Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
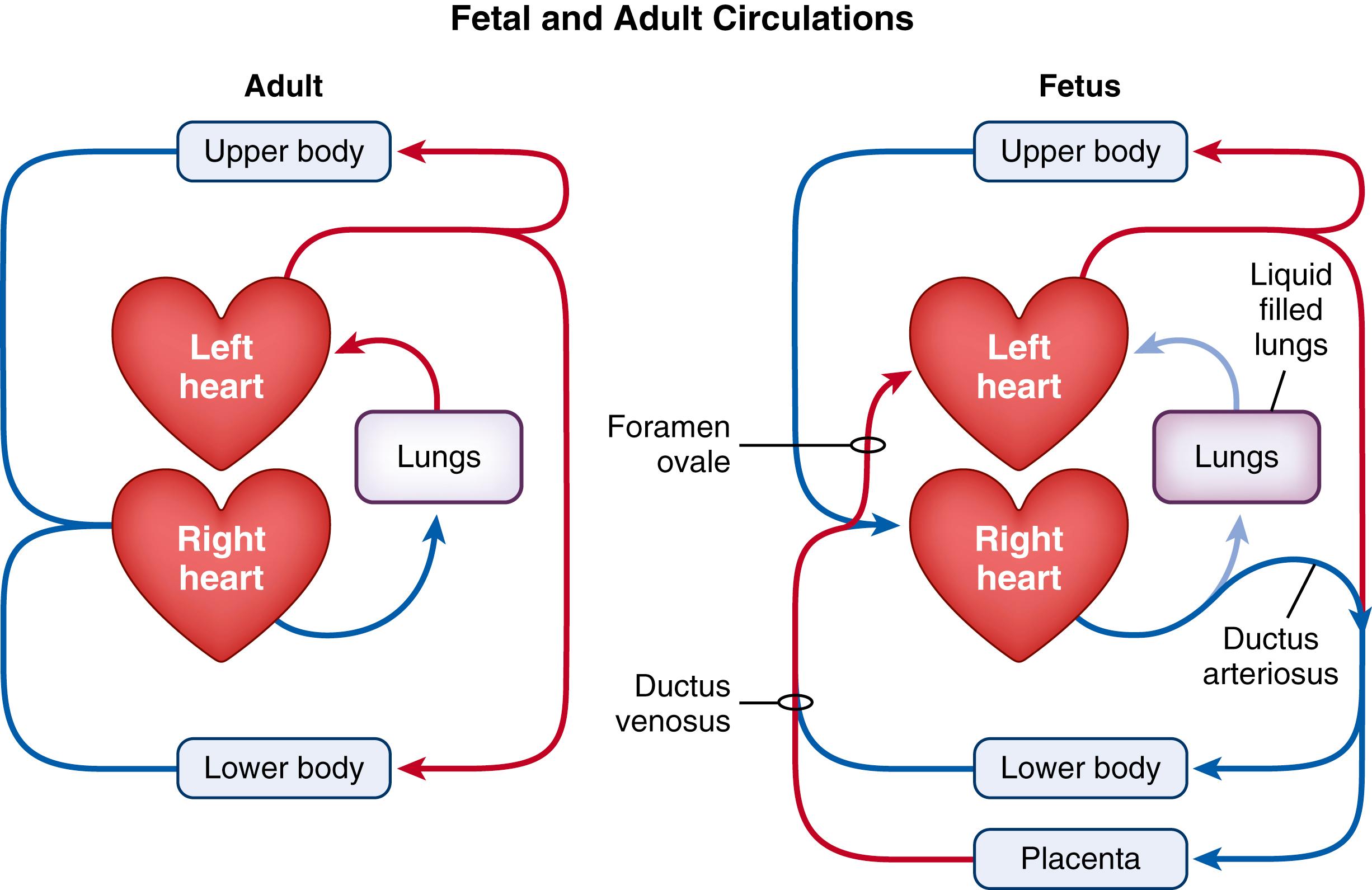
The transition to newborn life represents one of the greatest physiologic challenges that humans face during their lives. Before birth, the future airways of the lungs are liquid-filled and the lungs play no role in gas exchange. Instead, gas exchange occurs across the placenta and the majority of right ventricular output bypasses the lungs and passes through the ductus arteriosus (DA) to enter the descending aorta ( Fig. 150.1 ). As much of this blood is directed through the placenta, the right ventricle provides the majority of blood flow through the organ of gas exchange in the fetus, just as it does in the adult (lung). Similarly, much of the umbilical venous blood returning from the placenta passes through the ductus venosus (DV) and foramen ovale (FO) to directly enter the left atrium. Thus, the left ventricle in the fetus receives highly oxygenated blood from the organ of gas exchange, just as occurs in adults (see Fig. 150.1 ). As such, the transition to newborn life at birth primarily involves transferring the role of gas exchange from the placenta to the lungs, which requires a major reorganization of the circulatory system. Both of these physiologic changes are interlinked, commencing with lung aeration, which then triggers the circulatory transition by increasing pulmonary blood flow (PBF).
