Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The oocyte is usually fertilized near the distal end of the fallopian tube, at approximately day 15 of the menstrual cycle, or the day after ovulation. During the next week, as the zygote travels through the fallopian tube, it undergoes progressive cellular divisions to form first a 16-cell, raspberry-like morula and then a 32-cell, centrally cystic blastocyst. Under normal circumstances, on days 23 to 26 of the menstrual cycle (days 9–12 postconception), the blastocyst attaches to and then implants into the superficial endometrium. Subsequently, the developing inner cell mass transforms into a bilayered and then trilayered embryonic disc. The lateral edges of the embryonic disc are contiguous with two cystic structures, the dorsal amniotic sac and ventral yolk sac ( Fig. 29.1A ). Hematopoiesis arises in the wall of the yolk sac. Eventually, part of the yolk sac is incorporated into the embryonic body to establish the enteric circulation, whereas the remainder is exteriorized to form part of the body stalk (future umbilical cord), sometimes visible as remnant vessels peripheral to the umbilical vessels or as a secondary yolk sac (a vestigial, often mineralized, collapsed cyst within the placental chorionic plate; see Fig. 29.1B ). As the amniotic sac expands, its epithelium comes to cover the ventral body stalk (umbilical cord).
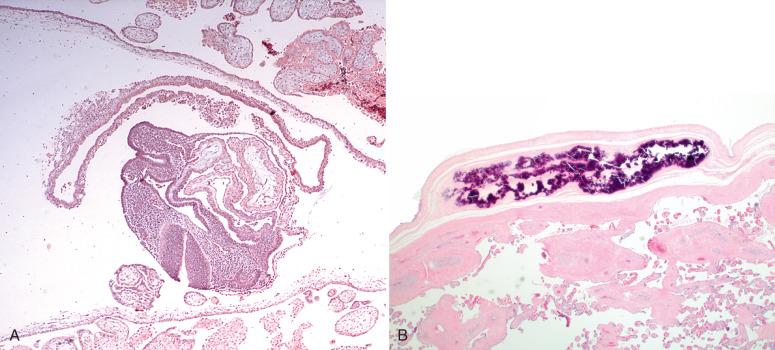
In this manner, the inner cell mass of the blastocyst eventually forms the embryo, yolk sac, and amnion. It also gives rise to the mesenchymal portion of chorionic villi, including the fetal vasculature in the placenta and umbilical cord. Hence, these structures are evidence of embryogenesis proper.
In turn, the outer trophoblastic shell of the blastocyst ( Fig. 29.2A ) gives rise to all the trophoblast subtypes, including the villous trophoblast, outer layers of chorionic villi, chorionic trophoblast in fetal membranes, and extravillous trophoblast within the placental disc and at the implantation site. The blastocyst trophoblastic shell consists of the outer mitotically inactive syncytiotrophoblast layer, termed the primitive syncytium, and inner, actively proliferating cytotrophoblast layer (see Fig. 29.2B ), which functions as a primordial trophoblast. The primitive syncytium develops multiple cytoplasmic lacunae, which expand and coalesce to eventually become the maternal intervillous (vascular) space, perfused by decidual spiral arterioles and drained by decidual veins. Primitive connective tissue, the chorionic stroma (see Fig. 29.2B ), develops subjacent to the cytotrophoblast. As this sphere of chorionic stroma thickens and expands, peripheral finger-like villous extensions develop. Initially, the gestational sac is evenly covered by chorionic villi, 360 degrees. As the chorionic sphere expands, the villi located farthest from the umbilical stalk gradually regress, forming the avillous chorionic membrane (chorion laeve); this membranous villous regression takes place between 7 and 15 weeks' gestational age, although remnants of these regressed first-trimester villi can be found in the fetal membranes of most term placentas (see below). Perfused chorionic villi continue to proliferate around the umbilical insertion site, eventually forming the parenchyma of the placental disc proper (chorion frondosum).
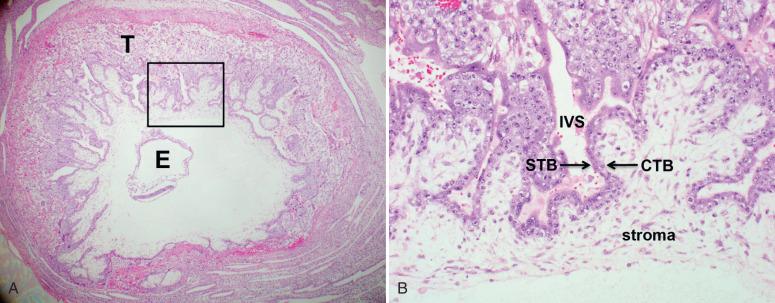
Primary chorionic villi form during the second week of gestation from invagination of the proliferating cytotrophoblast. Once invaded by mesenchymal cells, these invaginations are called secondary villi and, following proliferation of mesenchymal cells, tertiary villi. By the third week of gestation, tertiary villi are composed of primitive fetal blood vessels and mesenchymal and Hofbauer cells (fetal macrophages), and are surrounded by two layers of trophoblast, an inner layer of proliferative cytotrophoblasts and outer layer of multinucleated syncytiotrophoblasts ( Fig. 29.3A ). The latter derive from fusion of cytotrophoblast cells and are considered true syncytiotrophoblasts; how closely they resemble the primitive syncytium is poorly understood. The villous syncytiotrophoblast is a terminally differentiated, mitotically inactive cell type, with amphophilic to eosinophilic cytoplasm and multiple small, hyperchromatic nuclei. The syncytiotrophoblast cytoplasm may be finely to coarsely vacuolated; when multiple large vacuoles (lacunae) are present, the cytoplasm has an irregular lacy appearance. Syncytiotrophoblasts cover all chorionic structures (i.e., chorionic plate and villi), where they interface with the maternal blood, lining the entire maternal intervillous (vascular) space much like an endothelial layer.

