Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Somatosensory innervation of the oral cavity is provided by cranial nerve V (trigeminal nerve), second and third divisions, and cranial nerve IX (glossopharyngeal nerve): (1) V2—maxillary, hard, and soft palates; oral mucosa of the maxillary vestibule; and maxillary teeth, gingivae, and periodontal ligaments; (2) V3—mandibular, oral mucosa of the cheek and mandibular vestibule; anterior two thirds of tongue; mandibular teeth, gingivae, and periodontal ligaments; and (3) IX—oropharynx and posterior one third of the tongue.
Receptors on the periodontal ligament—the ligament that separates tooth from bone in healthy, functioning teeth—initiate oral reflexes of jaw opening and salivation and, together with receptors in the temporomandibular joint, contribute to interdental force discrimination and oral stereognosis. Therefore, bite force is lessened with a corresponding decrease in these periodontal ligament receptors (i.e., with periodontal disease, tooth extraction, and consequent denture construction).
Dental pain is mediated by C fibers (dull, burning) and A delta fibers (sharp, bright) located in the pulp chamber, the “nerve” of a tooth, and extending a short distance into the dentinal tubules (A delta only). In the hydrodynamic theory of dental pain, when enamel is breached by decay, fracture, or wear, the fluid in the tubules responds to stimuli and activates these nerve endings.
Chewing, swallowing, and breathing are produced by brainstem central pattern generators that control the fundamental rate and pattern of muscle contractions that define each function. Coordination of muscle activity occurs among functions, among motor groups, and within the muscles themselves and is affected by peripheral feedback mechanisms.
The main function of the masticatory muscles is to break down solid food into amounts small enough to be swallowed. These strong muscles that open and close the jaw generate significant forces across short distances and apply them via teeth. These forces must be controlled precisely and effectively—by cortical preprogrammed movement patterns, reflex stimulation, and peripheral input/feedback loops—to allow for smooth movements and successful deglutition.
Recent molecular and functional data show that no specific tongue “map” exists for taste buds; responsiveness to the five tastes—sweet, sour, bitter, salty, and umami—is present in all areas of the tongue supplied with taste buds.
Sweet taste sensations are associated with simple carbohydrates, sour taste is generated by weak organic acids, salty taste is stimulated mostly by sodium chloride (sodium ions), bitter taste arises from stimulation by plant alkaloids (potential toxins), and umami taste is associated with amino acids and peptides.
The biology of taste perception is complex, mediated by taste receptor cells in the taste buds and innervated by cranial nerves VII, IX, and X; however, there is no argument that the clinical recognition of taste affects our survival.
The oral cavity is a complex organ that comprises muscle, glands, teeth, and specialized sensory receptors. For most animals, the orosensory and oromotor apparatus is critical for successful defense, reproduction, exploration, nutrition, and vocalization. In humans, vocalization has evolved into complex speech production, but other human behaviors depend less on the mouth and tongue than on the eye and hand. In all animals, however, the mouth is essential for the ingestion of nutrients. The incorporation of nutrients by mastication and drinking involves a high degree of coordination within and among different oral motor systems. Chewing requires the reciprocal activation of antagonist trigeminal muscles to open and close the jaws and the tongue to position food between the teeth. A diverse array of highly specialized sensory systems guides these complex oromotor responses and initiates secretion of digestive enzymes. Mechanoreceptors in the tongue, palate, and periodontal ligament (PDL) all contribute to a three-dimensional stereognostic perception of the oral cavity. The sense of taste serves in both food selection and protection from ingesting potentially toxic substances.
Recent reviews provide comprehensive coverage of specific aspects of oral function; these include mastication, swallowing, oral mechanoreception, and the sense of taste. In addition, several recent articles have reviewed oral pain and taste dysfunction.
This chapter provides a concise overview of orosensory and oromotor function. A brief synopsis of orosensory function describes the innervation and sensitivity of the oral cavity and a summary of central pathways; a section on sensorimotor function includes a discussion of masticatory, lingual, and autonomic reflexes followed by a discussion of mastication and the oral phase of deglutition. The sense of taste is treated separately.
