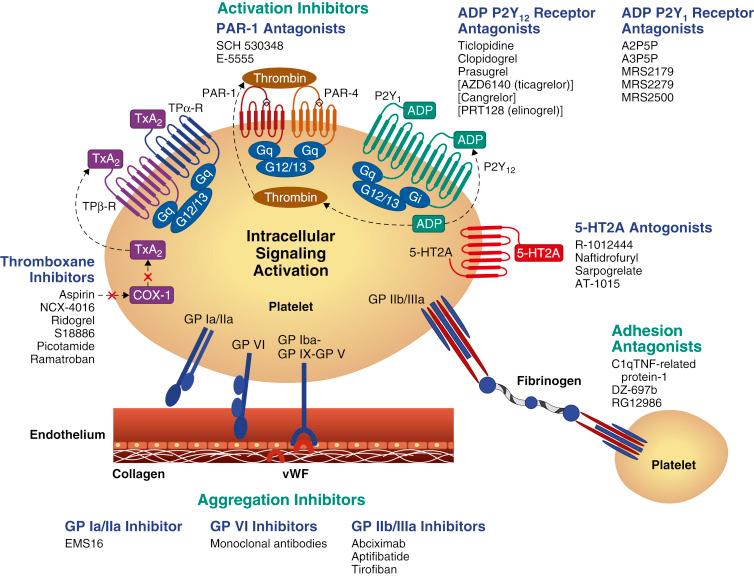
The mechanisms by which antiplatelet drugs interfere with platelet function involve targeting enzymes or receptors critical for synthesis or targeting the action of important mediators of these functional responses. Current and investigational oral antiplatelet therapies target key platelet-signaling pathways ( Fig. 10.2 ). This chapter reviews the mechanisms of action, efficacy, and safety of antiplatelet agents that inhibit key platelet-signaling pathways and their components—including the TXA 2 pathway, the P2Y 12 and PAR-1 receptors, phosphodiesterase III, and the GP IIb/IIIa receptor—and focuses on their roles in PCI.
Aspirin irreversibly inactivates the cyclooxygenase activity of prostaglandin H (PGH) synthase 1 and 2, also referred to as COX-1 and COX-2 . PGH synthase 1 and 2 convert arachidonic acid to PGH 2 , which acts as a substrate for the generation of several prostanoids, including TXA 2 and prostacyclin (PGI 2 ). Aspirin enters the COX channel and acetylates the amino acid serine at positions 529 and 516 of COX-1 and COX-2, respectively, thereby preventing arachidonic acid access to the catalytic site of the enzyme through steric hindrance. Mature platelets express only COX-1 and are the primary sources of TXA 2 , which is released by the platelet in response to a variety of agonists and induces platelet aggregation through the G protein–coupled TXA 2 receptor, TP. Other cells, including the vascular endothelium, express both COX-1 and COX-2. COX-1 is responsible for the production of cytoprotective prostaglandins PGE 2 and PGI 2 in the gastric mucosa, and COX-2 is the main source of vascular PGI 2 . Unlike mature platelets, gastric mucosal cells can synthesize COX-1 and therefore recover the ability to produce prostaglandins within hours after aspirin exposure. Compared with COX-1, higher levels of aspirin are required to inhibit COX-2; therefore low-dose aspirin is sufficient to inhibit platelet TXA 2 production but is insufficient to affect the generation of vascular PGI 2 , which is a platelet inhibitor and vasodilator. In addition to its primary effect on platelet aggregation through inhibition of the PGH synthase COX-1 activity, it has been suggested that aspirin also exerts antiplatelet effects via COX-1–independent pathways.