The growth and expansion of the villous placenta proceeds through a coordinated process of branching angiogenesis of the fetal vessels, as well as proliferation and differentiation of the overlying trophoblast. Over time, because the latter process lags behind growth of the villous stroma, the inner cytotrophoblastic layer becomes discontinuous, covering only 20% of the villous surface area at term (see Fig. 29.3B ). The remaining 80% of the area is covered only by multinucleated syncytiotrophoblasts; as these cells mature, they lose cytoplasmic mass and undergo apoptosis, giving rise to syncytial knots, which are shed into the maternal circulation. Loss of the continuous cytotrophoblast layer also translates into a decrease in maternofetal diffusion distance over time, from about 20 µm in the first trimester to 5 µm by term. This further accommodates the demands of the growing fetus during the latter half of pregnancy.
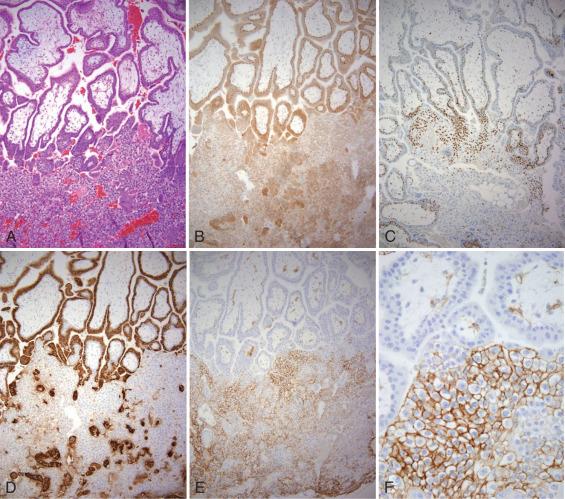
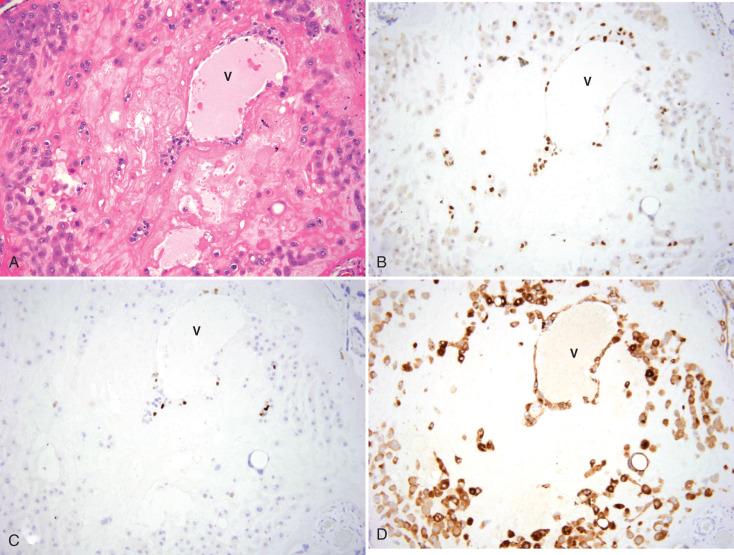
Cytotrophoblasts, the inner layer of villous trophoblasts, are composed of proliferative stemlike cells, characterized by expression of p63. The isoform of p63 predominantly expressed in these cells is the N-terminal truncated (dNp63) form, the same isoform expressed in stem cells of other stratified epithelia (including ectocervical and vulvar epithelia). As cytotrophoblasts fuse and form multinucleated syncytiotrophoblasts, p63 expression is abruptly lost (see Fig. 29.3C and D ). Instead, syncytiotrophoblasts acquire expression of the pregnancy hormones, human chorionic gonadotropin (hCG; see Fig. 29.4D ) and placental lactogen (also termed chorionic somatomammotropin ), as well as inhibin (see Fig. 29.3E and F ). hCG and inhibin are more highly expressed in syncytiotrophoblasts in the first trimester, whereas placental lactogen expression increases as gestation progresses. Finally, cytotrophoblasts and syncytiotrophoblasts also strongly express cytokeratins.
Adjacent to the cytotrophoblast and syncytiotrophoblast cells at the periphery of the chorionic sphere, a dense layer of eosinophilic proteinaceous material (fibrinoid, Nitabuch's fibrin) separates the gestational sac from the decidua. At this fetal–maternal interface, the cytotrophoblast cells in the anchoring villi proliferate to form cell columns, extending toward the uterine wall ( Figs. 29.4 and 29.5 ). As these cells distance themselves from the mesenchymal villous core, they lose their epithelial morphology, as well as their proliferative activity, and differentiate into extravillous trophoblasts. These cells acquire mesenchymal features, including migratory and invasive capabilities, as they differentiate. The typical implantation site of an extravillous trophoblast is a polyhedral to spindle-shaped cell, larger than the cytotrophoblast, with eosinophilic cytoplasm. Most have a single central nucleus, but binucleate and multinucleated forms can be prominent. In comparison with syncytiotrophoblasts, the nucleus of the trophoblast implantation site is larger and more reactive-appearing, with prominent chromatin and nucleolus. Smudging of chromatin and sharp angulations of nuclear shape are common; characteristics of extravillous trophoblasts are intranuclear cytoplasmic inclusions.
