Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter is a rewritten and updated form of a chapter appearing in a prior edition of this work, written by Dr. Hans-Georg Frank. Portions of the figures and writing remain Dr. Frank’s words. The authors are indebted to Dr. Frank’s contribution to the knowledge of placental structure, histopathology, and development as the scientific community understands it today.
For millennia, the remarkable ability of the fetus to derive nourishment and “breath” from within the womb has been a source of wonder for scientists, philosophers, physicians, artists, religious leaders, and lay men and women. That this capability was related to the placenta—an organ of great significance to the fetus, yet no longer used by the neonate—was acknowledged early on, whether it be as the “seat of the external soul,” the fetus’s “alter ego,” or the fetal equivalent of the liver, lung, and kidney, was evident. Indeed, it has long been certain that the fetus is expert at obtaining sustenance from the mother in the form of “uterine milk” and/or maternal blood, and that the placenta must be a key player in this task.
The gradual elucidation of the physiologic role of the human placenta as we understand it today has progressed in stages commensurate with advances in physiology, cell and molecular biology, chemistry, epidemiology, medicine, and importantly, technology. Central to an understanding of exactly how the placenta functions to support fetal life and growth was the anatomic question of how the maternal and the fetal vasculatures are interrelated: does maternal blood flow freely into the fetus, thereby directly sating the young with the “spirits” and nutrients therein, or does the placenta somehow extract nutrients from maternal blood, and if so, by what means? The question is extraordinarily difficult to answer based on gross anatomic features alone: anatomists and physicians disagreed, sometimes vehemently, on this matter. Not helping the situation was the intermittent legality of dissection of human cadavers in medical schools. This central question was, however, finally resolved in the 18th century when the famous experimentalist William Harvey (1578–1657) found that injection of uterine arteries and veins with red and blue wax, respectively, failed to reveal entry of wax into the umbilical vessels or the fetus; likewise, no wax entered the mother upon injection of the umbilical vessels. Further understanding of placental function was enabled with Malphigi’s (1628–1694) discovery that capillary beds connect arteries and veins, together with the isolation of elemental oxygen and its exchange with carbon dioxide by Priestly (1733–1804) and Lavoisier (1743–1794), respectively.
Following conception and embryo implantation into the uterus, the placenta is the first organ of the embryo and fetus to become fully functional. Together with the extraplacental membranes and umbilical cord, the placenta is derived entirely extrafetally and is remarkably large. As such, it is essential that placental development occurs early and promptly, so that as the metabolic demands of rapid fetal growth increase, the placenta is ready to deliver. The functions of the placenta are many and include selective delivery of nutrients, immunity, and oxygen to the fetus, return of metabolic waste to the mother for excretion, and serving as a barrier to environmental toxins.
This chapter will begin with a description of the gross appearance of the placenta at term and how it is perfused. We will review in detail how this fully functional structure is the culmination of a developmental plan that starts even prior to conception with hormonal priming of the maternal uterus, allowing implantation and differentiation of extraembryonic structures that make up the placenta.
The term placenta is disc-shaped and large, with a mean diameter of ∼220 mm and weight of 470 g in healthy term infants ( Table 8.1 ). It is no wonder that early anatomists faced challenges in elucidating the functional anatomy of the placenta; it can be described on its maternal surface ( basal plate ) as lobular and, inside, spongy, with millions of chorionic villi —the functional units of the placenta—stemming from the chorionic plate (the fetal-facing surface of the placenta). Obvious vascular connections between the villi, chorionic plate, and umbilical cord are discernable at the gross level only in the latter two, as consecutive branching of vessels within the villi becomes ever smaller. Peripherally attached are the extraplacental membranes that, in utero, reflect away from the maternal surface and around the fetus. The membranes attached to the periphery of the placenta consist of a layer of maternal uterine decidual cells fused with the embryo-derived chorion, connective tissue, and amnion. Owing to the distinct embryologic origins of these layers, the amnion and chorion are easily separable; it can be further appreciated at the gross level that they are avascular.
