Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In biology, pigments are defined as substances occurring in living matter which absorb visible light (electromagnetic energy within a narrow band which lies approximately between 400 and 800 nm). The various pigments may greatly differ in origin, chemical constitution and biological significance. They can be either organic or inorganic compounds which remain insoluble in most solvents. Minerals are naturally occurring homogeneous, inorganic substances having a definite chemical composition and characteristic crystalline structure, color and hardness. In biology they are necessary for growth and development.
Endogenous pigments
These substances are produced either within tissues and serve a physiological function, or are by-products of normal metabolic processes. They can be further subdivided into:
Hematogenous (blood-derived) pigments
Non-hematogenous pigments
Endogenous minerals.
Artifact pigments
These are deposits of artifactually produced material caused by the interactions between certain tissue components and some chemical substances, such as the fixative formalin. Formalin and malaria pigments are sometimes classified as a subdivision of endogenous pigments.
Exogenous pigments and minerals
These substances gain access to the body accidentally through a variety of methods e.g. carbon anthracotic pigment, which is seen in lung and lymph nodes. These pigments and minerals serve no physiological function. Entry is gained either by inhalation into the lungs or by implantation e.g. tattoos, into the skin. Most exogenous pigments are minerals, few of which are pigmented.
The above classification, although not scientifically precise, is used as a convenient aid in the identification of pigments. It is important to bear in mind that the same pigment may present itself in tissue sections in a variety of ways. Iron, for example, may be present as an endogenous pigment in liver sections in an iron overload condition, and as an exogenous pigment in the case of a shrapnel wound/prosthetic implantation. It is advisable to note a pigment’s morphology, tissue site and relevant clinical data before carrying out the various stains and histochemical reactions available in order that a pigment can be identified.
This group contains the following blood-derived pigments:
Hemosiderins
Hemoglobin
Bile pigments
Porphyrins.
These pigments are seen as yellow to brown granules and normally appear intracellularly. They contain iron in the form of ferric hydroxide which is bound to a protein framework and is unmasked by various chemicals. Iron is a vital component of the human body as it is an essential constituent of the oxygen-carrying hemoglobin found in red blood cells, where 60% of the body’s total iron content resides. It also occurs in myoglobin and certain enzymes, such as cytochrome oxidase and the peroxidases.
Dietary iron is absorbed in the small intestine and attached to a protein molecule for transfer to the sites in the body where it is to be utilized or stored. Approximately 30% is stored within the reticuloendothelial system, especially the bone marrow. The bone marrow is the main site of iron utilization in the body and where it is incorporated into the hemoglobin molecule during red cell formation. The normal breakdown of worn-out red cells results in the release of iron which is recirculated back into the various areas of iron storage for further utilization. Under normal conditions, this efficient system of recycling usually means that iron deficiency rarely occurs. There is a minimal loss of iron by the way of epithelial desquamation, hair loss and sweating. The main reason for loss of iron from the body is hemorrhage in the form of either chronic bleeding (e.g. a peptic ulcer or bowel neoplasia), or in the female by menstruation, with approximately 25% of females being iron deficient. The small intestine normally only absorbs sufficient iron from the excess iron in the diet to counteract any losses, but in excessive blood loss the dietary content may be relatively inadequate for the need, and even though the absorption mechanism is working at full efficiency, a state of clinical iron deficiency occurs. In iron deficiency, the iron stores in the bone marrow become depleted, insufficient hemoglobin is produced because of the lack of iron, and anemia develops in which the red cells contain diminished amounts of hemoglobin. The iron deficiency is characteristically demonstrated by the absence of stainable iron in the bone marrow.