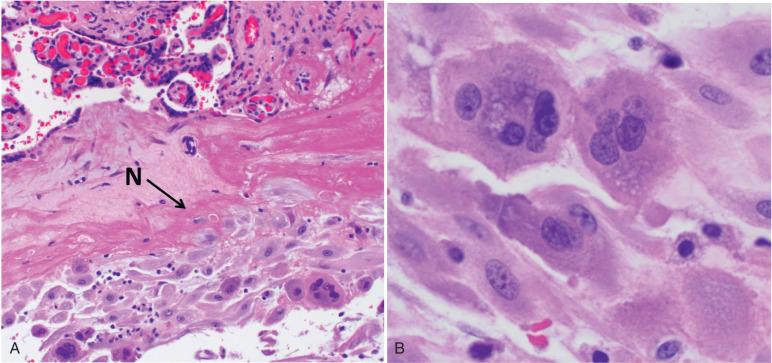
Extravillous trophoblast permeate the endomyometrium, as interstitial trophoblasts, and spiral arterioles, as endovascular trophoblast. The interstitial trophoblasts invade through the inner third of the myometrial wall; at this point, some develop into multinucleated giant cells (see Fig. 29.5 ). Meanwhile, the endovascular trophoblasts form plugs, which occlude maternal spiral arterioles. At the end of the first trimester, the cells migrate from the plugs along the arteries, allowing for conversion, the process whereby the maternal spiral arterioles are physiologically transformed from normal muscularized resistance arteries, responsive to changes in maternal systemic blood pressure, into dilated trophoblast-lined vessels, which passively and continuously divert increasingly larger percentages of the maternal cardiac output (as much as 25% at term) to the intervillous maternal vascular space. Studies have suggested that following conversion, the spiral arteries become re-endothelialized by maternal cells.
The deposition of fibrin-type fibrinoid around chorionic villi is a normal process, which leads to loss of syncytiotrophoblasts and differentiation of the underlying cytotrophoblast layer into extravillous trophoblasts. The extravillous trophoblasts, in turn, secrete matrix-type fibrinoid, leading to the formation of trophoblast cell islands; these structures are composed of a variable number of extravillous trophoblast, often surrounding a degenerated villus, encased in fibrinoid ( Fig. 29.6 ). Larger islands can also contain cystic structures, which are filled by secreted proteins emanating from extravillous trophoblasts.
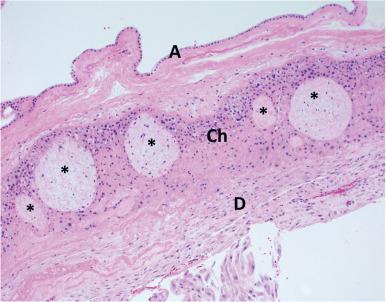
Although the gestational sac is fully covered by chorionic villi during early development, the villi located farthest from the umbilical stalk regress as the chorionic sphere grows, between 7 and 15 weeks' gestational age, forming the chorion laeve. The amnion epithelium and stroma become passively apposed to the inner surface of the chorion laeve between 9 and 11 weeks' gestation. Hence, the amnion forms a continuous innermost lining within the amniotic cavity, in direct contact with the amniotic fluid (see Fig. 29.1 ).
Finally, between 17 and 20 weeks' gestation, the expanding chorioamniotic membrane fuses with the endometrium at the opposite end of the uterus, providing a sturdy structure and maternal blood supply to this tissue. At term, the chorionic portion of fetal membranes often contains remnants of early villi, surrounded by extravillous trophoblasts of various morphology ( Fig. 29.7 ). Chorionic trophoblasts can appear highly variable, depending on their maturity (see below) and the intrauterine environment to which they are exposed ( Fig. 29.8 ).
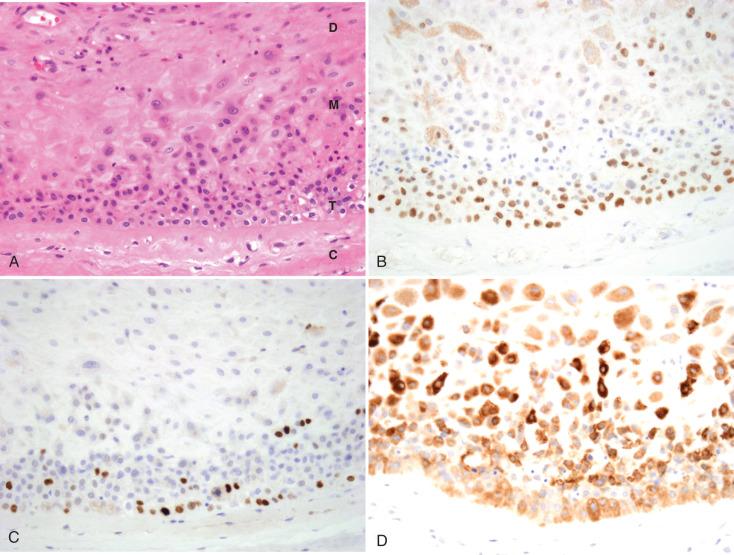
Extravillous trophoblasts (EVTs) can assume various morphologies, as noted in Figs. 29.4 through 29.8 . As cytotrophoblasts differentiate down the extravillous pathway, they lose expression of p63 and epidermal growth factor receptor (EGFR), in addition to their proliferative capacity, and gain surface expression of the nonclassic human leukocyte antigen (HLA), HLA-G, a molecule that induces a tolerogenic environment within the implantation site.1 However, before reaching maturity, they initially undergo a transitional phenotype, assuming a vacuolated epithelioid morphology, with some retention of proliferative activity and nuclear staining for p63; in contrast to cytotrophoblasts, these cells show variable staining for inhibin and MelCAM (see Figs. 29.4, 29.6, and 29.8 ). Although also present in the cell columns of early-gestation placentae (see Fig. 29.4 ), such transitional EVTs at term are most prominently seen in fetal membranes and within trophoblast islands. At both these sites, transitional EVTs are noted at the interface of the villus and perivillous fibrin deposits and gradually evolve into mature EVTs peripherally (see Figs. 29.6 and 29.8 ). Although transitional and mature EVTs are strongly immunoreactive for cytokeratins, only mature EVTs are uniformly positive for MelCAM, inhibin, and human placental lactogen (hPL). Mature EVTs are only weakly reactive for hCG.