| Preganancy Week (Postconception) | 1–2 | 3–6 | 7–10 | 11–14 | 15–18 | 19–22 | 23–26 | 27–30 | 31–34 | 35–38 |
|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy month (postmenstruation) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Diameter of chorionic sac (mm) | — | 5–33 | 34–65 | 66–99 | — | — | — | — | — | — |
| Placental diameter (mm) | — | — | — | 50–69 | 75–94 | 100–119 | 125–144 | 150–169 | 175–194 | 200–220 |
| Placental weight (g) | — | 6 | 8–26 | 32–60 | 70–112 | 126–180 | 198–252 | 270–324 | 342–396 | 414–470 |
| Placental thickness (postpartum [mm]) | — | — | — | 10–12 | 12–15 | 15–18 | 18–20 | 20–22 | 22–24 | 24–25 |
| Placental thickness, including uterine wall, measured by ultrasound in vivo (mm) | — | — | — | — | 28 | 29–34 | 35–38 | 39–42 | 42–44 | 44–45 |
| Length of the umbilical cord (mm) | 2 | 4–20 | 33–126 | 158–240 | 264–330 | 350–404 | 424–464 | 477–520 | 530–557 | 565–585 |
| Fetal weight (g) per g of placental weight | — | 0.18 | 0.25–0.65 | 0.72–1.00 | 1.29–2.23 | 2.54–3.11 | 3.28–3.97 | 4.19–4.78 | 4.97–5.81 | 6.04–7.23 |
| Villous volume (g) per placenta | — | 5 | 18 | 28 | 63 | 102 | 135 | 191 | 234 | 273 |
| Villous surface (cm 2 ) per g of villous tissue | — | 166 | 168 | 194 | 235 | 275 | 313 | 377 | 432 | 458 |
| Maternofetal diffusion distance (μm) | — | 55.9 | — | 40.2 | 22.4 | 21.6 | — | 20.6 | 11.7 | 4.8 |
| Villous trophoblastic thickness (μm) | — | 18.9 | 19.1–21.6 | — | 11.6 | — | 9.7 | — | 5.2 | 4.1 |
| Fetal vessel lumina per villous volume (%) | — | 2.7 | 3.0–4.0 | 6.0 | 6.3–6.6 | — | 9.1 | — | 21.3 | 28.4 |
The basal surface of the term placenta (basal plate) is characterized by 10 to 40 slightly protruding areas called maternal cotyledons , lobes , or lobules . They are minimally separated from each other by the so-called placental septa ( Fig. 8.1 ). From the chorionic plate at term, 60 to 70 villous stems ( trunci chorii ) arise. Each of these trunks branches into a villous tree, and at least one tree occupies a maternal cotyledon (see Fig. 8.1 ). The superficial cotyledonary borderlines are adjacent, to a large extent, to those of a corresponding group of villous trees.
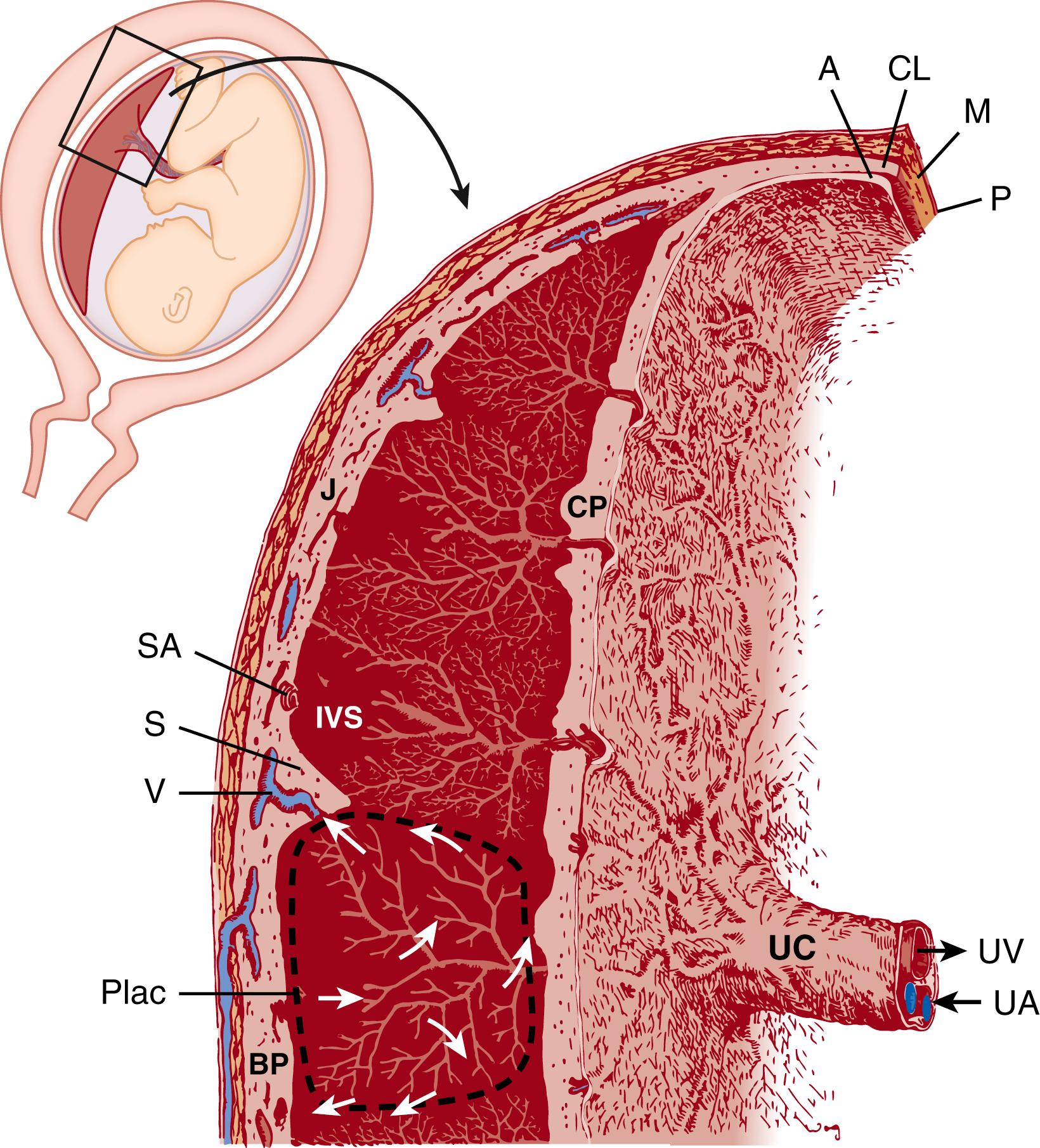