Aspirin is rapidly absorbed in the stomach and small intestine and achieves peak plasma levels in 30 to 40 minutes. Esterases in the gastrointestinal (GI) mucosa and liver hydrolyze aspirin into salicylic acid, which then interacts with platelets in the portal circulation. The half-life is short, approximately 20 to 30 minutes, but the pharmacodynamic effect is prolonged given the permanent inactivation of platelet COX-1 activity. Low-dose aspirin requires several days to effectively suppress TXA 2 production; therefore a loading dose (LD) is needed to quickly achieve effective platelet inhibition in aspirin-naïve subjects; however, LDs greater than 300 mg do not provide additional pharmacodynamic benefit at 2 hours after ingestion.
The American College of Cardiology Foundation (ACCF)/American Heart Association (AHA)/Society for Cardiovascular Angiography and Interventions (SCAI) 2011 percutaneous coronary intervention (PCI) guidelines state that patients not already taking daily long-term aspirin therapy should be administered 325 mg nonenteric aspirin before PCI (class I, level of evidence [LOE] B), and patients already on daily aspirin therapy should take 81 to 325 mg before PCI (class I, LOE B).
In healthy individuals, maintenance aspirin dosages as low as 30 mg daily are adequate to completely inhibit serum TXB 2 production, a marker of platelet thromboxane production. Collaborative meta-analyses of the clinical benefit of long-term aspirin regimens in high-risk patients show that dosages greater than 75 to 150 mg daily are no more effective in reducing ischemic events but are associated with a greater risk of bleeding. However, patients undergoing PCI and receiving stents are not represented in these studies. The ACCF/AHA/SCAI 2011 PCI guidelines state that after PCI, it is reasonable to use aspirin 81 mg/day in preference to higher maintenance doses (MDs) (class IIa, LOE B).
The impact of different aspirin dosages on ischemia and bleeding in stented patients was examined in a post hoc observational analysis of the PCI cohort of the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial. This analysis suggests that doses of aspirin below 200 mg might be optimal after PCI with a bare-metal stent (BMS). The study stratified the 2658 patients who underwent PCI for acute coronary syndrome (ACS) in the CURE trial into three groups: high-dose (≥200 mg), medium-dose (>100 to <200 mg), and low-dose aspirin (≤100 mg). No differences were found in the unadjusted or adjusted rates of death, myocardial infarction (MI), or stroke among the groups (high dose vs. low dose, adjusted hazard ratio [HR] 1.00; 95% confidence interval [CI], 0.67 to 1.48; medium dose vs. low dose, adjusted HR 1.09; 95% CI, 0.73 to 1.60). Unadjusted and adjusted rates of major bleeding were significantly greater with high-dose aspirin compared with low-dose aspirin (adjusted HR 2.03; 95% CI, 1.15 to 3.57).
The Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events—Seventh Organization to Assess Strategies in Ischemic Syndromes (CURRENT–OASIS 7) examined the safety and efficacy of higher-dose aspirin (300 to 325 mg daily) compared with lower-dose aspirin (75 to 100 mg daily) in 25,086 patients with ACS treated with an invasive strategy. All patients received an aspirin LD of 300 mg or more the day of randomization, and patients were also randomized in a two-by-two factorial design to standard-dose or double-dose clopidogrel. In the overall cohort, the rate of cardiovascular death, MI, or stroke at 30 days was not different between higher-dose and lower-dose aspirin regimens (4.2% vs. 4.4%, HR 0.97; 95% CI, 0.86 to 1.09; P = .61). The incidence of major bleeding as defined by the trial was not different among groups (2.3% vs. 2.3%, HR 0.99; 95% CI, 0.84 to 1.17; P = .9). Minor bleeding was more frequent with higher-dose aspirin (5.0% vs. 4.4%, HR 1.13; 95% CI, 100 to 1.27; P = . 04), as was GI bleeding (0.4% vs. 0.2%, P = . 04). The findings were similar among those patients who underwent PCI, approximately 42% of whom received a drug-eluting stent (DES), and no difference was found in the incidence of stent thrombosis within the PCI cohort. Therefore a treatment strategy of lower-dose aspirin in invasively managed patients with ACS for 30 days appears to provide ischemic outcomes similar to higher-dose aspirin with less minor bleeding and less GI bleeding. However, longer treatment durations have not been examined in a randomized fashion.