There is no active method of iron excretion from the body. Iron excess is a much less common condition; under normal conditions the intestine will not absorb iron from the diet when there is already a surplus within the body. However, this controlling mechanism may be bypassed when iron is given therapeutically, such as in the form of either iron injections or a blood transfusion. If excess iron is given this way, the iron stores may become overloaded, and excessive amounts of hemosiderin may be deposited in the organs with a prominent reticuloendothelial component e.g. spleen, bone marrow or liver. This condition is called hemosiderosis . A rarer cause of iron overload is the genetic disease hemochromatosis , in which the controlling mechanism at the small intestine absorption stage becomes impaired, and the iron is absorbed indiscriminately in amounts irrelevant to the state of the body’s iron stores. In this disorder, large quantities of hemosiderin are deposited in many of the organs, often interfering with those organs’ structure and function.
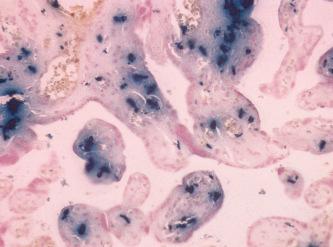
In unfixed tissue, hemosiderin is insoluble in alkalis but freely soluble in strong acid solutions. After fixation in formalin, it is slowly soluble in dilute acids, especially oxalic acid. Fixatives which contain acids but no formalin can remove hemosiderin or alter it in such a way that reactions for iron are negative. Certain types of iron found in tissues are not demonstrable using traditional techniques. This is because the iron is tightly bound within a protein complex. Both hemoglobin and myoglobin are examples of such protein complexes and, if treated with hydrogen peroxide (100 vol), the iron is released and can then be demonstrated using Perls’ Prussian blue reaction ( Fig. 14.1 ). A similar result is obtained if the acid ferrocyanide solution is heated to 60°C in a water bath, oven or microwave oven. However, the use of heat will sometimes cause a fine, diffuse, blue precipitate to form on both the tissue section and slide. This precipitate will not occur when the slides are stained at room temperature. Metallic iron deposits, or inert iron oxide seen in tissues because of industrial exposure, are not positive when treated with acid ferrocyanide solutions. As a consequence of the tissue response, various mechanisms release some of the iron in a demonstrable form, and such deposits are almost invariably surrounded by hemosiderin. In almost all the instances where demonstrable iron appears in tissues, it does so in the form of a ferric salt. On those rare occasions when iron appears in its reduced state as the ferrous salt, then method may be used to achieve the Turnbull’s blue reaction to visualize its presence in tissue sections ( Fig. 14.2 ).
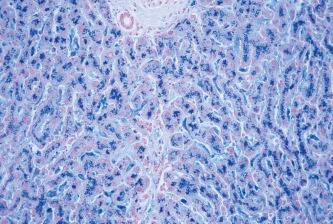
An interesting and sometimes useful modification of a serum iron technique was introduced by to demonstrate both ferrous and ferric iron in tissue sections. This method was claimed to be a more sensitive demonstration for the detection of both ferric and ferrous salts, but has not succeeded in replacing the more traditional method for the demonstration of iron. The method uses bathophenanthroline and the resultant color of any iron present in tissues is bright red.
This method is considered to be the first classical histochemical reaction. Treatment with an acid ferrocyanide solution will result in the unmasking of ferric iron in the form of the hydroxide, Fe(OH) 3 . The ferric iron then reacts with a dilute potassium ferrocyanide solution to produce an insoluble blue compound, ferric ferrocyanide (Prussian blue).
Avoid the use of acid fixatives. Chromates will also interfere with the preservation of iron.
Works well on all types of section, including resin.
| 1% aqueous potassium ferrocyanide | 20 ml |
| 2% aqueous hydrochloric acid | 20 ml |
Preferably freshly prepared, mix just before use.
Take a test and control section to water.
Treat sections with the freshly prepared acid ferrocyanide solution for 10–30 minutes (see Note a below).
Wash well in distilled water.
Lightly stain the nuclei with 0.5% aqueous neutral red or 0.1% nuclear fast red.
Wash rapidly in distilled water.
Dehydrate, clear and mount in synthetic resin.
| Ferric iron | blue |
| Nuclei | red |
Depending on the amount of ferric iron present, it may be necessary to vary the staining times.
Some laboratories keep the two stock solutions made up separately and stored in the refrigerator. The two solutions must not be stored for prolonged periods; this precaution will ensure that the solutions retain their viability.