The Arias-Stella reaction is defined as nuclear enlargement and hyperchromasia of the secretory endometrial lining cells. It is presumably the consequence of a hormonal effect and stems from polyploidy of the lining cells. Its presence usually signifies pregnancy, although high levels of pharmaceutical exogenous or pathologic endogenous progestin exposure can produce the same effect. In the context of pregnancy, it will not establish the site of gestational implantation, an important consideration for ectopic pregnancy ( Fig. 29.9 ).
Similar to endometrial glands, the stroma also undergoes changes during pregnancy secondary to progestins. These changes include flattening of the usually spindled cells, with round regular nuclei, resulting in a fried egg appearance (see Fig. 29.9 ). Unless showing reactive changes secondary to inflammation, the nuclei of decidual cells have fine chromatin and pinpoint nucleoli, which helps distinguish them from those of invasive extravillous trophoblasts, which often have nuclei with irregular contours and coarse chromatin. Similar to the Arias-Stella reaction of endometrial glands, decidualization of endometrial stroma can occur secondary to exogenous or pathologically endogenous progestins and does not distinguish between intrauterine and extrauterine pregnancy.
Clinicians and surgical pathologists frequently work together to resolve patient-related questions concerning disorders in pregnancy. In the previable period, meaning gestation of less than 24 weeks, these disorders fall into several categories.
In most cases, the clinician is eager to identify causes that could affect future pregnancies. A pathologic examination may be helpful, but the role of the pathologist is usually to confirm an intrauterine pregnancy and secure material for cytogenetic evaluation. Uncommonly, and particularly with recurrent pregnancy loss, a pathologic clue may be identified.
Here, the role of the pathologist is to confirm intrauterine pregnancy, and, if indicated, secure material for cytogenetic evaluation.
In practice, every pathologic examination of a supposed product of conception entails excluding an ectopic pregnancy. If this is suspected, the clinician and pathologist are drawn concurrently into a decision-making exercise that requires identifying the site of implantation and thus determining an appropriate clinical response.
In this gestational age group, one general consideration is exclusion of a molar pregnancy or other trophoblastic neoplasia; this consideration must be kept in mind not only for every early pregnancy loss, but even for cases of elective termination of pregnancy and ectopic gestation. Early molar gestations may be difficult to recognize morphologically; this topic is considered in detail in Chapter 30 .
The patient with intrauterine demise. The purpose of pathologic evaluation of the fetus and placenta is to identify the cause of death.
In cases of fetal demise, followed by dilation and evacuation. Here, pathologic evaluation is often hampered by fragmentation and fetal and placental autolysis, both of which may obscure antemortem pathology.
In cases of preterm premature rupture of membranes, followed by spontaneous or induced delivery of a well-preserved fetus.
In these cases, the cause of pregnancy loss is almost always extrinsic to the fetus and is attributable to errors of the intrauterine environment. Often, the cause is due to extreme preterm delivery associated with an inflammatory abruption sequence, the pathologic correlate of cervical incompetence (see Chapter 33 for a detailed discussion).
In cases of fetal anomalies in the above scenarios, it is most important to review ultrasonographic findings for optimal clinicopathologic correlation. Anomalies usually signify a genetic condition, except in the case of amniotic band sequence, an acquired disorder in a previously normally developing fetus (see Chapter 33 for a detailed discussion). The purpose of examination is to document fetal anomalies and, if the cause is unknown clinically, to suggest or identify the underlying disorder and thus a potential genetic disease. Formal evaluation by clinical geneticists following release of the pathology report is the ideal resource for follow-up counseling and should be suggested in the pathology report discussion.
The patient with elective termination of pregnancy. In the second trimester, these are mostly performed in cases of fetal anomalies.
Thus, a thorough evaluation, including cytogenetic analysis and correlation with ultrasonographic findings, is required. Often, evaluation is nevertheless hampered by fragmentation.
In the second trimester, there are two additional issues to consider. First, in this gestational period, all forms of abnormal pregnancy outcome overlap with those in the flanking trimesters. Early second-trimester losses more closely approximate the genetic causes of most first-trimester losses, whereas late second-trimester losses are usually attributable to disorders that are not intrinsic to the fetus but rather are caused by those of the intrauterine environment. For a detailed discussion of the latter, please see Chapter 32 .
Second, the proper disposition of embryonic and fetal tissues arriving in the pathology department must comply with the family's wishes. In many states, this entails express legal mandates or hospital policy guidelines for written consent to examine and preserve tissues for burial or cremation or to direct hospital disposition. This is a complex legal, sociologic, and psychological concern, and the pathologist should be mindful of such scenarios, realizing that families may even wish access to paraffin-embedded tissue from early pregnancy losses for burial. Release of such tissues must be made with care, in accordance with state regulations or hospital policies, and with the interests and desires of the family in mind. Discussion of this topic in detail is beyond the scope of this text.