Somatosensory innervation of the oral cavity is provided by the maxillary and mandibular branches of the trigeminal nerve and by the glossopharyngeal nerve.
Mandibular nerve: oral mucosa of the cheek, anterior two thirds of the tongue, mandibular dentition, PDL, gingiva, and anterior mandibular vestibule.
Maxillary nerve: hard and soft palates, the oral mucosa of the maxillary vestibule, and the maxillary dentition, gingiva, and PDL.
Glossopharyngeal nerve : back of the tongue and oropharynx.
Although the entire oral cavity is densely innervated with sensory fibers, considerable evidence indicates that the innervation is not uniform. Specialized oral tissues—including the lips, teeth, PDL, tongue, and palate—each display specific patterns of sensitivity. Although specific parts of the oral cavity rival the hand in terms of absolute psychophysical thresholds for tactile and thermal sensitivity, the structure and function of somesthetic correlations so painstakingly deduced for the hand have little predictive veracity in the mouth.
Overall, the anterior oral cavity displays greater tactile sensitivity than does the posterior oral structure. The tip of the tongue is particularly sensitive, with a discriminative capability equivalent to that of the digits ( Fig. 86.1 ). The midline of the palate and tongue are more sensitive than lateral regions, and a similar pattern of sensitivity applies to the teeth. Adults with complete dentition