It is essential that a positive control is used with all test sections. The choice of material which is most suitable as a control specimen is important. A useful control would be postmortem lung tissue which contains a reasonable number of iron-positive macrophages (heart failure cells). Freshly formed deposits of iron may be dissolved in the hydrochloric acid.
Avoid the use of acid fixatives. Chromates will also interfere with the preservation of iron.
Paraffin wax, frozen and resin.
Take test and control sections to distilled water.
Dissolve 400 mg of potassium ferrocyanide in 40 ml of 0.5% hydrochloric acid when testing for ferric iron. For testing ferrous iron, substitute 400 mg of potassium ferricyanide. Prepare just before use. Expose sections for 30 minutes.
Wash well in distilled water.
Stain nuclei with 0.1% aqueous nuclear fast red for 5 minutes.
Rinse in distilled water.
Dehydrate, clear, and mount in synthetic resin.
| Ferric iron | dark Prussian blue |
| Ferrous iron | dark Turnbull’s blue |
| Nuclei | red |
Not critical but avoid prolonged exposure in acid fixatives.
All types of tissue section may be used including resin.
| Bathophenanthroline (4, 7-diphenyl-1, 10-phenanthroline) | 100 mg |
| 3% aqueous acetic acid | 100 ml |
Place together in oven at 60°C for 24 hours, agitating at regular intervals. Cool to room temperature and filter. This solution is stable for about 4 weeks. Before use, add thioglycolic acid to a concentration of 0.5% (this should be replenished each time before use as it rapidly undergoes oxidation on exposure to air).
Take test and control sections to distilled water.
Stain sections in bathophenanthroline solution for 2 hours at room temperature.
Rinse well in distilled water.
Counterstain in 0.5% aqueous methylene blue for 2 minutes.
Rinse well in distilled water.
Stand slides on end until completely dry.
Dip slides in xylene and mount in synthetic resin.
| Ferrous iron | red |
| Nuclei | blue |
It is important that the bathophenanthroline is completely dissolved prior to use.
Dehydration with alcohol will remove the resultant red coloration.
Hemoglobin is a basic conjugated protein which is responsible for the transportation of oxygen and carbon dioxide within the bloodstream. It is composed of a colorless protein, globin, and a red pigmented component, heme. Four molecules of heme are attached to each molecule of globin.
Heme is composed of protoporphyrin, a substance built up from pyrrole rings and combined with ferrous iron. Histochemical demonstration of the ferrous iron is possible only if the close binding in the heme molecules is cleaved. This can be achieved by treatment with hydrogen peroxide, but this has no practical use. As hemoglobin appears normally within red blood cells its histological demonstration is not usually necessary. The need to demonstrate the pigment may arise in certain pathological conditions e.g. casts in the lumen of renal tubules in cases of hemoglobinuria or active glomerulonephritis.
Two types of demonstration method can be used to stain hemoglobin in tissue sections. The first demonstrates the enzyme, hemoglobin peroxidase, which is reasonably stable and withstands short fixation and paraffin processing. This peroxidase activity was originally demonstrated by the benzidine-nitroprusside methods, but because of the carcinogenicity of benzidine these methods are not recommended and are no longer used. introduced the patent blue method which was later modified by ( Fig. 14.3 ). Tinctorial methods have also been used for the demonstration of hemoglobin; the amido black technique ( ) and the kiton red-almond green technique ( ) are worth noting.
Formalin is best; poor preservation with Heidenhain’s Susa has been noted by .
| 1% aqueous patent blue V (CI 42045) | 25 ml |
| Powdered zinc | 2.5 g |
| Glacial acetic acid | 0.5 ml |
Mix well on a magnetic stirrer. The solution will become pale green-blue. Filter and store in a refrigerator at 3–6°C. The solution is stable for about 1 week.
| Stock solution | 10 ml |
| Glacial acetic acid | 2 ml |
| 3% hydrogen peroxide | 1 ml |
Prepare immediately before use.
Take test and control sections to distilled water.