The platelet P2Y 12 receptor plays a central role in amplifying the effect of various stimuli on platelet activation, and it promotes thrombus growth and stability. It is an inhibitory G protein–coupled receptor (Gα12) activated by ADP, which is released from dense granules after platelet activation from a variety of stimuli. ADP binding to the P2Y 12 receptor leads to a series of intracellular signaling events that result in further granule release and amplification of platelet activation, conformational changes of the GP IIb/IIIa receptor, and stabilization of the platelet aggregate. P2Y 12 activation further amplifies other responses to platelet activation including P-selectin expression, microparticle formation, procoagulant changes in the surface membrane, and potentiation of shear stress–induced platelet aggregation. The intracellular effect of P2Y 12 receptor activation is mediated by signal transduction via a secondary messenger system that activates phosphoinositide 3-kinase (PI3K) and inhibits adenylyl cyclase. PI3K activation leads to GP IIb/IIIa receptor activation through activation of a serine-threonine protein kinase B (PKB/Akt) and RAP1B guanosine triphosphate (GTP) binding protein. Inhibition of adenylyl cyclase decreases intracellular levels of cAMP, a key cofactor for the phosphorylation of vasodilator-stimulated phosphoprotein (VASP). Dephosphorylated VASP helps promote the conformational change of the GP IIb/IIIa receptor to its active state. Therefore through its action on cAMP levels, P2Y 12 activation drives the dephosphorylation of VASP and in turn drives GP IIb/IIIa activation ( Table 10.1 ).
| Trial Name | N | Population Studied | Intervention | Control | Primary End Point | F/U | Treatment Effect b | |
|---|---|---|---|---|---|---|---|---|
| Clopidogrel | ||||||||
| PCI-CURE a | 2658 | NSTE-ACS | Clopidogrel 300 mg LD, then 75 mg/day + ASA | Clopidogrel open-label for 28 days + ASA | CV death, MI, or revasc. | 9 months | RR 0.70 [0.50–0.97] P = .03 |
|
| CREDO | 2116 | Stable CAD and unstable angina | Clopidogrel 300 mg pre-PCI, then 75 mg/day + ASA | Clopidogrel 75 mg/day for 28 days + ASA | CV death, MI, or stroke | 1 year | RRR 26.9% [3.9%–44.4%] P = .02 |
|
| PCI-CLARITY a | 1863 | STEMI treated with fibrinolytics w/PCI 2–8 days later | Clopidogrel 300 mg before PCI, then 75 mg/day + ASA | Open-label clopidogrel starting at time of PCI + ASA | CV death, MI, or stroke | 30 days | OR, 0.54 [0.35–0.85] P = .008 |
|
| CURRENT–OASIS 7 | 25,807 | NSTE-ACS and STEMI with intended PCI | Clopidogrel 600 mg before angiography, 150 mg/day for 6 days, then 75 mg/day + ASA | Clopidogrel 300 mg before angiography, then 75 mg/day + ASA | CV death, MI, or stroke | 30 days | Overall cohort: HR 0.94 [0.83–1.06] P = .30 PCI cohort a : ( n = 17,263) HR 0.86 [0.74–0.99] P = .04 a |
|
| GRAVITAS | 2214 | Patients with high on-treatment reactivity to standard clopidogrel 12–24 hours after PCI | Clopidogrel 600 mg LD, then 150 mg/day + ASA | Clopidogrel 75 mg + ASA | CV death, nonfatal MI, stent thrombosis | 6 months | HR 1.01 [0.58–1.76] P = .97 |
|
| Prasugrel | ||||||||