Every maternal cotyledon possesses at least one placentome, that is, the fetomaternal circulatory unit. Each placentome consists of a villous tree and the surrounding intervillous space, which is centrifugally perfused with maternal blood (see Fig. 8.1 ). The centers of typical placentomes exhibit loosely arranged villi that provide a large intervillous space for maternal arterial inflow. Placentomes are separated by narrow intervillous clefts. Near the chorionic plate and at the border of neighboring placentomes, the villous arrangement provides space for the venous backflow toward the venous openings in the basal plate. Supporting the placentome concept, ultrasonographic technologies have measured flow velocities at the spiral artery opening into the intervillous space. Blood flows from typical spiral arteries are described as “jets” that resemble the patterns of flow into the intervillous space that were previously estimated from histologic specimens and simple computational models. ,
Variations within individual villous trees must be kept in mind during histopathologic and functional evaluation of the placenta. The centers of the placentomes act as growth zones of villous trees where new formation and differentiation of villi take place and consist of types of villi called immature intermediate villi (see detailed descriptions below). At term, the centers of the placentome are the remainders of immature villous trees from earlier stages of pregnancy and can be observed in at least some placentomes until term. They may disappear completely only in cases of preterm hypermaturity of the placenta. By contrast, the peripheral portions of placentomes represent the metabolically active area in which fetomaternal exchange occurs.
The gross anatomy described above is the culmination of developmental processes that involve a high level of coordination between maternal and fetal elements. Implantation and placental development are critical to pregnancy success; however, so are the series of coordinated events, controlled initially entirely by the mother, called decidualization . This prepares the superficial layer of the uterus—that is, the endometrium—into a receptive state that will provide nutrition, trophic support, and immune privilege for the implanting embryo and developing fetus. Early descriptions describe the “union” between the decidua and the chorionic villi of the early placenta, such that these layers function as one.
