Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Placental uptake, metabolism, and transfer of amino acids to the fetus are critical for both placental and fetal development and growth to produce a successful pregnancy. Placental amino acid transfer is mediated by active transport and is performed by over 20 distinct amino acid transport systems that are present on the maternal facing apical, microvillous, and fetal facing, basal plasma membranes of the placental syncytiotrophoblast. The transport systems are made up of proteins that are members of the solute carrier (SLC) superfamily. These SLCs exhibit a degree of specificity for certain classes of amino acids, but there also is overlap among the systems. Expression of the amino acid transporters is time dependent across gestation and is regulated by a host of factors including hormones, adipokines, and cytokines and is influenced by adverse in utero conditions. These systems can operate as accumulative transporters, exchangers, or facilitative transporters. Placental amino acid transfer requires the interplay of all three types of transporters. The interplay of these systems in conjunction with an increase in their number and activity rates over the course of placental development during gestation promote an adequate amino acid supply for placental and fetal metabolism and growth. This chapter focuses on describing the protein systems associated with placental amino acid transport as well as examining aspects of placental and fetal amino acid metabolism in animals and humans.
Amino acid supply to the placenta and the fetus involves active, energy-dependent transport of amino acids across the placental membranes that is mediated by transport proteins ( Fig. 41.1 ). The transport of amino acids is altered qualitatively and quantitatively by membrane transporter location and activity, competition among circulating amino acids for transporters, placental metabolism of amino acids, and relative concentrations of the amino acids in maternal and fetal plasma. Furthermore, changes in the overall placental surface area and architecture of the placental tissues also impact placental amino acid supply.
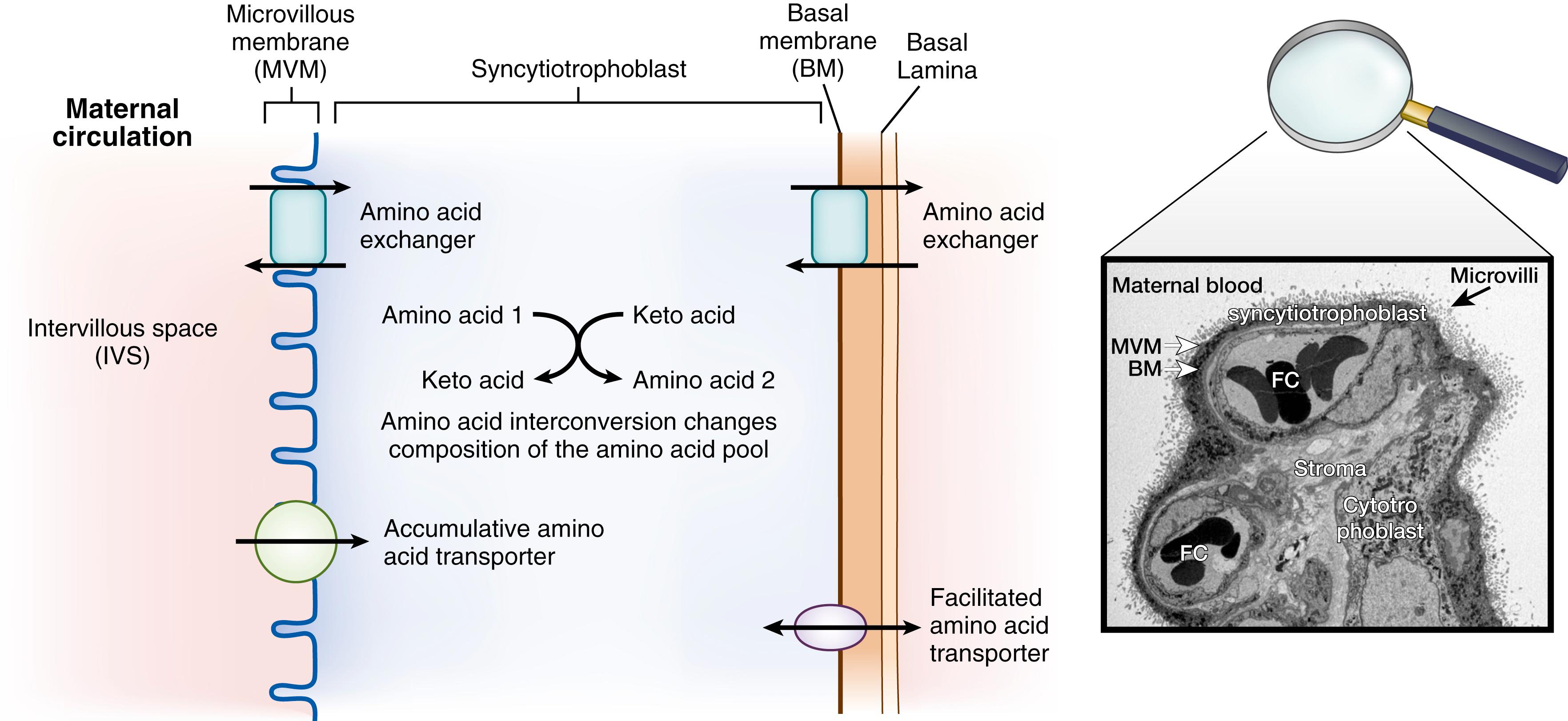
The placental membranes through which amino acids are transported into the placenta and into the fetus are the maternal and fetal surfaces of the syncytial epithelium of the human placenta, or the syncytiotrophoblast, a polarized multinucleate epithelium (see Fig. 41.1 ). The maternal-facing, apical surface of this epithelium has a microvillus membrane (MVM); the basal surface facing the fetus is termed the basal membrane (BM). The transport of amino acids across the syncytiotrophoblast involves three steps: (1) uptake from the maternal circulation across the MVM, (2) movement through the trophoblast cytoplasm, and (3) transport out of the trophoblast across the BM into the placental villous stroma (see Fig. 41.1 ).