| TRITON–TIMI 38 | 13,608 | NSTE-ACS and STEMI with planned PCI | Prasugrel 60-mg LD, then 10 mg/day MD + ASA | Clopidogrel 300-mg LD, then 75 mg/day MD + ASA | CV death, MI, or stroke | 450 days | HR 0.81 [0.73–0.90] P < .001 |
|
| ACCOAST | 4003 | NSTE-ACS planned for coronary angiography | Pretreatment with prasugrel 30-mg LD, additional 30-mg LD/10-mg MD if PCI performed | Pretreatment with placebo, 60-mg LD/10-mg MD if PCI performed | CV death MI, stroke, urgent revasc., or GP IIb/IIIa bailout at 7 days | 30 days | HR 1.02 [0.84–1.25], P = .81 | |
| Ticagrelor | ||||||||
| PLATO Invasive | 13,408 (PCI in 77%) | NSTE-ACS and STEMI, intended early invasive management | Ticagrelor 180-mg LD, then 90 mg bid + ASA | Clopidogrel 300- to 600-mg LD, then 75 mg/day + ASA | CV death, MI, or stroke | 12 months | HR 0.84 [0.75–0.94] P = .0025 |
|
| ATLANTIC | 1862 | STEMI | 180-mg LD in the ambulance, 90 mg bid thereafter + ASA | Placebo in the ambulance, 180-mg LD in the catheterization laboratory, 90 mg bid thereafter + ASA | Co-primary: Lack of ST-segment resolution; TIMI flow <3 in infarct-related artery | In-hospital | ST-segment resolution: OR 0.93 [0.69–1.25], P = .63 TIMI flow: OR 0.97 [0.75–1.25], P = .82 |
|
| ASA + clopidogrel 600 mg post PCI | Death, MI, or revasc. | 48 h | OR 0.87 [0.71–1.07] P = .17 |
|||||
| ASA + clopidogrel 600 mg pre-PCI | Death, MI, or revasc. | 48 h | OR 1.05 [0.88–1.24] P = .59 |
|||||
The thienopyridines—ticlopidine, clopidogrel, and prasugrel—are prodrugs that require biotransformation into an active metabolite to exert their antiplatelet effect. The active metabolite irreversibly binds and antagonizes the P2Y 12 receptor for the platelet’s life span (7 to 10 days). Differences in the rapidity and magnitude of platelet inhibition between the thienopyridines are predominantly the result of differences in prodrug metabolism that affect the efficiency of active metabolite formation. Because the interaction between the active metabolite and the P2Y 12 receptor is irreversible, a substantial waiting period for platelet functional recovery is required after thienopyridine exposure, which appears to be related to the magnitude of the initial inhibition.
Ticlopidine was the first thienopyridine to be introduced into clinical practice. It has a slow onset of action, is poorly tolerated, and its use is associated with blood dyscrasias. The incidence of neutropenia has been reported to be 2.4%, peaking at 4 to 6 weeks after the start of therapy; the incidence of aplastic anemia is 1 in 4000 to 8000 patients; and the incidence of thrombotic thrombocytopenic purpura is approximately 1 in 2000 to 4000 patients. The onset of hematologic disorders is rare after 3 months of therapy; therefore hematologic monitoring is required before initiation and for the first 3 months of exposure. The Stent Anticoagulation Restenosis Study (STARS) demonstrated that the combination of aspirin and ticlopidine significantly reduced the rate of death, angiographically evident stent thrombosis, MI, or revascularization at 30 days by 85% compared with aspirin alone and by 80% compared with the combination of aspirin and warfarin. Ticlopidine has been widely replaced by clopidogrel, given its better tolerability and lack of blood-monitoring requirements.