The endometrium undergoes cyclic decidualization, sloughing, and regeneration under the control of the ovarian hormones estrogen and progesterone. Rising concentrations of estrogen produced by ovarian follicles during the proliferative phase of the menstrual cycle stimulate proliferation of endometrial stromal and epithelial cells, and “prime” the uterus by inducing expression of progesterone receptors in these cells. Following ovulation, progesterone produced by the corpus luteum dominates the endocrine environment, and under its influence the endometrial stromal cells stop proliferating, and instead differentiate. This results in formation of the predecidua late in the menstrual cycle, about 13 days after ovulation. To this end, stromal cells enlarge, accumulating glycogen, glycoproteins, and lipids in their cytoplasm; prolactin and insulin-like growth factor binding protein (IGFBP)-1 also become strongly expressed. These changes are accompanied by increased vascular permeability and leukocyte infiltration; uterine natural killer (uNK) and macrophage cells become particularly abundant, the former eventually comprising about 40% of the total cells of the decidua.
Uterine NK cells will be critical for vascular remodeling at the maternal-fetal interface (discussed in detail below), an event that commences during the first trimester and is complete by the early second trimester of pregnancy. Because these cells recognize human leukocyte antigen (HLA)-C on placental trophoblast cells, they are also believed to play a role in allorecognition of the fetus. Macrophages also accumulate in the decidua and are believed to aid in trophoblast invasion and remodeling of the maternal-fetal interface. Additional changes important to creating an immune-privileged site for the fetus occur: decidual genes for T-cell chemoattractants are epigenetically silenced, and trafficking of dendritic cells between the uterus and draining lymph nodes is blocked.
While decidualization commences with the rise of ovarian progesterone independently of pregnancy, embryo implantation completes the process. This is due to multiple factors produced by the embryo, including interleukin (IL)-1β, cyclooxygenase-2, and matrix metalloproteinases (MMP), all of which promote the functional and morphologic differentiation of endometrial stromal cells. Critically, the decidual endometrial glands begin production of several cytokines, including IL-11 and leukemia inhibiting factor (LIF), which act to bring the uterus into a receptive state, to complete decidualization, and later, to play an indispensable role in allowing embryonic attachment and implantation. ,
As these changes occur, the decidua becomes receptive, but only transiently, to the implanting embryo. During this critical window of implantation, the uterus and embryo are synchronized in maturation and development; lack of synchronicity results in implantation failure. During the implantation window, the embryo becomes apposed to the luminal epithelium, to which a loose, then stronger, adherence is formed; finally, the embryo penetrates the epithelium and invades the stroma. In women, this occurs around days 20 to 24 of the menstrual cycle, or 6 to 10 days after ovulation.
Implantation constitutes the first contact between the developing blastocyst and the uterine mucosa; it begins around 6 to 7 days after conception ( Fig. 8.2A ). At this contact zone, the placenta develops rapidly and continuously during the course of pregnancy, and from just after implantation the placenta controls fetomaternal exchange of nutrients, gasses, and waste products. While this exchange is common to all placentas, the gross and microanatomic structure, patterns of maternal-fetal interdigitation, and degree of integration of the fetal and maternal layers constituting the placenta vary considerably between mammalian species. The human placenta is of the most invasive type characteristic of those species with interstitial implantation—that is, that in which the blastocyst burrows completely beneath the surface of the uterine epithelium. Placental trophoblast cells invade as far as the myometrium, eroding maternal tissue so extensively that they become completely surrounded by, and in direct contact with, maternal blood. This type of placentation is called hemochorial .