could detect a 1-g von Frey hair applied to the anterior (midline) teeth, but they would require nearly 10 g to detect stimulation of the first molar. The sensitivity to warm and cold stimuli also varies widely across oral tissues. Sensitivity to warm stimuli is relatively high on the tip of tongue but not particularly so on either the palate or buccolabial surfaces. In contrast, the sensitivity to a cool stimulus is less differentiated within the oral cavity, and the sensitivity of the tongue tip, palate, and buccolabial surfaces is essentially equal. In general, the sensitivity to cool stimuli is greater compared with warm stimuli.
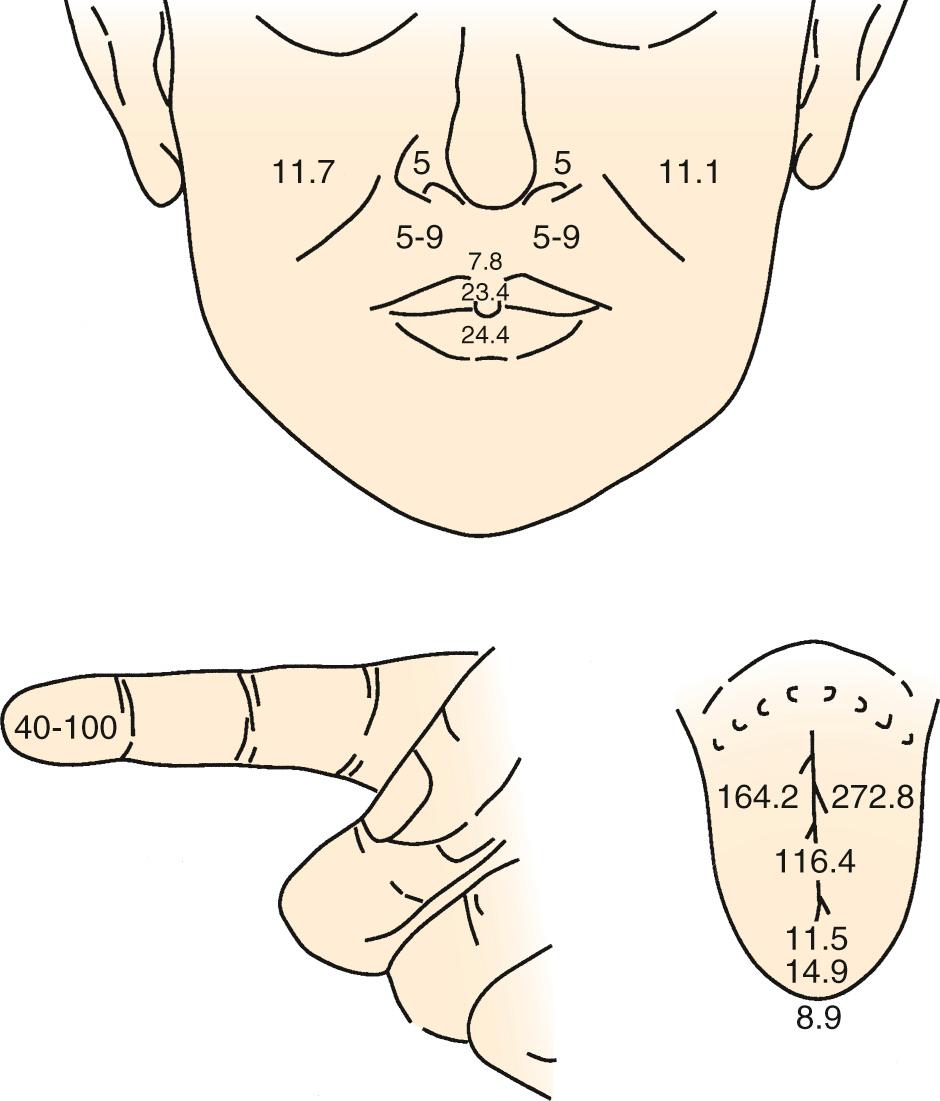
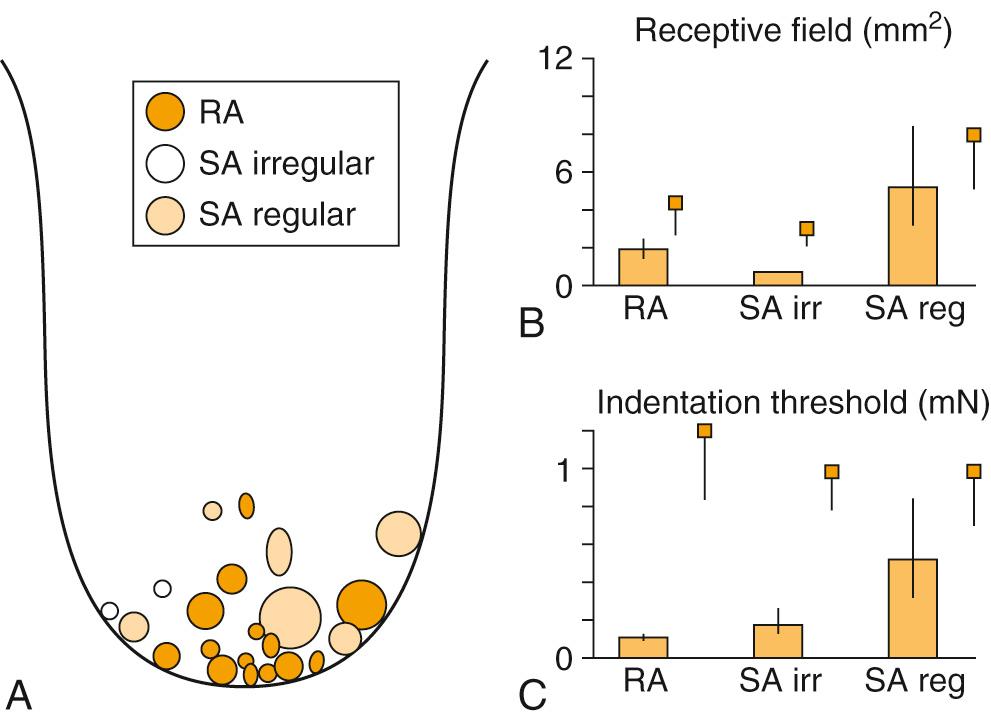
Recording from single human lingual fibers that innervate the anterior tongue confirms the small receptive fields and high sensitivity to low-threshold forces perceived psychophysically ( Fig. 86.2 ). On the basis of their small receptive fields and low thresholds, lingual fibers could be divided into:
Fibers innervating superficial (mucosal) surface of the tongue: the majority of the superficial fibers are rapidly adapting—a characteristic they have in common with other highly sensitive structures used in exploratory activity (e.g., the hand)
Fibers innervating deeper muscle tissue of the tongue: the deeper receptors were all slowly adapting, and these deeper receptors provide information about the position of the tongue.