Considering the complexities involved, it is remarkable that most infants easily make the transition to life after birth. Nevertheless, approximately 20% of infants need some form of respiratory support at birth, and a small percentage require significant intervention. While assisting infants at birth is commonly referred to as neonatal resuscitation , the need for “resuscitation” is rare. Instead, most infants usually just require assistance to transition from a physiologic state consistent with in utero life into one consistent with ex utero life. As such, the phrase neonatal stabilization rather than neonatal resuscitation is probably more appropriate, to avoid the impression that intervention is required when it is only assistance and support that are needed.
The type and degree of assistance infants require at birth can vary considerably depending on the degree of development the infant has achieved at delivery and whether the infant has been exposed to adverse conditions, such as severe asphyxia, prior to birth. As our understanding of the physiologic challenges infants face at birth has improved, it has become clear that extrapolating treatments from one group of infants to another is inappropriate. For instance, the approaches required to assist asphyxiated term infants are very different to those required for very preterm infants, particularly as the physiologic challenges facing term and preterm infants are fundamentally different.
In this chapter, we discuss the general physiologic principles of the fetal-to-neonatal transition, before discussing how these principles are differentially affected by lung immaturity, abnormal lung development, and severe birth asphyxia.
Before birth, the future airways of the lung are liquid-filled. This liquid is secreted by the lung, enters the airways across the distal airway surface and leaves the lungs by flowing out of the trachea, whereby it is either swallowed or enters the amniotic sac. , Liquid efflux from the lungs is mostly unidirectional, flowing out of the lungs primarily during fetal breathing movements (FBMs) when the glottis is open and upper airway resistance is low. , While liquid can enter the upper airways, this is uncommon, with significant volumes only entering the airways during periods of prolonged hypoxia, thereby facilitating meconium entry into the lower airways before birth. During fetal apnea (approximately 50% of the time) the fetal glottis adducts, which restricts the efflux of liquid from the airways. This plays a vital role in fetal lung growth and development as the retention of liquid within the airways maintains the lung in a distended state (see Chapter 57 ). Increasing the degree of lung distension is a potent stimulus for fetal lung growth, whereas a reduction in lung expansion causes fetal lung growth to cease (see Chapter 57 ). However, at birth this liquid must be cleared from the airways to allow the entry of air and the onset of pulmonary gas exchange.
Airway liquid has a profound effect on respiratory function at birth, with the lung passing through two distinct phases before it starts to function like a newborn lung. During the first phase, which is usually quite short (30 to 60 seconds), the airways are liquid-filled, which prevents pulmonary gas exchange and greatly increases airway resistance. During the second phase, the liquid has been cleared from the airways into the surrounding lung tissue, but still remains within the thoracic cavity. As the liquid can take 4 to 6 hours to clear from lung tissue, it can adversely influence respiratory function well into the newborn period, which is a largely unrecognized problem.
Phase contrast (PC) x-ray imaging ( Figs. 150.2 and 150.3 ) has led to a new understanding of the timing and mechanisms of airway liquid clearance by visualizing the air entering the lungs at birth. , Indeed, it is now clear that airway liquid clearance does not simply result from Na + reabsorption induced by the stress of labor. Instead, up to three mechanisms are involved, with the primary mechanism depending upon the timing and mode of delivery. Two of the mechanisms are active during labor, and the third is active after birth (see below). More importantly, only one of these mechanisms results in the loss of liquid from the respiratory system, as the other two involve the reabsorption of liquid into lung tissue, which may interfere with lung function after birth.