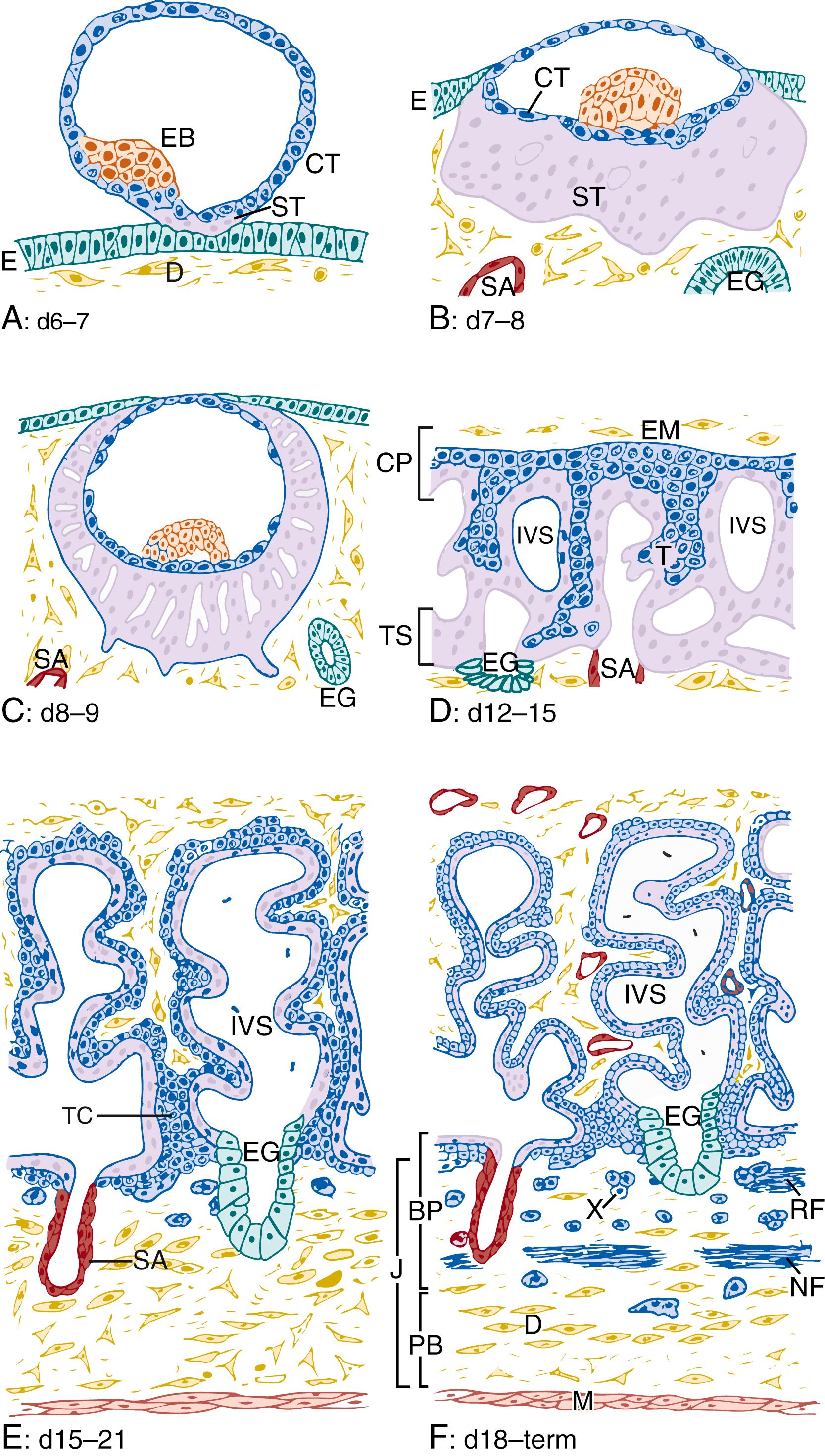
Prior to implantation, the blastocyst consists of a single outer layer of epithelium, called the trophectoderm , and a cluster of inner cells called the embryoblast (see Fig. 8.2A ). The trophectoderm is the direct precursor of all the trophoblast cells in the placenta, and ultimately forms the interface between maternal cells, maternal blood, and fetal cells. The embryoblast, on the other hand, contributes placental vasculature and surrounding mesenchyme, , interior to the trophoblast layers.
The trophectoderm consists of a single layer of trophoblast cells, more specifically called cytotrophoblast cells . At implantation, cytotrophoblast cells at the embryonic pole of the blastocyst adhere to the uterine epithelium, proliferate, and the outermost cells begin to fuse with each other (see Fig. 8.2A ). This process results in a nascent multinucleated syncytium that, unlike the syncytium of the later chorionic villi, functions as an invasive structure, coordinating the entry of the blastocyst into the decidual stroma. Continued proliferation of the inner trophoblast cells, together with subsequent fusion of daughter cells with the overlying syncytium, is responsible for rapid and enormous increase in volume of the syncytiotrophoblast mass (prelacunar period; see Fig. 8.2B ). As the blastocyst becomes more deeply embedded into the decidua, the syncytiotrophoblastic mass rapidly spreads along the outer walls of the blastocyst. Through this action, the blastocyst becomes completely encased within the decidual stroma, below the uterine surface epithelium (see Fig. 8.2C ).