Trigeminal nerve endings that mediate somesthetic and thermal sensitivity of the tongue and palate can be any of the following:
Free nerve endings
An intermediate group of “semiorganized” endings
More highly organized endings variously referred to as Krause end bulbs, mucocutaneous end organs, or coiled terminations.
All investigators agree that no Pacinian corpuscles exist in the oral mucosa. Based on ultrastructural criteria, Munger referred to many highly organized oral mucosa endings as Meissner corpuscles, similar to those found in glabrous skin of the hand. However, despite all this variation in nomenclature, many of the illustrations of the specialized endings are quite similar and show “finely wound nonmyelinated fibers” without a clearly defined capsule. Ultrastructural studies further reveal that some of these organized endings in the palate, but not in the lingual epithelium, send axonal processes into the overlying epithelial pegs and are associated with Merkel cells. In the hand, Merkel cells are correlated physiologically with slowly adapting mechanoreceptors; however, a similar correlation has not been made in the palate, and their apparent absence in the lingual epithelium does not preclude slowly adapting mechanoreceptors in this structure (see Fig. 86.2 ). Thus, unlike the hand, a correlation between the morphology of oral receptor endings and their response properties as rapidly or slowly adapting has not been demonstrated.
Mechanoreceptors in the PDL have been studied in some detail. In addition to detecting forces directed against the teeth, PDL receptors initiate oral reflexes of jaw opening and salivation and, together with receptors in the temporomandibular joint (TMJ), contribute to interdental discrimination and oral stereognosis. As many as six varieties of receptor morphology are found in the PDL, ranging from complex branched endings to free nerve endings. The cell bodies for PDL receptors are located peripherally in the trigeminal ganglion and centrally in the mesencephalic trigeminal nucleus. Mesencephalic trigeminal innervation of the PDL is primarily in the apical region near the root and consists mostly of small, myelinated, Ruffini-like endings. Trigeminal ganglion innervation extends from the apical region to the more superficial region and includes small unmyelinated nerve endings.
Both rapidly and slowly adapting mechanoreceptors are found in the PDL, and it is likely that the location of the receptor in the ligament determines its response characteristic. Because the tooth rotates about its fulcrum, forces directed laterally to the crown will translate to greater stretch at the root of the tooth compared with the fulcrum. Thus it is perhaps not surprising that lower-threshold fibers are found near the root and that they tend to be slowly adapting compared with receptors located near the fulcrum. In addition, individual Ruffini endings are not uniformly distributed around the tooth, and thus they display directional sensitivity to the force required to activate them. Recordings from human nerves (microneurography) demonstrate the directional sensitivity of PDL receptors ( Fig. 86.3 ) and further indicate mechanical coupling between the teeth. Single fibers respond to stimulation of multiple (adjacent) teeth; however, no anatomic evidence suggests that individual fibers innervate multiple teeth.