Stain in patent blue solution for 5 minutes at room temperature.
Rinse in distilled water.
Lightly counterstain in 0.5% aqueous neutral red or 0.1% aqueous nuclear fast red for 1 minute.
Rinse in distilled water.
Dehydrate, clear, and mount in synthetic resin.
| Hemoglobin peroxidase (red blood cells and neutrophils) | dark blue |
| Nuclei | red |
Experience has shown that the hemoglobin demonstrated by this method tends to be more of a green-blue color.
In some instances, if the staining solution is left on the section for too long, it may start to decolor a positive reaction.
Fixation in excess of 36 hours may give rise to unreliable results.
This method demonstrates peroxidase activity, including the peroxidases in other blood cells, particularly in the lysosomes of polymorphonuclear leukocytes and their precursors. Tissue peroxidases are also demonstrated.
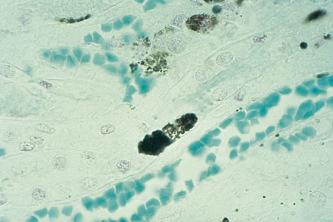
Red blood cells are broken down in the reticuloendothelial system when they have reached the end of their useful life, usually after 120 days. Hemoglobin is released after the red cell membrane has been ruptured. The protein, globin and iron components are released for recycling within the body after the hemoglobin has been broken down. After the heme portion has been split from the globin, the tetrapyrrole ring of the heme molecule is cleaved and opened out into a chain composed of four linked pyrrole groups. With the opening out of the tetrapyrrole ring, the iron component is removed to be stored in those tissues which specialize in iron storage. This iron component is now free to be incorporated into the hemoglobin molecule during red cell formation. The opened tetrapyrrole ring, which has had its iron component removed, is known as biliverdin. This residue is formed in the phagocytic cells which populate the reticuloendothelial system, particularly the bone marrow and spleen. Biliverdin is transported to the liver, where it is reduced to form bilirubin. In this form, the bilirubin is insoluble in water, but after conjugation with glucuronic acid it forms a water-soluble compound, bilirubin-glucuronide. This process takes place in liver hepatocytes due to the activity of the enzyme glucuronyl transferase. The conjugated bilirubin passes from the hepatocytes into the bile canaliculi, and then via the hepatic ducts into the gallbladder which acts as a reservoir. The bilirubin passes along the common bile duct to be released into the duodenum via the ampulla of Vater.
The term bile pigments used by many authors when discussing the various (generic) staining techniques can be applied to all bile pigments. In using this terminology, it is implied that all bile pigments react in an identical manner, but this is not the case. Contained within the group ‘bile pigments’ are both conjugated and unconjugated bilirubin, biliverdin, and hematoidin, all of which are chemically distinct and show different physical properties, particularly with regard to their solubility in water and alcohol. Microscopic examination of any liver section which contains ‘bile pigments’ will almost certainly reveal a mixture of biliverdin and both conjugated and unconjugated bilirubin. This is particularly likely when the liver contains an excess of bile pigments, either through bile duct obstruction, e.g. due to a stone or tumor, an abnormality of biliverdin-bilirubin metabolism in the rare congenital enzyme disorders, or where there is extensive liver cell death or degeneration. The non-specific term bile will be used to include biliverdin and both conjugated and unconjugated bilirubin in the following text.
In a hematoxylin and eosin (H&E) stained section of liver, bile, if present, is most commonly seen in the hepatocytes in the early stages as small yellow-brown globules and then subsequently within the bile canaliculi as larger, smooth, round-ended rods or globules commonly referred to as bile thrombi . The latter, if present, in liver sections is a histopathological indication that the patient has obstructive jaundice due to a blockage in the normal flow of bile from the liver into the gallbladder and subsequently into the bowel, probably because of gallstones or a carcinoma of the head of pancreas. Masses of bile in the canaliculi of liver sections are easily distinguished microscopically because of their characteristic morphology and their situation. Bile in hepatocytes must be distinguished from the lipofuscins which are also commonly seen within these cells and can appear as small yellow-brown globules. The need to distinguish between bile and lipofuscin in hepatocytes is particularly important in liver biopsies taken from liver transplant patients where sepsis is suspected. Bile is difficult to identify in the sections of normal liver. It is important to note that both bile and lipofuscin can be positive with Schmorl’s ferric ferricyanide reduction test ( ). Bile is also seen in H&E-stained sections in the gallbladder where it can appear as amorphous, yellow-brown masses adherent to the mucosa or included as yellow-brown globules within the epithelial-lined Aschoff-Rokitansky sinuses in the gallbladder. Bile is also present, together with cholesterol, in gallstones.