The lacunar period of placental development (day 8 to day 13 after conception) begins with the appearance of a system of vacuoles within the syncytiotrophoblastic mass (see Fig. 8.2C ). The syncytium erodes maternal capillaries and endometrial glands. As a result, lacunae fill with maternal cell–free blood components and glandular secretions, which together provide histiotrophic nutritional support of the embryo. While it is difficult or impossible to precisely elucidate the precise mechanisms by which the early syncytium coordinates implantation and invasion studies using human embryonic stem cells suggest that these cells do so by production of large quantities of MMPs, tissue inhibitors of matrix metalloproteinases (TIMPs), and through production of its own matrix proteins. Further, these cells likely provide nutritive support to the embryo through transporters that carry ions, water, sugars, amino acids, and lipids across cellular membranes.
The areas of syncytiotrophoblast surrounding the lacunae form trabeculae, and this system of trabeculae and lacunae constitute the antecedents to the primary chorionic villi. Around day 12 after conception, proliferating cytotrophoblast cells start to push into the syncytial trabeculae toward the outermost trophoblast (commonly referred to as the trophoblastic shell) (see Fig. 8.2D ). This expansion of cytotrophoblast accounts for longitudinal growth and branching of the trabeculae. Branches that end blindly and protrude into the lacunae are the primary villi (see Fig. 8.2D ), while the trabeculae form anchoring villi , which connect the villus with the trophoblastic shell and not long afterwards, the decidua. With the appearance of the first primary villi, which remain encased by syncytium, the still-expanding lacunar system is called the intervillous space .
Around day 14 after conception, extraembryonic mesodermal cells grow out and migrate from the embryoblast and form a loose connective tissue layer above the primary chorionic plate.
These cells begin to spread out from the embryonic disk along the inner trophoblastic surface of the blastocyst cavity, forming a loose network of branching cells, the extraembryonic mesenchyme. This movement of cells thus adds another layer to the primary chorionic plate, which now consists of three layers: (1) the newly added extraembryonic mesoderm, (2) a middle layer of cytotrophoblast, and (3) an outer layer of syncytiotrophoblast facing the intervillous space (see Fig. 8.2D and E ).
Between days 15 and 20 after conception, cells of the expanding extraembryonic mesenchyme push into the center of the primary villi, establishing a connective tissue core inside the villi, which were formerly purely trophoblastic. This mesenchymal core establishes them as secondary villi. This mesenchyme never fully reaches the trophoblastic shell: the segments of the anchoring villi that connect them to the trophoblastic shell remain wholly trophoblastic. These trophoblastic segments are called the trophoblast cell columns and consist of a voluminous core of proliferating cytotrophoblast cells and an incomplete and interrupted syncytial cover (see Fig. 8.2E ). The column cytotrophoblast cells are the main source for longitudinal growth of the anchoring villi. In addition, these cells are the source of invasion: upon contact with the maternal uterine decidua, the trophoblast cells further differentiate and penetrate deeply into the uterus, forming an admixture of maternal and fetal cellular components—the so-called junctional zone (see later section, “Extravillous Trophoblast Invasion”).
As the placenta continues along its developmental program, the mesenchymal cells constituting the core of the villi give rise to several cell types. In addition to forming fibroblasts, which supply much of the connective tissue of the villous cores, some cells differentiate into macrophages (Hofbauer cells) (see later in this chapter). Others form cords of hemangioblastic cells underlying the trophoblastic epithelium. These endothelial precursors connect via desmosomes or tight junctions and gradually form lumina and vessels. Around day 20 after conception, the first fetal capillaries become apparent, a change that marks the transition from secondary into tertiary villi (see Fig. 8.2F ). The villous endothelial cells are of the non-fenestrated type and are linked by junctional complexes including both tight and adherens junctions, suggesting that in early gestation capillaries are highly plastic, permeable, and easily remodeled. Thus, tertiary villi are comprised of the trophoblastic epithelial layers, mesenchyme, and vascular networks: all the basic constituents of the placental barrier.
Concomitantly with the formation of villous capillaries, the vascularized allantois, which arises from embryonic hindgut, comes into contact and fuses with the mesenchyme of the chorionic plate. Allantoic vessels rapidly grow out over the chorionic plate—that is, the fetal side of the placenta. With further branching and growth, these vessels grow into the villi and anastomose with the locally spreading networks of intravillous capillaries. Complete by around the fifth week after conception, these events establish the fetoplacental circulation. In newly formed villous capillaries, continued hematopoiesis can be observed even after this stage.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here