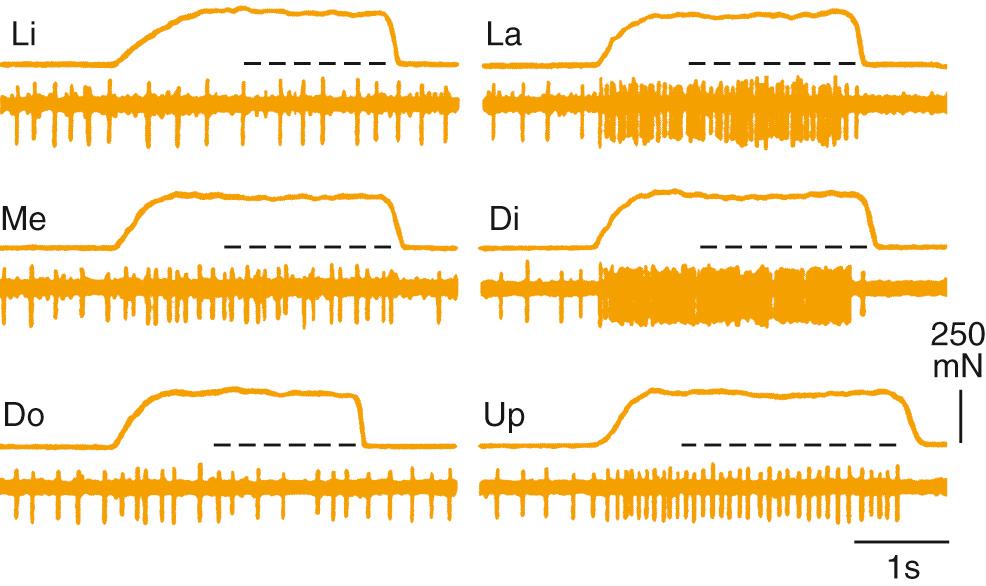
The PDL is innervated by two different receptors that have functional significance:
The Trigeminal Ganglion Receptors:
Include slowly adapting mechanoreceptors (position detectors) and high-threshold C fibers (nociceptors) in addition to rapidly adapting mechanoreceptors.
Because these periodontal receptors from the trigeminal ganglion terminate centrally in the sensory trigeminal complex, the source for the ascending sensory pathway to the thalamus and cortex, they provide information about tooth displacement and dental pain to the forebrain.
The Mesencephalic Trigeminal Nucleus Receptors:
Are primarily medium and rapidly adapting receptor types, and many have directional sensitivity.
The central termination of these mesencephalic force detectors includes inhibitory connections to trigeminal jaw-closer motoneurons via the supratrigeminal area.
Thus these receptors serve a protective role in preventing potentially damaging tooth contact during mastication.
Although mechanoreceptors in the PDL are not encapsulated, their response characteristics are influenced by the elastic properties of the ligament. When the attachment of the ligament is compromised, such as during periodontitis that loosens the connective attachments of the ligament, a corresponding loss in interdental force discrimination is observed. Periodontal receptors also contribute to the regulation of bite force. Individuals with dentures cannot bite as hard as normal dentulous subjects and cannot perceive variations in their own bite force. Similar results were obtained by anesthetizing the inferior alveolar nerve. In contrast, anesthetizing the TMJ does not affect bite-force discrimination, but it does impair jaw-positioning performance. Thus different populations of oral receptors may regulate sensation of jaw position and control of bite force during mastication.
Stimulation of the oral cavity with high concentrations of salts, acids, alkaloids, and other compounds elicits intense taste sensations but also evokes nontaste sensations that range from stinging and burning to warm, cool, and painful. This sensitivity of the oral cavity, mediated by nonspecialized free nerve endings and shared by all mucosal membranes, is referred to as the common chemical sense , or chemesthesis ; this should not be confused with taste. Although free nerve endings respond to many traditional gustatory stimuli, they typically display a much lower sensitivity. Electrophysiologic recordings from the lingual nerve, for example, indicate that single fibers require concentrations of sodium chloride (NaCl) a thousand times higher than those necessary to elicit a response from a gustatory fiber in the chorda tympani nerve. However, much lower concentrations of other types of chemical stimuli, such as menthol, are adequate to elicit a response in trigeminal nerve fibers. The types of chemical stimuli that elicit low-threshold responses in trigeminal fibers suggest that one function of the common chemical sense is to protect the oral cavity. Responses to common chemical stimuli include reflex salivation and coughing that function to diffuse and remove offending stimuli from the mouth. The common chemical sense is not purely protective, however. Spices such as horseradish, ginger, and red pepper are effective stimuli for trigeminal afferent fibers and contribute to the flavor of food. In 2001, one of the receptors for chemesthetic stimulation was cloned. A member of the transient receptor potential (TRP) family of G-protein–coupled receptors, the vanilloid receptor termed VR1 responds to both noxious heat and low concentrations of protons in addition to vanilloid compounds such as capsaicin, found in chili peppers. Stimulation of this receptor results in the opening of a cation channel and thus depolarizes the afferent fiber.