Amino acids must diffuse across the villous stroma before crossing the fetal capillary endothelium into the umbilical circulation. It is not clear whether amino acid transfer across the capillary endothelium is primarily mediated through the paracellular routes (via cell-cell junctions) or a transcellular transporter mediated route. For most of the amino acids and in all mammalian species that have been studied, transport across the trophoblast membranes from maternal to fetal plasma occurs against a concentration gradient and involves energy-dependent transport proteins. The major membrane transport proteins and their location for amino acid supply to the placenta are discussed below and are reported in Table 41.1 . ,
| Gene/Protein (Traditional System) | Mechanism | Predominant Substrates | Reported Localization in the Syncytiotrophoblast |
|---|---|---|---|
| SLC1A1 /EAAT3 (System X AG ) | 3Na + /1H + /AA co-transport/1K + -antiport | E, D | MVM, BM |
| SLC1A2 /EAAT2 (System X AG ) | |||
| SLC1A3 /EAAT1 (System X AG ) | |||
| SLC1A4 /ASCT1 (System ASC) | Na + dependent amino acid exchange | A, S, C, T | ASC activity on the BM |
| SLC1A5 /ASCT2 (System ASC) | Na + dependent amino acid exchange | A, S, C, T, Q, N | |
| SLC7A1 /CAT1 (System y + ) | Facilitated but highly trans-stimulated | R, K, H | CAT activity on MVM, BM |
| SLC7A2 /CAT2B System y + | Facilitated | R, K, H | |
| SLC7A3P /CAT3 (System y + ) | Facilitated | R, K, H | |
| SLC7A5 /LAT1 (System L) | Exchanger Heterodimerize with 4F2hc (SLC3A2) |
Large neutral L-amino acids | MVM, BM |
| SLC7A8 /LAT2 (System L) | Neutral L-amino acids | MVM, BM | |
| SLC7A7 /y + LAT1 (System y + L) | exchanger, Heterodimerize with 4F2hc (SLC3A2) | Cationic amino acids and Na + -dependent transfer of neutral AA Favor efflux of cationic amino acids |
MVM |
| SLC7A6 /y + LAT2 (System y + L) | Exchanger, Heterodimerize with 4F2hc (SLC3A2) | MVM, BM | |
| SLC7A10 /Asc1 (System asc) | Exchanger Heterodimerize with 4F2hc (SLC3A2) |
Small neutral amino acids | MVM |
| SLC16A10 /TAT1 | Facilitated diffusion | F, Y, W | BM |
| SLC38A1 /SNAT1 (System A) | Na + /AA co-transport | Q, A, N, C, H, S | MVM/BM |
| SLC38A2 /SNAT2 (System A) | Na + /AA co-transport | A, N, C, Q, G, H, M, P, S | MVM/BM |
| SLC38A4 /SNAT4 (System A) | Na + /AA co-transport | A, N, C, G, S, T | MVM BM |
| SLC38A3 /SNAT3 (System N) | Na + /AA co-transport - H antiport | Q, H, A, N | Localization unclear |
| SLC38A5 /SNAT5 (System N) | Na + /AA co-transport - H antiport | Q, N, H, S | MVM |
| SLC43A1 /LAT3 | Facilitated diffusion | L, I, V | BM |
| SLC43A2 /LAT4 | Facilitated diffusion | L, I, V | BM |
Amino acid transporters are found to operate as accumulative transporters, exchangers, or facilitated transporters and placental amino acid transfer requires the interplay of all three types of transporters. Accumulative transporters mediate uptake of amino acids into the cell and can operate against the concentration gradient by coupling transport to electrochemical gradients, primarily the Na + gradient. Exchanger systems take up one amino acid while mediating the efflux of a second, while facilitated transporters mediate both uptake and efflux. Historically, these amino acid transporters were described in terms of systems that were based on common substrates (e.g., system L that transported leucine). , More recently, the ability to clone, sequence, and study the expression of individual transporter proteins has led to an explosion of data defining the molecular basis of amino acid transporter systems that are all members of the SLC superfamily.