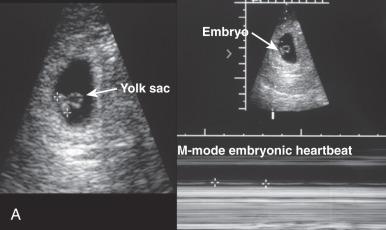
Sonographic evaluation of the conceptus plays a dual role of pregnancy confirmation and outcome prediction. Currently, transvaginal ultrasound permits detection of a 2- to 3-mm gestational sac, corresponding to 4.5 menstrual weeks. The sac diameter increases at a rate of approximately 1 mm/day from this point in normal pregnancies. Normally, the yolk sac is visible at 6 weeks. The absence of a yolk sac beyond 32 days or the presence of an abnormal (enlarged) yolk sac portend poor outcome. Cardiac activity is normally detectable shortly after sonographic yolk sac identification and is required to establish and maintain embryonic viability. As with abnormal yolk sacs, a heart rates of less than 85 beats/min at 6 to 8 weeks is also associated with a uniformly poor outcome. Additional ultrasound markers predictive of poor outcome include absence of an embryonic pole at 16- to 18-mm embryonic size ( Fig. 29.10 ), oligohydramnios in the first or second trimester, and large, early, subchorionic hemorrhage.
In most early-gestation products of conception (POCs), no recognizable embryonic or fetal tissues can be grossly identified. This results from specimen fragmentation, precluding gross recognition of embryonic and fetal parts, or from genetically abnormal gestations with early embryonic demise (empty sac, blighted ovum; Fig. 29.11A and B ; see Fig. 29.10B ). When an intact gestational sac is identified, a small malformed embryo may be found in approximately 25% of specimens, variously referred to as a nodular, amorphous, cylindric, or growth-disorganized embryo. In another 25% of intact sacs, a well-formed embryo or fetus may be found (see Fig. 29.11C and D ). When evaluating relatively normally formed embryos and fetuses, pathologists should determine the gestational age and assess whether landmarks of normal development and growth have been reached ( Tables 29.1 and 29.2 ). A gestational age of 10 weeks divides the embryonic period of organogenesis from the fetal period of growth and differentiation; a crown-rump length of 3 cm separates the embryo (<3 cm) from the fetus (>3 cm). In fragmented specimens, foot length is the standard gross pathologic measurement used for estimating gestational age; a foot length of 5 mm distinguishes an embryo (<5 mm) from a fetus (>5 mm). Additional foot length measurements are summarized in Table 29.2 .
| Developmental Age (days) a | Gestational Age (wk) | No. of Somites | Greatest Length (mm) | Main Characteristics |
|---|---|---|---|---|
| Embryonic Stage | ||||
| 20–21 | 5 | 1–3 | 1.5–3.0 | Deep neural groove and first somites present; head fold evident |
| 22–23 | 4–12 | 2.0–3.5 | Embryo straight or slightly curved; neural tube forming or formed opposite somites, but widely open at rostral and caudal neuropores; first and second pairs of branchial arches visible | |
| 24–25 | 13–20 | 2.5–4.5 | Embryo curved owing to head and tail folds; rostral neuropore closing; otic placodes present; optic vesicles formed | |
| 26–27 | 21–29 | 3.0–5.0 | Upper limb buds appear; caudal neuropore closing or closed; three pairs of branchial arches visible; heart prominence distinct; otic pits present | |
| 28–30 | 6 | 30–35 | 4.0–6.0 | Embryo has C-shaped curve; upper limb buds are flipper-like; four pairs of branchial arches visible; lower limb buds appear; otic vesicles present; lens placodes distinct; attenuated tail present |
| 31–32 | 33–36 | 5.0–7.0 | Upper limbs are paddle-shaped; lens pits and nasal pits visible; optic cups present | |
| 33–36 | 7 | 7.0–9.0 | Hand plates formed; lens vesicles present; nasal pits prominent; lower limbs are paddle shaped; cervical sinus visible | |
| 37–40 | 8.0–11.0 | Foot plates formed; pigment visible in retina; auricular hillocks developing | ||
| 41–43 | 8 | 11.0–14.0 | Digital, or finger, rays appear; auricular hillocks outline future auricle of external ear; trunk beginning to straighten; cerebral vesicles prominent | |
| 44–46 | 13.0–17.0 | Digital, or toe, rays appearing; elbow region visible; eyelids forming; notches between finger rays; nipples visible | ||
| 47–48 | 9 | 16.0–18.0 | Limbs extend ventrally; trunk elongating and straightening; midgut herniation prominent | |
| 49–51 | 18.0–22.0 | Upper limbs longer and bent at elbows; fingers distinct but webbed; notches between toe rays; scalp vascular plexus appears | ||
| 52–53 | 22.0–24.0 | Hands and feet approach each other; fingers are free and longer; toes distinct but webbed; stubby tail present | ||
| 54–55 | 23.0–28.0 | Toes free and longer; eyelids and auricles of external ears more developed | ||
| Fetal Stage Begins | ||||
| 56 | 10 | 27.0–31.0 | Head more rounded and shows human characteristics; external genitalia still have gender-less appearance; distinct bulge still present in umbilical cord, caused by herniation of intestines; tail has disappeared | |
| Gestational Age (wk) | Mean (mm) | Minimum FL (mm) | Maximum FL (mm) |
|---|---|---|---|
| Embryonic Stage | |||
| 8.5 | 4.2 | 3.8 | 4.6 |
| 9 | 4.6 | 4.2 | 5.0 |
| Fetal Stage a | |||
| 10 | 5.5 | 5.0 | 6.0 |
| 11 | 6.9 | 6.0 | 7.8 |
| 12 | 9.1 | 7.5 | 10.8 |
| 13 | 11.4 | 9.8 | 13.0 |
| 14 | 14.0 | 12.5 | 15.5 |
| 15 | 16.8 | 15.2 | 18.5 |
| 16 | 19.9 | 18.2 | 21.6 |
| 17 | 23.0 | 21.0 | 25.0 |
| 18 | 26.8 | 24.8 | 28.8 |
| 19 | 30.7 | 28.5 | 33.0 |
| 20 | 33.3 | 31.0 | 35.7 |
| 21 | 35.2 | 32.5 | 38.0 |
| 22 | 39.5 | 36.0 | 43.0 |
| 23 | 42.2 | 39.0 | 45.5 |
| 24 | 45.2 | 42.0 | 48.5 |
a For practical purposes, a foot length of ≥5 mm distinguishes a fetus (≥10 weeks' gestational age) from an embryo (<10 weeks).