Clopidogrel is a prodrug that requires hepatic conversion into an active metabolite to exert its antiplatelet effect ( Fig. 10.3 ). Approximately 85% of absorbed clopidogrel is hydrolyzed by human carboxylesterase in the liver into an inactive carboxylic acid metabolite, so only a fraction of absorbed clopidogrel is available for conversion into the active metabolite by the cytochrome P450 (CYP) system. Hepatic biotransformation of absorbed clopidogrel into the active metabolite is thought to occur through a two-step process. The thiophene ring of clopidogrel is first oxidized to 2-oxo-clopidogrel, which is then hydrolyzed to a highly labile active metabolite (R-130964) that forms a disulfide bond with the P2Y 12 receptor as platelets pass through the liver. The first metabolic step involves the isoenzymes CYP2C19, CYP2B6, and CYP1A2; the second step involves the isoenzymes CYP2C19, CYP2B6, CYP3A4, CYP3A5, and CYP2C9. An alternative pathway for the oxidative biotransformation of clopidogrel that does not involve CYP2C19 has been suggested. In this formulation, CYP-catalyzed oxidation of clopidogrel to 2-oxo-clopidogrel is mediated by CYP1A2, CYP2B6, and CYP3A, and conversion of 2-oxo-clopidogrel to the thiol active metabolite is mediated by the esterase paraoxonase 1 (PON-1). The catalytic activity of PON-1 is proposed to be the rate-determining step for the active metabolite formation of clopidogrel. However, this alternative pathway has not been independently validated; paraoxonase may catalyze the formation of a minor isomer of the active metabolite, rather than the major isomer, and several studies have shown no influence of genetic polymorphisms of PON1 on clopidogrel active metabolite levels or on clopidogrel’s antiplatelet effects.
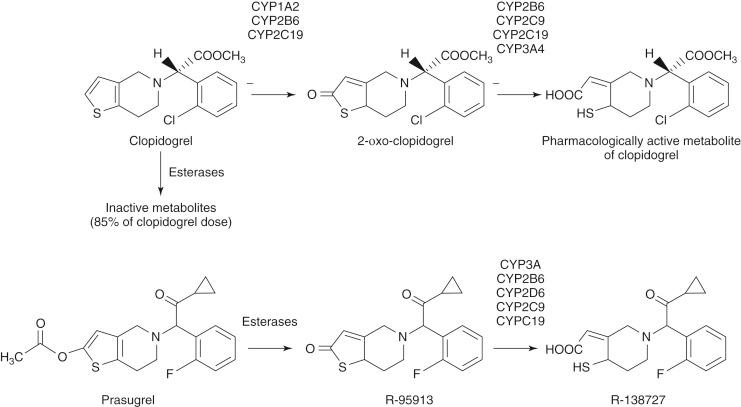
A daily dose of clopidogrel 75 mg requires 3 to 7 days to reach steady-state platelet inhibition, whereas an LD provides a rapid onset of action. However, the clinical benefit of a 300-mg LD may not be seen for 6 hours to as long as 15 hours after administration. Larger doses provide higher circulating levels of active metabolite, more rapid onset, and more intense inhibition. Peak inhibition after a 600-mg LD occurs at 4 to 6 hours after exposure. A 900-mg LD may or may not provide more rapid and additional suppression of platelet function compared with a 600-mg dose because the intestinal absorption of clopidogrel may be limited at doses greater than 600 mg. An MD regimen of 150 mg daily is associated with greater inhibition than a dose of 75 mg daily. Variability is wide among individuals in regard to the antiplatelet effect of clopidogrel after either an LD or an MD. Higher doses of clopidogrel reduce, but do not eliminate, this variability. The pharmacodynamic response to clopidogrel has been associated with CYP2C19 genotype, age, diabetes mellitus, body mass index, sex, ACS presentation, active smoking, renal dysfunction, pretreatment reactivity, and concomitant therapy with calcium channel blockers or proton pump inhibitors (PPIs). However, clinical characteristics and the CYP2C19 genotype only partly explain the variability in on-treatment reactivity. The level of ADP-induced platelet reactivity measured by several ex vivo platelet function tests have been associated with clinical outcomes in clopidogrel-treated patients undergoing PCI.