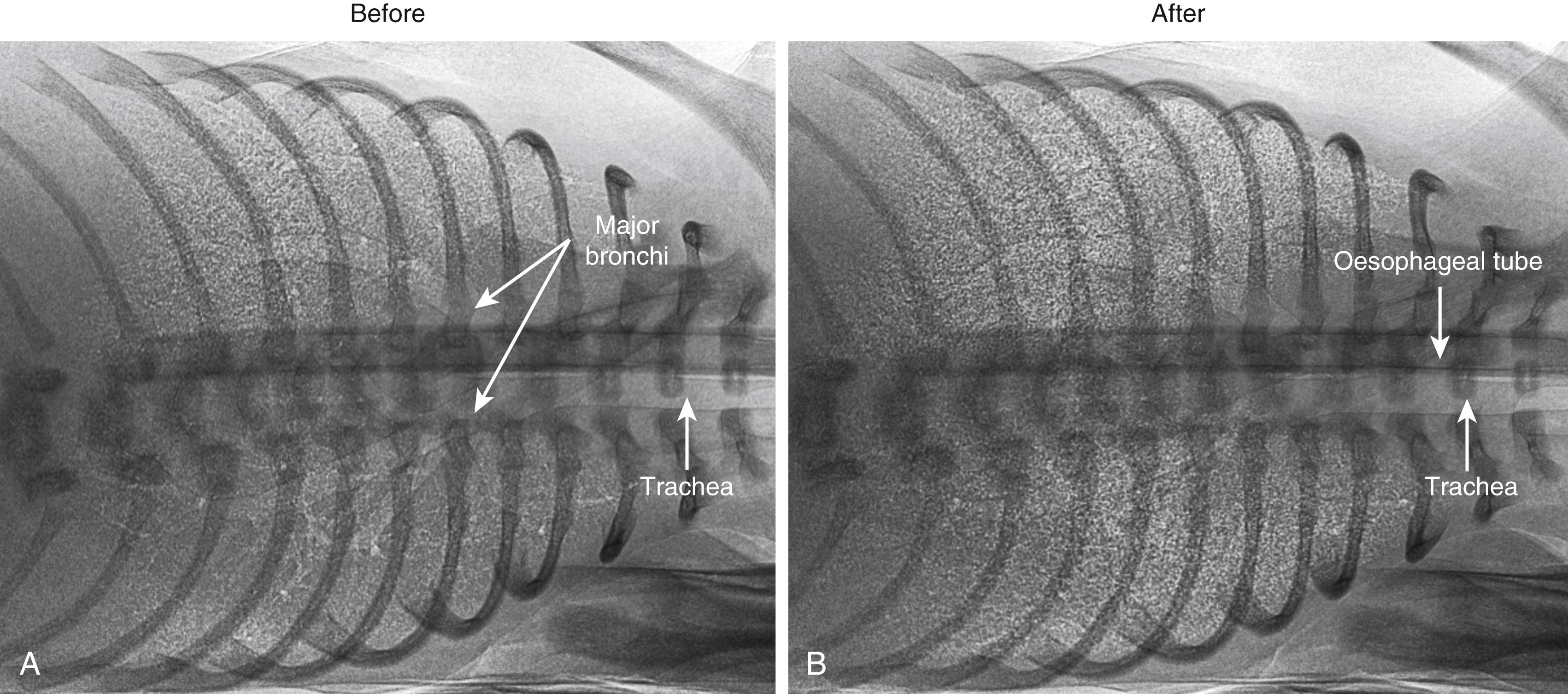
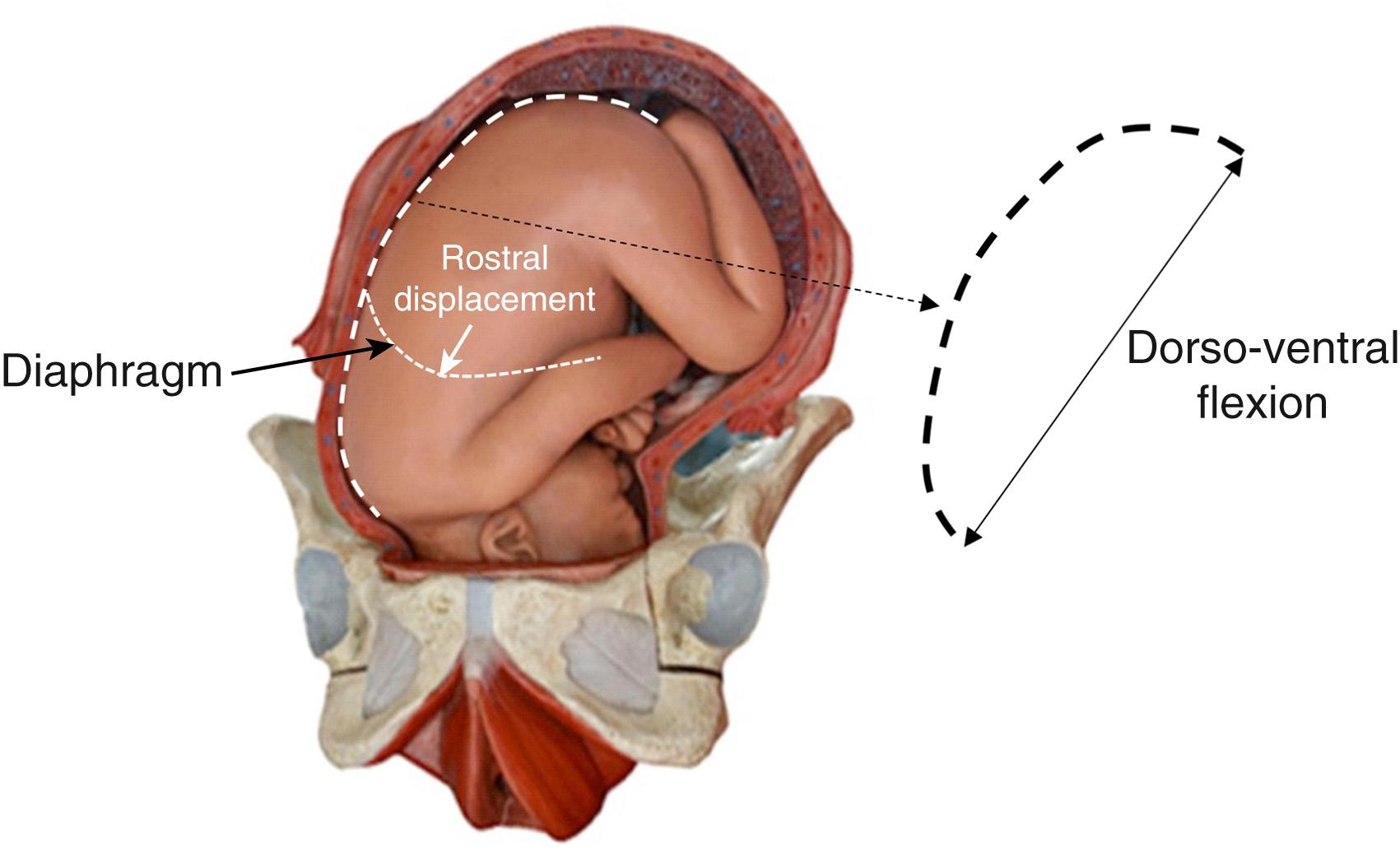
Some have argued that liquid clearance begins days to weeks before labor onset, but the evidence supporting this concept was questioned when the relationship between reduced amniotic fluid volumes and reduced lung liquid volumes was fully explored. , Nevertheless, it is clear that the volume of liquid present in the airways before birth varies considerably between individuals and largely depends on the available intrauterine space. Reducing this space, as occurs with reduced amniotic fluid volumes and uterine contractions, reduces fetal lung liquid volumes due to increased spinal flexion ( Fig. 150.4 ). Other factors, such as a twin, may have a similar effect.
The stress of labor (particularly during vaginal delivery of the head) is thought to stimulate fetal adrenaline release, which acts on β-adrenoceptors located on the basolateral surface of alveolar epithelial cells to increase intracellular cyclic adenosine monophosphate (cAMP) concentrations. , This stimulates opening of luminal surface Na + channels (ENaCs), leading to increased Na + and Na-linked Cl − transport from the lung lumen into lung tissue. , This reverses the osmotic gradient across the lung epithelium, leading to liquid reabsorption from the airways. However, Na + -induced liquid reabsorption is slow (maximum rates of 10 mL/kg/h), only develops late in gestation, and requires high levels of adrenaline, which must remain elevated well (hours) into the newborn period to clear all of the liquid. Thus, this mechanism is not active in preterm infants and if adrenaline levels were to remain elevated for hours after birth, this should be reflected by elevated heart rates. However, heart rate nomograms over the newborn period are not consistent with this concept and if airway liquid clearance occurred by Na + reabsorption alone, it would take hours before gas exchange could occur in term infants and preterm infants would not be able to aerate their lungs.