first described extracellular yellow-brown crystals and amorphous masses within old hemorrhagic areas, which he called hematoidin. reviewed the histochemistry of bile pigments. Microscopically, hematoidin frequently appears as a bright yellow pigment in old splenic infarcts, where it contrasts well against the pale gray of the infarcted tissue. Hematoidin can also be found in old hemorrhagic areas in the brain. Bearing in mind the differences discussed above, it is almost certain that hematoidin is related to both bilirubin and biliverdin, even though it differs from them both morphologically and chemically. It is thought that heme has undergone a chemical change within these areas which has led to it being trapped, thus preventing it from being transported to the liver to be processed into bilirubin.
The need to identify bile pigments arises mainly in the histological examination of the liver, where distinguishing bile pigment from lipofuscin may be of significant importance. Both appear yellow-brown in H&E-stained paraffin wax sections, and it is worth remembering that the green color of biliverdin is often masked by eosin. In such cases, unstained paraffin wax or frozen sections, lightly counterstained with a suitable hematoxylin (e.g. Mayer’s), will prove of value. Bile pigments are not autofluorescent and fail to rotate the plane of polarized light (monorefringent), whereas lipofuscin is autofluorescent. The most commonly used routine method for the demonstration of bile pigments is the modified Fouchet technique ( ), in which the pigment is converted to the green color of biliverdin and blue cholecyanin by the oxidative action of the ferric chloride in the presence of trichloroacetic acid ( Fig. 14.4 ). The Fouchet technique is quick and simple to carry out, and when counterstained with van Gieson’s solution the green color is accentuated.
Any fixative appears suitable.
Any.
| 25% aqueous trichloroacetic acid | 36 ml |
| 10% aqueous ferric chloride | 4 ml |
Freshly prepared before use.
Dissolve 100 mg of acid fuchsin (CI 42685) in 100 ml of saturated aqueous picric acid (see Note c below).
Take test and control sections to distilled water.
Treat with the freshly prepared Fouchet’s solution for 10 minutes.
Wash well in running tap water for 1 minute.
Rinse in distilled water.
Counterstain with van Gieson solution for 2 minutes.
Dehydrate, clear and mount in synthetic resin.
| Bile pigments | emerald to blue-green |
| Muscle | yellow |
| Collagen | red |
Two control sections are stained with the test section, one stained with Fouchet’s reagent and van Gieson, and the other with Fouchet’s reagent alone.
Although the solutions used in Fouchet’s reagent have a reasonable shelf life, experience has shown that a freshly prepared solution gives a more reliable result.
Bile which may be present in situations outside the liver such as that seen in the Aschoff-Rokitansky sinuses or in hemorrhagic and infarcted areas is likely to show no color change with this method. This type of pigment can be shown using the Gmelin (see below) or the Stein technique. Sirius Red F3B (CI 35780) may be substituted for acid fuchsin. Bile is a reducing substance and therefore will stain with the Masson-Fontana and Schmorl techniques.
This technique is the only method which shows an identical result with liver bile, gallbladder bile and hematoidin. The method tends to be messy, capricious, and gives impermanent results. Deparaffinized sections of tissue containing bile pigments are treated with nitric acid, and a changing color spectrum is produced. It can be unreliable and so it is advisable to repeat the test at least three times before a negative result is acceptable. A popular modification of this technique is that of , in which bromine in carbon tetrachloride is used as an oxidant.