The longer-term ischemic benefit of clopidogrel in patients who present with ACS was established by the CURE trial, which randomized 12,562 patients with NSTE-ACS to aspirin and clopidogrel (300-mg LD followed by 75 mg daily) or aspirin alone for 3 to 12 months. The composite end point of cardiovascular death, nonfatal MI, or stroke occurred in 9.3% of patients in the clopidogrel group and in 11.4% of patients in the placebo group ( P < .001). Clopidogrel therapy was associated with an increased rate of major bleeding as defined by the trial (3.7% vs. 2.7%, P = . 001). In the population of patients enrolled in CURE who underwent PCI (17% of the overall cohort, 82% of whom received a BMS), pretreatment with clopidogrel for a median of 6 days reduced the rate of cardiovascular death, MI, or urgent target-vessel revascularization within 30 days from 6.4% to 4.5% ( P = . 03).
The Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction (CLARITY–TIMI 28) trial randomized 3491 patients 75 years of age and younger who received aspirin and fibrinolytic therapy within 12 hours of an ST-elevation MI (STEMI) to clopidogrel 300 mg followed by 75 mg daily or placebo. All patients underwent mandated angiography 2 to 8 days later. Clopidogrel significantly reduced the rates of occluded infarct–related artery, death, or recurrent MI before angiography (15.0% vs. 21.7%, P < .001) without increasing TIMI-defined major bleeding, minor bleeding, or intracranial hemorrhage. A prespecified analysis of patients who underwent PCI demonstrated that clopidogrel significantly reduced ischemic events from randomization through 30 days, from PCI through 30 days, and from randomization to PCI. This trial supports the use of clopidogrel in patients 75 years and younger who present with STEMI and are treated with aspirin and fibrinolysis.
The rationale for clopidogrel pretreatment is based on the slow onset of a substantial pharmacodynamic effect even after a clopidogrel LD. The Clopidogrel for the Reduction of Events During Observation (CREDO) trial randomized 2116 patients with stable coronary artery disease (CAD), unstable angina (UA), or recent ACS to a clopidogrel 300-mg LD or placebo 3 to 24 hours before PCI. All patients received clopidogrel 75 mg daily for 28 days thereafter; patients in the control arm did not receive an LD. Pretreatment did not significantly reduce the primary composite end point of death, MI, and urgent target revascularization at 28 days (6.8% vs. 8.3%, P = . 23). Post hoc analysis suggested that longer durations of pretreatment were associated with improved outcomes, but little benefit was achieved when the treatment duration was less than 12 hours. A prospectively planned analysis of the 1863 patients in CLARITY–TIMI 28 who underwent PCI after mandated angiography showed that pretreatment for a median duration of 3 days in patients with STEMI treated with aspirin and fibrinolysis significantly reduced the incidence of cardiovascular death, MI, or stroke following PCI (3.6% vs. 6.2%, P = . 008) and from randomization through 30 days (7.5% vs. 12.0%, P = . 001). Unfortunately, the use of a 300-mg LD in CREDO, PCI-CURE, and the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention (PCI) in Patients With ST-Elevation Myocardial Infarction Treated With Fibrinolytics (PCI-CLARITY) studies and the prolonged duration of pretreatment in PCI-CURE and PCI-CLARITY limit their applicability to current practice patterns for both elective and urgent PCI. The ischemic benefit of a shorter pretreatment duration of high-dose clopidogrel before PCI has not been examined in a large, randomized, placebo-controlled trial. Post hoc analysis of the Intracoronary Stenting and Antithrombotic Regimen–Rapid Early Action for Coronary Treatment (ISAR-REACT) trial, which compared abciximab with placebo in elective PCI patients treated with clopidogrel 600 mg for at least 2 hours before intervention, showed no incremental benefit from durations of pretreatment greater than 2 to 3 hours. The PRAGUE-8 study randomized 1028 patients undergoing coronary angiography and potential ad hoc PCI for stable angina to either clopidogrel 600 mg more than 6 hours before angiography or clopidogrel 600 mg in the catheterization laboratory only in the case of PCI. No differences were found in the rate of death, MI, stroke, or reintervention among groups at 7 days, not in the entire population or in the subgroup undergoing PCI (0.8% vs. 1.0%, P = . 7; 1.3% vs. 2.8%, P = . 4, respectively), but bleeding was increased in the pretreatment group (3.5% vs. 1.4%, P = . 025). The findings of this small trial support a strategy of “on the table” clopidogrel loading before ad hoc PCI in elective patients, although the findings must be interpreted within the context of the relatively small sample size and very low event rates. The findings are supported by another smaller trial, Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty (ARMYDA) PRELOAD, which randomized 409 patients (39% with ACS) to a 600-mg clopidogrel LD 4 to 8 hours before PCI or a 600-mg LD given in the catheterization laboratory after coronary angiography and before PCI. The rates of major adverse cardiovascular events (MACEs) at 30 days were similar between groups and occurred in 10.8% of patients pretreated, compared with 8.8% in the patients receiving clopidogrel, in the laboratory ( P = . 7). No differences in the rates of bleeding were reported.