People usually describe dental pain as either dull and burning or sharp. Sensations of dull burning pain are associated with stimulation of C fibers that terminate in the pulp chamber, whereas sharp, “bright” dental pain is associated with A delta fiber innervation that extends a short distance into the dentinal tubules matrix interposed between the pulp chamber and the enamel covering of the tooth ( Table 86.1 ). Unmyelinated C fibers constitute the majority of pulpal innervation (50% to 75%); however, endings within the pulp chamber may be unmyelinated terminals of A delta (myelinated) afferent fibers. Polymodal C fibers that innervate the pulp chamber respond to thermal stimuli and, in particular, to inflammatory mediators that include histamine and bradykinin, endogenous factors associated with pulp pathology. C fibers that innervate the pulp chamber contain neuropeptides such as substance P and calcitonin gene-related peptide. The peripheral release of these neuropeptides on C fiber activation produces local vasodilation and thus increases the pressure within the rigid pulp chamber and further augments C fiber activation (i.e., peripheral sensitization). The release of substance P in infected teeth has been directly measured in human patients using microdialysis, and patients with irreversible pulpitis had significantly higher levels of substance P in the pulp chamber of infected teeth compared with noninfected teeth. Although the release of neuropeptides augments pain, evidence suggests that it may also reduce inflammation and promote recovery. In experiments with animals, elimination of the afferent terminal release of neuropeptides by denervation of the teeth reduced wound healing after lesions were experimentally induced.
| C-Fibers | |
|---|---|
| 1 | Mediate dull burning pain |
| 2 | Unmyelinated fibers constitute the majority of pulpal innervation (50%–75%) |
| 3 | Terminate in the pulp chamber |
| 4 | Respond to thermal stimuli and to inflammatory mediators (e.g., histamine, bradykinin) |
| 5 | Contain and release neuropeptides upon activation (e.g., substance P, calcitonin gene-related peptide), which augments pain and may reduce inflammation and promote recovery |
| 6 | Peripheral sensitization: the release of neuropeptides produces local vasodilation increasing the pressure within the pulp, which further augments C fiber activation |
| A-delta Fibers | |
| 1 | Mediate sharp, “bright” dental pain |
| 2 | Myelinated fibers |
| 3 | Extend 0.2–0.3 mm into the dentinal tubules that encase the pulp chamber |
| 4 | Respond to heat, mechanical, and osmotic stimuli applied to the distal end of the dentinal tubules |
| 5 | Supports the “hydrodynamic” theory of dental pain, which offers an explanation of dental hypersensitivity |
| 6 | Hydrodynamic theory: dentinal tubules are filled with a fluid, the fluid transmits mechanical, thermal, and osmotic stimuli to the proximal end of the dentinal tubules, where the nerve endings are located generating a sharp pain stimulus |
Sharp pain is mediated by A delta fibers that extend 0.2 to 0.3 mm into the dentinal tubules that encase the pulp chamber. These nerve fibers respond to heat, mechanical, and osmotic stimuli applied to the distal end of the dentinal tubules that become exposed to environmental stimuli when the enamel layer is breached. Because the dentinal tubules are filled with a fluid, the fluid transmits mechanical, thermal, and osmotic stimuli to the proximal end of the dentinal tubules, where the nerve endings are located. This “hydrodynamic” theory of dental pain is supported by a growing body of anatomic, physiologic, and psychophysical evidence and further offers an explanation of dental hypersensitivity. When the dentinal tubules are exposed by a cavity or other lesion, patients report sharp pain in response to innocuous stimuli such as mild temperature or osmotic stimuli (e.g., sweet compounds). However, the theory predicts that if the tubules are covered, thus limiting exposure to environmental stimuli, stimulated pain should be reduced. This had been experimentally assessed in human volunteers, in whom a small cavity in a tooth scheduled for removal was prepared, and a conical chamber was positioned over the cavity through which regulated air pressure could be delivered. Creating a smear layer of amorphous tooth particles in the cavity or dissolving it away with solvents controlled the interface between the exposed dentinal tubules and the air pressure stimulus. When the smear layer was intact and covered the dentinal tubules, it took more air pressure to induce the perception of sharp pain than when the smear layer was dissolved.