Paraffin wax embedded.
Take sections to distilled water and mount in distilled water.
Place mounted section under the microscope using an objective with reasonable working distance.
Place 2–3 drops of concentrated nitric acid to one side of the coverglass and draw under the coverglass by means of a piece of blotting paper on the opposite side.
Remove excess solution and observe pigment for color changes.
Bile pigments will gradually produce the following spectrum of color change: yellow-green-blue-purple-red.
This method is impermanent, thus preventing storage of sections.
The reaction can occur rapidly, but by using a 50–70% solution of nitric acid it can be slowed down.
Sulfuric acid can also be used in this method.
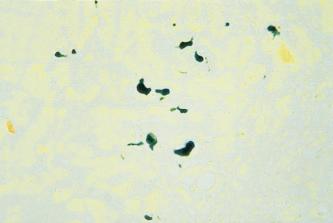
Oxidation methods aim to demonstrate bilirubin by converting it to green biliverdin. In practice they fail to produce the bright blue-green color seen in the more popular Fouchet technique and tend to be a dull olive green color. These oxidation methods are of little value in routine surgical pathology and are rarely used. Another group of methods which have been used to demonstrate bile pigments are the diazo methods which are based on a well-known technique previously used in chemical pathology, namely the van den Burgh test for bilirubin in blood. The method is based on the reaction between bilirubin and diazotized sulfanilic acid. modified the method for use on cryostat sections but the reagents are complex to make up and section loss may be high, therefore, its use is limited.
These substances normally occur in tissues in only small amounts. They are considered to be precursors of the heme portion of hemoglobin. The porphyrias are rare pathological conditions which are disorders of the biosynthesis of porphyrins and heme.
In erythropoietic protoporphyria, porphyrin pigment can be seen as focal deposits in liver sections. The pigment appears as a dense, dark brown pigment and in fresh frozen sections exhibits a brilliant red fluorescence which rapidly fades with exposure to ultraviolet light. The pigment, when seen in paraffin wax sections and viewed using polarized light, appears bright red in color with a centrally located, dark Maltese cross.
This group contains the following:
Melanins
Lipofuscins
Chromaffin
Pseudomelanosis (melanosis coli)
Dubin-Johnson pigment
Ceroid-type lipofuscins
Hamazaki-Weisenberg bodies.
Melanins are a group of pigments whose color varies from light brown to black. The pigment is normally found in the skin, eye, substantia nigra of the brain and hair follicles (a fuller account of these sites is given later). Under pathological conditions, it is found in benign nevus cell tumors and malignant melanomas. The chemical structure of the melanins is complex and varies from one type to another. Melanin production is not fully understood but the generally accepted view is that melanins are produced from tyrosine by the action of an enzyme tyrosinase (DOPA oxidase). This enzyme acts on the tyrosine slowly to produce the substance known as DOPA (dihydroxyphenylalanine) which is subsequently rapidly acted upon by the same enzyme to produce an intermediate pigment which then polymerizes to produce melanin. The later stages of melanogenesis remain largely speculative, and it is beyond the scope of this chapter to evaluate the many studies relating to melanin biosynthesis which have been carried out recently. gives a more detailed account of melanin production.
The melanins are bound to proteins, and these complexes are localized in the cytoplasm of cells within so-called ‘melanin granules’. described these granules as the end stage of the development of the melanosome as seen at ultrastructural level.
There are four recognized stages of melanosome maturation:
Tyrosine is synthesized in the Golgi lamellae and pinched off into vesicles with no melanin present.
The characteristic lattice-like appearance becomes evident at this stage.
Melanin deposition is first observed.
The fully mature granule has its structure obscured by melanin pigment.
Ultrastructurally the lamellar structures become increasingly difficult to see following melanin deposition. By the time the melanosome reaches stage 4 the lamellae structures are completely obscured ( Fig. 14.5 ).