Aside from foot length and determination of gestational age, evaluation of fetal tissue is best guided by the clinical question. In cases with documented fetal anomalies, careful gross examination, complemented by magnification with a dissecting microscope, can identify a sizable portion of congenital malformations, particularly in second-trimester cases. These examinations should include radiography, when warranted, because even in fragmented cases, diagnostic findings may be obtained ( Fig. 29.12 ). The most commonly recognized abnormalities include neural tube defects and distal extremity malformations (e.g., polydactyly); areas of discrepancy often include central nervous system (CNS) and cardiovascular abnormalities, as well as abdominal wall defects. It is important to note that although such examinations are mostly viewed as quality control for ultrasonographers and perinatal radiologists, a detailed evaluation, particularly by a trained perinatal pathologist, can often provide additional useful information. Consultation by a resident dysmorphologist and/or clinical geneticist can further contribute to an optimal evaluation of recurrence risk. Depending on the differential diagnosis, karyotyping, DNA microarray, and/or exome sequencing may be performed (see below).

Similar to a gross examination, histologic sampling in these cases is also best guided by the clinical question. Histologic evaluation of tissues can be helpful in the timing of intrauterine demise, evaluation for fetal infection, further documentation of specific fetal anomalies, and identification of intrauterine stress.
Early POC specimens are admixtures of mostly decidual and placental tissues. Placental tissues during this early gestational period (<10 weeks) are grossly identified as pale pink to tan-white, delicate, velvety fragments, with a spongelike consistency. Vessels are typically not identified in the chorionic villi with the naked eye or dissecting microscope; this property distinguishes villi from planar fragments of decidua or endometrium. Decidual tissue possesses a more uniform consistency to the touch and, through the dissecting microscope, can be identified by a smooth surface punctuated by indentations of penetrating decidual vessels. Fragments of the implantation site cannot normally be distinguished from decidua but may have a slightly more rubbery consistency because of the presence of abundant Nitabuch's fibrin. Examining the past or curetted specimen in a Petri dish of sterile saline may help identify potential chorionic villi, which expand and float, compared with the denser decidua, which settle to the bottom. A cystlike, intact, collapsed chorionic sac is sometimes found; it will have a roughened, velvety exterior surface and a smooth, shiny interior lining. Intact sacs are usually seen in spontaneously passed specimens as opposed to curettings ( Fig. 29.13 ).

For all early POC specimens, at least one or two blocks of villous (and decidual) tissues should be submitted for histologic assessment. In many specimens, particularly early spontaneous abortions, no grossly identifiable gestational tissue is present, and sampling should take this uncertainty into account. When villous and embryonic tissues are not grossly evident, the specimen is small, or the clinician raises the suspicion of an ectopic gestation, the entire specimen should be histologically examined. Examination of the tissue through the dissecting microscope may be helpful in selecting the most likely villous tissue ( Fig. 29.14 ). For more voluminous specimens, particularly if ectopic gestation is not suspected clinically, several blocks of tissue can be submitted initially, followed by additional sampling or, if necessary, submission of the entire specimen. Obtaining levels on tissue blocks to search for products of conception is rarely fruitful.
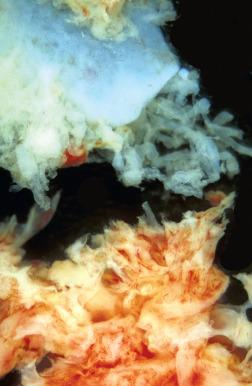
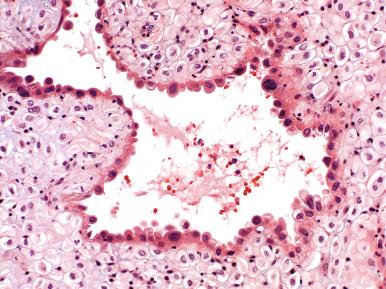
Many specimens submitted to confirm intrauterine pregnancy consist principally of blood clots and, on gross examination, may not appear promising. However, small amounts of gestational tissue may be found centrally within clotted blood. The pathologist should direct careful sampling of these clots, submitting sections that contain small cystic or lucent central foci. Microscopic examination may disclose villous tissue in these sites ( Fig. 29.15 ). Of note, the most commonly identified cells on microscopic examination within clotted blood are individual syncytiotrophoblasts. These cells confirm pregnancy, although they do not unequivocally identify the location of the gestational sac. For example, syncytiotrophoblasts may in theory fall into the uterus from a tubal gestation. Correlation with the clinical history (e.g., abundant tissue passed at home before curettage, representing the gestational sac) or radiographic findings is often informative.