Afferent fibers of the trigeminal nerve enter the brainstem in the pons, bifurcate, and either terminate in the principal sensory nucleus or descend to terminate in the spinal trigeminal complex in the medulla. The bifurcation of the trigeminal nerve at the level of the pons reflects a tendency toward a segregation of function. In general, low-threshold mechanoreceptors predominate in the principal trigeminal sensory nucleus, indicative of a tactile discriminative function. In contrast, considerable evidence implicates the subnucleus caudalis in orofacial pain mechanisms, and many neurons in the subnucleus caudalis respond to noxious stimuli applied to the head and neck. These neurons include those specifically activated by noxious stimuli (nociceptive-specific neurons) and wide-dynamic-range neurons responsive to both low- and high-intensity stimulation.
Because the receptive fields for many nociceptive neurons in the subnucleus caudalis are large and include responses to nociceptive stimuli applied to the masticatory muscles, tooth pulp, and TMJ, a role for these neurons in referred pain has been suggested. Anatomic studies confirm that afferent fibers that innervate the oral cavity, tooth pulp, oropharynx, TMJ, masticatory muscles, and superficial skin all converge in the subnucleus caudalis. In many patients, lesions in mandibular teeth have a high likelihood of producing referred pain to the maxillary region, cheek, and ear in addition to the mandible itself. Likewise, lesions in the maxillary teeth are often referred to the mandible and to the maxilla, temple, and orbital region.
In addition to the subnucleus caudalis, other parts of the sensory trigeminal complex are also involved in trigeminal pain. Nociceptive responses have been obtained from extensive areas of the sensory trigeminal complex, and destruction of the subnucleus caudalis does not prevent all trigeminal pain function. Case studies of patients who have undergone trigeminal tractotomy for intractable pain associated with cancer are completely analgesic on the face, but pulpal pain is intact. Likewise, when the principal trigeminal nucleus and subnucleus oralis were damaged after a stroke, oral and perioral pain sensitivity was diminished, as was normal tactile sensitivity from these structures.
Neurons in both the rostral sensory trigeminal complex (subnucleus oralis) and the subnucleus caudalis may also form a substrate for “central sensitization,” in which central neurons in the pain pathway have their response characteristics magnified as a result of peripheral stimulation. These changes can last a variable amount of time and potentially contribute to both short-term hyperalgesia and long-term chronic pain. Fundamental to the concept of central sensitization is that some neurons initially responsive to only high-threshold (nociceptor) input become responsive to low-threshold, nonnociceptive input. The increased responsiveness is thought to be mediated by A-β (nonnociceptive) input that becomes functionally only active after intense peripheral nociceptor input. One neural mechanism for the nascent response to nonnociceptive input has been studied in great detail. An intense afferent barrage of nociceptor input following peripheral tissue damage or inflammation “sensitizes” a central neuron via structural modification of an N -methyl- d -aspartate (NMDA) glutamate receptor. NMDA receptors are voltage sensitive and will not pass ions, even in the presence of a ligand, unless the cell is sufficiently depolarized. However, the central release of a neuropeptide such as substance P by nociceptor afferents may provide sufficient depolarization to modify NMDA glutamate receptors via intracellular signaling pathways, thereby allowing glutamate released by nonnociceptive (A-β) input to activate central neurons; this activation thus provides a neural mechanism for allodynia, and similar mechanisms have been demonstrated in the brainstem sensory trigeminal complex and may provide a substrate for chronic oral and facial pain. Experimental studies demonstrate that neuropharmacologically blocking NMDA receptors prevents TMJ and tooth pulp afferents from inducing hyperactivity in central trigeminal neurons (i.e., central sensitization).