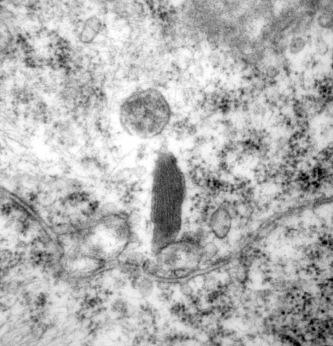
The enzyme tyrosinase cannot be demonstrated in the mature granule.
Skin , where it is produced by cells called melanocytes which are usually scattered within the basal layer of the epidermis. In certain inflammatory skin diseases melanin may also be found in phagocytic cells (‘melanophages’) in the upper dermis. The melanophages may also phagocytize other material such as lipofuscins and lipoproteins, thus producing a mixture which after denaturation may give unexpected staining results. Pathological deposition of melanin occurs in a benign lesion called a nevus or ‘mole’. The malignant counterpart to the nevus is the malignant melanoma; it is in the diagnosis of this important tumor and its metastases that histological demonstration of melanin finds its most important practical application ( Fig. 14.5 ). Melanin is also found in the hair follicles of dark-haired people.
Eye , where it is found normally in the choroid, ciliary body and iris. Similar brown-black pigment is found in the retinal epithelium but its identity with melanin is uncertain. Melanomas can occur in the eye but these tumors are rare.
Brain , where it is found particularly in the substantia nigra in such quantities that this structure is macroscopically visible as a black streak on both sides of the mesencephalon. In patients with long-standing Parkinson’s disease this area is markedly reduced. Black melanin is also found in patches in the arachnoid covering some human brains, and has been described as having a ‘sooty’ appearance.
A number of methods can be used for the identification of melanin and melanin-producing cells. The most reliable of these are:
Reducing methods such as the Masson-Fontana silver technique and Schmorl’s ferric-ferricyanide reduction test.
Enzyme methods e.g. DOPA reaction.
Solubility and bleaching methods.
Fluorescent methods.
Immunohistochemistry.
Melanin and its precursors are capable of reducing both silver and acid ferricyanide solutions. It also shows the marked physical property of being completely insoluble in most organic solvents which is almost certainly due to the fact that formed melanin is tightly bound to protein within the melanosome. The other physical characteristic shown by melanin is its ability to be bleached by strong oxidizing agents. This property is particularly useful when trying to identify nuclear detail in heavily pigmented melanocytic tumors. Further reference to these procedures will follow the conventional demonstration techniques for melanin. These two physical characteristics relate to formed melanin and not to melanin precursors.
The enzyme tyrosinase can be demonstrated by the DOPA reaction and is demonstrable in any cell capable of synthesizing melanin. Cells which have produced an abundance of melanin, and in which the melanosomes are filled with pigment, are said to no longer show tyrosinase activity, but some workers have found that tyrosinase is active in most cells, even though melanin may be present in large quantities.
The fluorescent method depends upon the ability of certain biogenic amines, including DOPA and dopamine, to show fluorescence after exposure to formaldehyde (formalin-induced fluorescence). This method therefore demonstrates melanin precursors rather than formed melanin. Recent advances in antibody production have produced a wide range of antibodies which recognize antigens in the melanin synthesis pathway, e.g. tyrosinase or tyrosinase related protein 1 and 2 (TRP 1 and 2), or those recognizing melanocyte activation antigens, e.g. gp100 (HMB 45) and Mart-1 (Melan A). The use of enzyme histochemical procedures is now rarely required.
Melanin is a powerful reducing agent and this property is used to demonstrate melanin in the following ways:
The reduction of ammoniacal silver solutions to form metallic silver without the use of an extraneous reducer is known as the argentaffin reaction. method (using Fontana’s silver solution) and its various modifications, which also rely on melanin’s argentaffin properties, are now widely used for routine purposes. Melanins are blackened by acid silver nitrate solutions. Melanin is also argyrophilic, meaning that melanin is colored black by silver impregnation methods which use an extraneous reducer ( Fig. 14.6 ). This is not a property considered to be of diagnostic value.