Grossly evident villous hydrops is characterized by cystic structures that resemble clear spheres of a few to several millimeters in width and, when present, require further microscopic examination to exclude hydatidiform mole (see Chapter 30 for more details). When gross villous hydrops is present, or if a hydatidiform mole is clinically or sonographically suspected, flow cytometric or cytogenetic studies of villous tissues should be considered, if technically available. In addition, more extensive histologic sampling of villous and decidual tissues is warranted to distinguish among hydropic abortus and partial and complete hydatidiform moles.
Karyotypic analysis is usually considered in the event of recurrent spontaneous abortion (see below), although with societal factors affecting pregnancy, such as later age at first conception, a clinical request may reasonably be made to karyotype an initial pregnancy loss. If karyotyping is requested, the unfixed specimen should be dissected in a sterile fashion to identify villous tissue grossly; disposable suture removal sets or two sterile blades manipulated as one would use eating utensils work well for this purpose.
The selected sample is subsequently placed into Hanks' solution or another combination antibiotic and balanced salt solution, or sterile saline, and can be refrigerated but not frozen for up to 72 hours to maintain optimal viability for culture and preparation of chromosomes. In the cytogenetics laboratory, the submitted specimen is disaggregated and cultured for several days until a sufficient fraction of cells undergoes mitosis. Mitotic spindle formation is then blocked by adding colcemid (colchicine) to the culture, arresting cells in metaphase. The metaphase chromosomes are spread, stained, counted, and analyzed by their characteristic banding patterns. The pathologist's selection of appropriate material for karyotypic analysis is critical to ensure that the results reflect the chromosomal composition of the gestation and not the mother (i.e., not from decidual tissue). If villous tissue is not apparent to the unaided eye, a dissecting microscope can be useful for grossly identifying small fragments of embryonic or villous tissues (see Fig. 29.14 ). Ideally, samples of uncertain origin should be divided into separate cytogenetic samples and correlated with histology to optimize confirmation of the tissue source. In later gestation POCs, although culture of fetal tissue may be preferred—to exclude cases of confined placental mosaicism—this may not be possible, particularly in cases of prolonged retention of a demised fetus. In such cases, we routinely send fetal and placental (villous) tissues to our cytogenetics laboratory, where an attempt is made to derive cells from both sets of tissues and then proceeds to placental karyotyping only if no cells grow from the fetal tissues.
In addition to karyotyping, recently developed methods allow for the evaluation of genetic aberrations by direct isolation of DNA from fresh and paraffin-embedded fetal or placental tissues. These analyses include a DNA microarray, which can assess for loss or gain of portions of chromosomes, and exome sequencing, which is used for the detection of genetic mutations, including single nucleotide gain or loss. In the post–noninvasive prenatal testing (NIPT) era, many POC specimens are received along with an NIPT-based diagnosis. It is therefore important to keep in mind that even for common aneuploidies such as trisomy 21, NIPT is considered a screening test. Direct testing of fetal tissue is required for confirmation of the diagnosis as well as to rule out a potential rare case of confined placental mosaicism, in which the genetic aberration is confined to the placenta and the fetus is genetically normal. Finally, even these more advanced genetic tests are not meant to replace a thorough pathologic evaluation; rather, results of such tests should always be interpreted in light of gross and histologic findings on pathologic examination.
The incidence of ectopic pregnancies, defined as any implantation of a conceptus outside the uterus or in an abnormal position within the uterus, has dramatically increased. Ectopic pregnancies, which two decades ago occurred once in every 250 pregnancies, have increased in frequency sixfold and have accounted for 1% to 2% of all documented pregnancies in the United States.
Epidemiologic studies have shown that an ectopic pregnancy usually occurs in women who are older, urban dwellers, of African American descent, of lower socioeconomic status, and multiparous. Ectopic pregnancy is the leading cause of maternal death in the first trimester and accounts for 9% to 13% of pregnancy-related deaths. The annual mortality due to ectopic pregnancy has been estimated at 30 to 40 women in the United States and is on the decline.
In a study of 126 consecutive ectopic pregnancies, 35% had a history of infertility, 38% prior fallopian tube operation, 17% history of prior pelvic inflammatory disease, 16% prior ectopic pregnancy, and 13% prior pelvic operation. Importantly, no risk factor was identified in 47% of patients. Table 29.3 summarizes relative risk estimates from a large study. The fallopian tube is the most common site of ectopic implantation, accounting for 97% of all ectopic pregnancies.
| Risk Factor | Specific | Relative Risk |
|---|---|---|
| Prior tubal surgery | Yes | 4.0 |
| Smoking | >20 cigarettes/day | 3.9 |
| H/O STD | With PID | 3.4 |
| Prior SAB | >3 | 3.0 |
| Age | >40 | 2.9 |
| Prior TAB | Medical or surgical | 2.8 |
| H/O infertility | >2 years | 2.7 |
| Appendectomy | Yes or ruptured | 1.4 |
| Prior IUD | Yes | 1.3 |
Fallopian tube factors, which have been associated with ectopic pregnancy, include congenital abnormalities (e.g., diverticula, hypoplasia, accessory ostia), prenatal diethylstilbestrol (DES) exposure, which cause structural abnormalities, salpingitis isthmica nodosa, and tubal neoplasms, which are very rare. Nongranulomatous salpingitis, especially that caused by Chlamydia trachomatis and possibly Mycoplasma hominis, and granulomatous salpingitis, especially treated Mycobacterium tuberculosis, increases the risk of ectopic pregnancy, presumably through postinflammatory scarring and ciliary dysfunction of the lining epithelial cells. In contrast, condom use is associated with a lower incidence of tubal pregnancy. Women who have undergone prior tubal sterilization have an overall lower risk of ectopic pregnancy than the general population. However, postligation pregnancies, when they do occur, are much more likely to be ectopic (10%–15%). Similarly, following tuboplasty to reverse a tubal ligation, 5% to 10% of resulting pregnancies implant in the fallopian tube.