Pharmacodynamic studies have demonstrated that higher clopidogrel LDs and MDs provide more rapid onset of action and greater levels of inhibition compared with a 300-mg LD and a 75-mg MD, respectively. Two large randomized studies, CURRENT–OASIS 7 and GRAVITAS (Gauging Responsiveness With a VerifyNow Assay–Impact on Thrombosis and Safety), have examined the efficacy and safety of higher-dose clopidogrel in patients managed invasively or undergoing PCI.
The CURRENT–OASIS 7 trial examined the ischemic benefit of a higher-dose strategy in 25,086 patients with NSTE-ACS and ST-elevation ACS undergoing an early invasive strategy, of whom 17,263 underwent PCI. Before angiography, patients were randomized to receive either a 600-mg LD, followed by 150 mg daily for 6 days and 75 mg daily thereafter, or a 300-mg LD followed by 75 mg daily thereafter. Patients were also randomized to high-dose aspirin or low-dose aspirin in a two-by-two factorial design. The primary end point—a composite of cardiovascular death, MI, or stroke at 30 days—was no different with double-dose clopidogrel or standard-dose clopidogrel (4.2% vs. 4.4%, P = . 30). Major bleeding, as defined by the trial, was significantly greater in the patients randomized to double-dose clopidogrel (2.5% vs. 2.0%, HR 1.24; 95% CI, 1.05 to 1.46; P = . 01); however, no differences were noted in fatal bleeding, coronary artery bypass grafting (CABG) related bleeding, or TIMI-criteria major bleeding. Within the subgroup of patients who underwent PCI, high-dose clopidogrel was associated with a 13% relative risk reduction in the primary end point (3.9% vs. 4.5%, P = . 04). However, the interaction test between patients who underwent PCI and those who did not failed to reach the prespecified threshold for statistical significance; therefore the possibility that the results of the PCI subgroup are a chance finding cannot be excluded.
The GRAVITAS trial tested whether an additional clopidogrel LD followed by a 6-month course of clopidogrel 150 mg daily would reduce thrombotic events compared with clopidogrel 75 mg daily in patients who had undergone PCI with a DES and displayed high on-treatment reactivity according to ex vivo platelet function testing (PFT) 12 to 24 hours after the intervention. Unlike the population examined by the CURRENT–OASIS 7 trial, the predominant indication for PCI in the enrolled population was stable CAD or low-risk UA. No difference was reported in the rate of cardiovascular death, nonfatal MI, or stent thrombosis at 6 months between groups (2.3% vs. 2.3%, P = . 9). The incidence of severe or moderate bleeding per the Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO) criteria was not increased with the high-dose regimen (1.4% vs. 2.3%, P = . 10). The higher-dose clopidogrel regimen had a significant but only modest effect on platelet inhibition in patients with high on-treatment reactivity to standard dosing, which may partly explain the similar outcomes of the two groups.