Somatosensory information reaches the ventrobasal complex of the thalamus from all major subdivisions of the trigeminal sensory complex. Many cells in the ventrobasal complex respond to low-intensity stimulation, indicative of a tactile discriminatory function; however, other neurons require high-intensity stimulation. The small receptive fields of both types of neurons suggest a role in localization. Other nuclei, including the posterior thalamic nuclei and the nucleus submedius, respond preferentially to high-intensity stimulation and may be involved in affective components of pain. Both nociceptive and nonnociceptive trigeminally activated neurons from the thalamus project to the somatosensory cortex. Electrophysiologic mapping studies in primates indicate a complex, sometimes discontinuous somatotopic map of the facial and oral region. In general, the face is represented medially on the cortical surface adjacent to the representation of the hand, with successively lateral representations of the teeth and tongue. Magnetic resonance imaging in humans confirms this somatotopic representation.
Oral motor functions include mastication, swallowing, respiration, and vocalization. This review will focus on mastication and the oral components of swallowing and respiration. One of the dominant concepts in oral motor physiology is central pattern generation. Chewing, swallowing, and breathing are each produced by brainstem central pattern generators that control the fundamental rate and pattern of muscle contractions that define each function. Although sensory pathways from the mouth play an intimate role in oral motor function, fundamental to the concept of central pattern generation is that afferent activity is not necessary to evoke rhythmic activity, and it does not provide the critical timing information for coordinated motor output. Although organized in the brainstem, central pattern generators for chewing, swallowing, and respiration are influenced by descending inputs from virtually all major regions of the neuraxis. Detailed reviews of oromotor central pattern generation can be found in works by Nakamura and Katakura, Rekling and Feldman, and Jean.
Transection studies that relied on electrical stimulation to induce fictive jaw movements have localized the central pattern generator for mastication to the medial core of the reticular formation. More recent studies using reversible pharmacologic lesion techniques in awake, freely moving (feeding) animal preparations indicate that a necessary substrate for rhythmic lingual/masticatory movements is in the lateral reticular formation in a region that overlaps substantial populations of preoromotor interneurons. This region of the brainstem reticular formation is also the target of descending projections from metabolic integrative substrates in the hypothalamus and from the motor cortex.
Fundamental to oral motor function is the complex interplay among behaviors that compete for the same muscles. Chewing, swallowing, and respiration all require the coordinated activity of masticatory, lingual, facial, and infrahyoid muscles. Swallowing and respiration further depend on pharyngeal and abdominal muscles, and motor coordination takes place on multiple levels. At a behavioral or molar level, swallowing and respiration must be coordinated to prevent aspiration of food into the airway. How this coordination is achieved is only beginning to be understood, but it likely involves both peripheral feedback and interactions among central pattern generators as explained later. However, individual oral motor functions also require a high level of coordination. Bolus formation during mastication requires the coordinated activity of masticatory, lingual, and facial muscles, which are innervated by motoneuron groups highly segregated in the brainstem. Although the jaw and tongue can function independently, often they appear inextricably “linked.” The nature of this linkage and whether it relies on interactions between central pattern generators, reflex control, or peripheral mechanical linkage represents a significant problem in oral motor control.
In addition to the complexity of coordination between functions and coordination between motor groups, another level of complexity in oral motor control can be found within the muscles themselves. Individual masticatory and lingual muscles are not homogeneously functioning units; muscles are often composed of multiple compartments, with muscle fibers oriented in multiple directions, and thus different parts of a muscle can be more or less active during a given behavior. Further adding to the complexity of oral musculature are the multiple isoforms of myosin heavy chain (MHC) proteins that form the contractile elements of the muscle fibers. The differential distribution of MHCs within different muscles and muscle compartments imparts additional degrees of freedom to motor output.
A myriad of “simpler” oral reflexes serve protective functions and contribute to complex rhythmic output. Muscle spindles in jaw-closer muscles, for example, may contribute to load regulation during chewing, and oral reflexes may assist coordination between the jaw and tongue. Autonomic oral reflexes modulate salivation and initiate digestive processes. Several recent reviews of oral reflex function are available.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here