The SLC1 family contains the accumulative anionic amino acid transporters that form System X AG and the neutral amino acid exchangers comprising the System ASC (alanine, serine, and cysteine). Through System X AG proteins, the amino acids glutamate and aspartate are taken up into the placenta by the highly accumulative excitatory amino acid transporters (EAATs; SLC1A1-3, 6, and 7). The EAATs mediate sodium-coupled uptake of anionic amino acids and are found on both the MVM and BM of the placenta. , The rat BM preparation is reported to have a higher maximal velocity, an observation that is consistent with the predominant flux of glutamate from the fetus to the placenta, as has been demonstrated in the sheep and human. , EAAT-1, EAAT-2, and EAAT-3 have been detected in human placental tissue. The regulation of these proteins appears to be under the control of growth hormone (GH) and insulin-like growth factor (IGF) family members, both of which are regulated by nutrient supply, as shown in rodent cell line culture nutrient deprivation studies. ,
ASCT1 (SLC1A4) and ASCT2 (SLC1A5) mediate sodium-dependent exchange of small neutral amino acids—alanine, serine, and cysteine—originally termed the ASC transport proteins. These transporters have been found in the MVM and in the BM. Hypoxia is reported to inhibit or limit their expression, and levels are reduced in placentae with growth-restricted fetuses. Both ASCT1 and ASCT2 are expressed in placental tissue , and play additional critical roles in cytotrophoblast fusion into the syncytiotrophoblast.
The taurine transporter (TAUT) (SLC6A6) has been found in MVM placental tissue and is thought to be important in maintaining the high intracellular concentrations of taurine observed in the human placenta. Taurine is essential for fetal growth and is thought to be important in maintaining osmotic balance and functioning as an antioxidant. In addition to transporting taurine into the placenta, the TAUT transporter potentially mediates the bilateral release of taurine into maternal and fetal circulations. Studies examining the impact of obesity on placental function have reported that placental TAUT activity at term is negatively related to maternal body mass index (BMI).
The SLC7 family contains a large number of amino acid transporters that are relevant to placental function, including the transporters that constitute System L, System y + , and System y + L. LAT1 (SLC7A5) and 2 (SLC7A8) are exchange transporters that mediate transfer of neutral amino acids and, characteristic of System L, mediate transfer of the L-system-specific substrate 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH). These transporters, especially LAT2, have a broad substrate specificity for the neutral amino acids. Functional System L activity occurs through specific light chain proteins associated with 4F2hc. System L activity is reported on both the MVM and BM. Immunologic and functional studies suggest that at term gestation, the light chain LAT1 is located predominantly in the MVM. , Furthermore, the L transport system phenotype associated with the MVM occurs when the LAT1 catalytic subunit is coexpressed with the 4F2hc, not LAT2. , Human LAT2 mRNA also has been reported in human choriocarcinoma BeWo cells and placental villous tissues. Placental BM studies concerning the inhibition of specific amino acid and/or synthetic amino acid uptake have determined that the BM L transport system phenotype is associated with the coexpression of LAT2 and 4F2hc, and not LAT2, as observed to occur in the MVM. , , Furthermore, regulation of placental System L activity does not seem to be under the control of hypoxia, although evidence exists that it may not be the case in other cell preparations. , Although evidence is lacking in placental preparations, IGF-1 appears to regulate System L activity where chronic infusions of IGF-1 have corrected impaired transport of leucine in the brush border of the rat intestine. Additionally, in a mouse model of intrauterine growth restriction (IUGR), System L remained at control levels after Ad-hIGF-1 administration.
The transport of cationic amino acids occurs through the CAT and the y + LAT transporters. The cationic amino acid transporter (CAT) activity is associated with System y + activity and in placental cell culture and human placental studies, CAT 1 (SLC7A1), 2B (SLC7A2), and 3 (SLC7A3) are expressed. , In contrast to the other SCL7 family transporters the CATs do not require a heavy chain for their activity. , CAT activity has been extensively studied in the rodent and human placenta. In human preparations, CAT activity has been localized to the MVM and BM, although conflicting data exist as to y + activity in the BM. ,
System y + L activity is mediated by the transporters y + LAT1 (SLC7A7) and y + LAT2 (SLC7A6). , , y + LATs are exchangers for cationic amino acids and sodium dependent exchangers for neutral amino acids. The effect of this selective Na-dependence is that they preferentially mediate efflux of cationic amino acids (as intracellular sodium concentrations are low, precluding efflux of neutral amino acids). y + LAT activity is localized to both the MVM and BM. , , This system may play an essential role in supplying cationic amino acids to the fetus by exchanging cationic amino acids within the trophoblast for neutral amino acids from the fetal circulation. , ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here