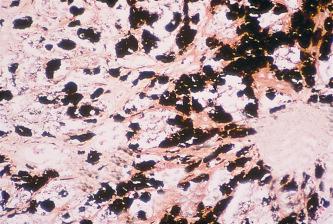
Melanin will reduce ferricyanide to ferrocyanide with the production of Prussian blue in the presence of ferric salts (the Schmorl reaction). This type of reaction ( Fig. 14.7 ) is also seen with certain other pigments e.g. some lipofuscins, bile and neuroendocrine cell granules.
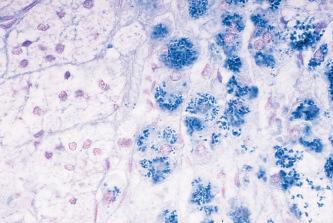
Other reducing methods for demonstrating melanin are Lillie’s ferrous ion uptake (described in ) and Lillie’s Nile blue A ( ).
Formalin is best; chromate and mercuric chloride should be avoided.
Works on all types of section, although some adjustment may be necessary for resin sections.
Place 20 ml of a 10% aqueous silver nitrate solution in a glass flask. Using a fine-pointed dropper pipette, add concentrated ammonia drop by drop, constantly agitating the flask until the formed precipitate almost dissolves. This titration is critical if the method is to work consistently well. The end point of the titration is seen when a faint opalescence is present, and is best viewed using reflected light against a black background. If too much ammonia is inadvertently added, then the addition of a few drops of 10% silver nitrate will restore the opalescence. To this correctly titrated solution add 20 ml triple distilled water and then filter into a dark bottle. Store the solution in the refrigerator and use within 4 weeks, but note that ammoniacal silver solutions are potentially explosive if stored incorrectly.
Take test and control sections to distilled water.
Treat with the ammoniacal silver solution in a Coplin jar which has been covered with aluminum foil, for 30–40 minutes at 56°C or overnight at room temperature.
Wash well in several changes of distilled water.
Treat sections with 5% aqueous sodium thiosulfate (hypo) for 1 minute.
Wash well in running tap water for 2–3 minutes.
Lightly counterstain in 0.5% aqueous neutral red or 0.1% aqueous nuclear fast red for 5 minutes.
Rinse in distilled water.
Dehydrate, clear and mount in a synthetic resin.
| Melanin, argentaffin, chromaffin and some lipofuscins | black |
| Nuclei | red |
Only use thoroughly clean glassware, as the silver solution will react with any residual contaminant left on glassware.
Prolonged exposure to 56°C may give rise to a fine deposit over the section.
Friable material may need to be coated with celloidin as the ammonia in the silver solution could lead to sections lifting off the slide.
10% buffered neutral formalin.
Paraffin wax embedded.
To 10 ml of 2% silver nitrate add 5 ml of 0.8% lithium hydroxide monohydrate. Then add 28% ammonium hydroxide, drop by drop with constant shaking, until the precipitate almost dissolves. Make up the solution to 200 ml with distilled water and store in a refrigerator at 3–6°C. The solution is stable for at least 1 month.
| 0.2% aqueous gold chloride |
| 2% aqueous sodium thiosulfate |
Take slides to distilled water.
Place slides in 40 ml of refrigerated cold ammoniacal silver solution in a plastic Coplin jar and microwave at a power setting of 360 W for 35 seconds. Gently agitate the Coplin jar for about 15 seconds. Microwave again at the same power for 35 seconds. Gently agitate the Coplin jar for about 15 seconds. Allow the slides to remain in the hot solution (approximately 80°C) for 2–3 minutes or until the sections appear a light brown.
Rinse in four changes of distilled water.
Place slides in 0.2% aqueous gold chloride for 1 minute.
Rinse in two changes of distilled water.
Place slides in 2% aqueous sodium thiosulfate for 1 minute.
Rinse in four changes of distilled water.
Counterstain with 0.1% aqueous nuclear fast red for 3 minutes.
Rinse in three changes of distilled water.
Dehydrate, clear, and mount in synthetic resin.
| Melanin, argentaffin, chromaffin, lipofuscin and other silver reducing substances | black |
| Nuclei | red |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here