Extratubal factors that have been reported to increase ectopic pregnancy rates include smoking and hormonal alterations, such as ovulatory dysfunction and low-dose, progestin-only oral contraception, presumably by altering epithelial cilia function. About 2% to 10% of assisted reproductive technology (ART)–derived pregnancies implant in the tube, but it is not clear whether this is secondary to exogenous gonadotropin exposure and subsequent hormonal alterations or is a manifestation of the underlying process causing infertility. In contrast, the overall incidence of ectopic pregnancy is not increased in patients using intrauterine devices (IUDs) compared with the nonuser population. However, 4% to 8% of pregnancies that occur when an IUD is in place are ectopic, and 10% of these implant in the ovary, likely reflecting the efficacy of the IUD in preventing intrauterine implantation.
In light of these risk factors, the increased rate of ectopic pregnancy observed in Europe and North America has been ascribed to the early treatment of salpingitis (preserving fertility but leading to tubal scarring rather than complete occlusion), increased use of ART, and wider use of sensitive imaging and laboratory techniques to diagnose early, potentially previously unrecognized, ectopic gestations.
The initial history and physical examination of a patient later found to have an ectopic pregnancy can be misleading, leading to a clinical misdiagnosis. The classic presenting symptoms of pelvic or abdominal pain and vaginal bleeding are not consistently present, especially if the ectopic pregnancy has not yet ruptured. Further obscuring the clinical presentation, fewer than 50% of patients give a history that includes any of the aforementioned ectopic pregnancy risk factors. Additionally, the pelvic examination is often not reliable. Furthermore, a history of vaginally passed tissue does not distinguish a missed intrauterine abortion from a sloughed decidual cast in a patient with an ectopic pregnancy. A low or slowly increasing serum β-hCG level cannot reliably distinguish an abnormal intrauterine from extrauterine pregnancy. Finally, although transvaginal ultrasound can be helpful, the results are often indeterminate. Therefore, for a timely clinical diagnosis, a high index of suspicion is required, particularly when a patient of reproductive age presents with abdominal pain.
When there is a high index of suspicion for an ectopic pregnancy and a positive serum β-hCG level, the diagnostic algorithm entails a transvaginal ultrasonogram, followed by sampling of the endometrium if no intrauterine gestational sac is seen. With the rare exception of heterotopic gestations (coexisting ectopic and intrauterine pregnancy; incidence estimated at ≈1 in 4000 pregnancies), the presence of products of conception in the uterine cavity confirms a failed intrauterine pregnancy and effectively excludes ectopic gestation. When an ectopic pregnancy is confirmed, clinically ( Fig. 29.16 ) or histologically, or an intrauterine pregnancy cannot be confirmed, treatment options include expectant management or methotrexate administration if the patient is stable or surgery.
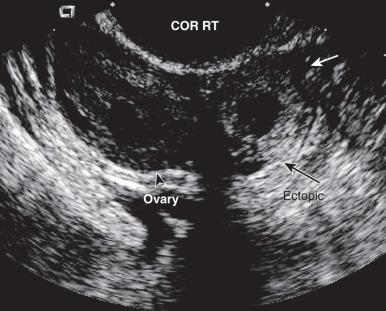
When the pathologist receives the uterine curettings from the clinical service, the first task is to determine if there is a clinical suspicion of ectopic pregnancy. If there is a low index of suspicion, the patient is clinically stable, and there are no plans to intervene surgically, the curettings should be grossly examined, the most promising areas culled into a single cassette, and the entire specimen processed for a rush reading the following day. However, if there is a high index of clinical suspicion of ectopic pregnancy, concerns about the stability of the patient's medical condition, or the specimen cannot be routinely processed and evaluated within 18 hours, the specimen can be evaluated by submission of all tissue for frozen section.
As discussed earlier, careful examination for the presence of immature villi, embryonic tissue, or fresh implantation site should be made histologically. The tissue should first be examined with the naked eye, preferably while floating in a Petri dish of sterile saline, with attention to the fine spongy features of immature villi. If a dissecting microscope is available, it can be used to identify tissues that may have the highest yield (see Fig. 29.14 ). However, at this point in the examination, the pathologist must be aware of the risk of confusing vascular or glandular epithelial structures emanating from fragments of decidua with chorionic villi. Evaluation of the former is particularly treacherous for the novice. For this reason, it is prudent not to declare the presence of chorionic villi without histologic verification. One or more of the following features must be identified histologically in the curettings—fetal parts, chorionic villi, and/or implantation site. It should be emphasized that ectopic gestation can coexist with any form of endometrial histology, ranging from a normal cyclic endometrium to gestational endometrium. In more than 60% of cases, the endometrium responds to hormones generated by the ectopic gestation, developing hypersecretory glands, decidua, or focal Arias-Stella reaction. Thus, observation of an Arias-Stella reaction or decidualized endometrium alone in the curettage specimen is not sufficient to indicate an intrauterine pregnancy. Moreover, as mentioned previously, the absence of hormonal endometrial changes does not exclude ectopic pregnancy.
See Box 29.1 and Figs. 29.17 and 29.18 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here