The antiplatelet effect of clopidogrel is dependent on the generation of an active metabolite through the hepatic CYP450 system. Patients who are carriers for genetic polymorphisms that reduce the catalytic activity of CYP2C19 have lower clopidogrel active metabolite levels and diminished platelet inhibition with treatment. Approximately 5% to 12% of the variability in ADP-induced platelet reactivity appears to be explained by carriage of the reduced function CYP2C19∗2 allele. The sensitivity of active metabolite generation to changes in the catalytic activity of CYP2C19 may be attributed to the important contribution of this enzyme to both steps in clopidogrel biotransformation. Decreased CYP2C19 function could lead to a bottleneck at the level of hepatic activation, thereby shunting the prodrug into the pathway that leads to an inactive carboxylic acid metabolite.
Patients can be classified on the basis of the predicted metabolic phenotype of the CYP2C19 genotype. The single-nucleotide polymorphisms that affect enzyme activity are described using the established “star allele” nomenclature. The CYP2C19∗1 allele denotes the lack of known polymorphisms and therefore is considered to be a wild type. CYP2C19∗2 is the most common reduced-function allele, with an allelic frequency of approximately 13% in whites, 18% in blacks, and 30% in Asians. CYP2C19∗3 is the second most common reduced-function allele; it has an allelic frequency of approximately 10% in Asians but is rare in other ethnicities. Much less common reduced-function alleles include ∗4, ∗5, ∗6, ∗7, ∗8, and ∗10. In addition, the ∗17 variant is associated with increased gene transcription and increased catalytic activity of the enzyme. The combination of two alleles (genotype) can be used to predict the metabolic phenotype of a particular individual ( Table 10.2 ). Metabolic phenotype is associated with the pharmacokinetics and pharmacodynamics of clopidogrel. In a study of healthy volunteers, ultra-rapid metabolizers had the highest exposure to active metabolite and the greatest platelet inhibition, and poor metabolizers had the lowest exposure and least platelet inhibition with both loading and MDs. The frequency of poor metabolizers is approximately 2% in the white population.
| CYP2C19 Genotype | Predicted Phenotype |
|---|---|
| ∗17/∗17 | Ultra-rapid metabolizer |
| ∗1/∗17 | Ultra-rapid metabolizer |
| ∗1/∗1 | Extensive metabolizer |
| ∗1/∗2–∗8 | Intermediate metabolizer |
| ∗17/∗2–∗8 | Intermediate metabolizer/unknown |
| ∗2–∗8/∗2–∗8 | Poor metabolizer |
A collaborative meta-analysis of nine studies involving 9685 patients, 91% of whom had a PCI, reported a significantly increased risk of the composite end point of cardiovascular death, MI, or ischemic stroke in carriers of at least one reduced-function CYP2C19 allele (HR 1.57; 95% CI, 1.13 to 2.16; P = . 006) and in patients with two reduced-function CYP2C19 alleles (HR 1.76; 95% CI, 1.24 to 2.50; P = . 002). Carriers of at least one reduced-function CYP2C19 allele had an increased risk of stent thrombosis (HR 2.81; 95% CI, 1.81 to 4.37; P < .0001); this risk was especially strong in patients with two reduced-function alleles (HR 3.97; 95% CI, 1.75 to 9.02; P = . 001). The influence of CYP2C19 genotype on outcomes is less apparent in populations treated with clopidogrel for indications other than PCI. In the genetic substudy of the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE A), a randomized comparison of aspirin and clopidogrel compared with aspirin alone for the prevention of thromboembolic events in atrial fibrillation (AF), the primary outcome was similar in carriers and noncarriers of the CYP2C19∗2 reduced-function alleles. Similarly, in the CURE trial, in which only 14% of patients who presented with ACS underwent PCI, no difference was reported in ischemic outcomes according to CYP2C19 genotype.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here