PC x-ray imaging has demonstrated that Na + channel blockade (with amiloride) does not affect the extent or the spatial and temporal pattern of airway liquid clearance after birth. However, it did increase the rate of airway liquid re-entry between inflations, indicating that Na + reabsorption may help to keep liquid out of the airways following its clearance, but the ongoing need for increased adrenaline levels to sustain this role is unclear. There is also much confusion over whether adrenaline levels are consistently increased during delivery and whether the mode of delivery (vaginal vs. cesarean section [CS]) influences adrenaline levels. The commonly cited reference found no increase in cord blood adrenaline levels and no difference between vaginally or CS-delivered infants. In contrast, asphyxiated and breech-delivered infants had markedly elevated adrenaline levels. Similarly, while knockout of the αENaC gene, but not the βENaC or δENaC genes, causes apparent respiratory failure and death within 40 hours of birth, these newborn mice also had poor respiratory efforts. Furthermore, increased wet lung tissue weights after death were cited as evidence for airway liquid retention, but this is a poor indicator of airway liquid retention, as it does not distinguish between liquid in the tissue or the airways or post-mortem movement between the two.
Forces imposed on the infant during uterine contractions (see Fig. 150.4 ) can also cause liquid to exit the lungs via the nose and mouth. Gushes of liquid from the nose and mouth have been observed upon delivery of the head, which likely results from increased dorsoventral flexion (knees to chest) causing compression of the fetal abdomen and chest during vaginal delivery (see Fig. 150.4 ). , Increased spinal flexion increases abdominal pressure and elevates the diaphragm, which increases transpulmonary pressure and forces liquid to leave the lung via the trachea. As the fetal respiratory system is highly compliant, only small increases in transpulmonary pressure are required to displace large volumes of liquid. However, the contribution of this mechanism to airway liquid clearance at birth is difficult to quantify and likely varies between individuals. For instance, infants delivered vaginally and exposed to strong uterine contractions during a protracted labor could lose large volumes of lung liquid via this mechanism. However, infants delivered by elective CS without labor may lose little or no liquid via this mechanism. Nevertheless, it is important to note that this mechanism of airway liquid clearance is the only known mechanism whereby liquid is completely lost from the respiratory system as a result of its clearance from the airways. Both other mechanisms (Na + reabsorption and inspiratory efforts, see below) involve the clearance of liquid into lung tissue, before it is eventually cleared from the tissue hours after birth.
Imaging studies have demonstrated that, after birth, the pressure gradients generated by inspiration rapidly and efficiently clear the airways of liquid (see Figs. 150.2 and 150.3 ), resulting in full aeration within 3 to 5 breaths (<30 seconds) in term newborns. , The calculated rates of liquid clearance during inspiration are very large (approximately 32 L/h/kg) and explain why infants can rapidly initiate pulmonary gas exchange after birth, even in infants born by CS without labor. , The air/liquid interface moves distally through the airways in a stepwise manner with each inspiration, with little or no distal movement between breaths (see Fig. 150.3 ). Some proximal movement can occur between breaths (see Fig. 150.3 ), which is considered to result from liquid re-entry into the airways. , Inspiration generates the pressure gradients that drive liquid movement distally through the airways and across the distal airway wall by reducing interstitial tissue pressures, a mechanism first described in humans. The resulting hydrostatic pressure gradient between the tissue and airways causes liquid to move out of the airways into the surrounding lung tissue. As similar pressure gradients can be generated by applying positive pressures to the airways, positive pressure ventilation can also rapidly clear airway liquid. , , While this confirms the rationale for providing positive pressure ventilation to infants at birth, deciding the best way to provide this support is unclear, but understanding the physiology provides important clues.
The initial resistance to air entry into the lung at birth is primarily governed by the resistance to moving liquid through the airways and across the distal airway wall. As lung liquid has a viscosity that is approximately 70× greater than air, the initial resistance is considerably (approximately 100-fold) greater than it is following lung aeration, and it rapidly decreases (approximately 100-fold) as the lung aerates ( Fig. 150.5 ). Preterm infants have smaller airways and, as the resistance increases exponentially (fourth power) with decreasing airway radius, the initial resistance to moving liquid through the airways is significantly greater in preterm compared to term newborns (see Fig. 150.5 ). Similarly, as the surface area of the distal airways increases exponentially with increasing gestational age ( Fig. 150.6 ), preterm infants have a markedly reduced distal airway surface area, which must increase the resistance for liquid movement across the distal airway wall into lung tissue. Despite the smaller airways and a reduced gas exchange surface area, it is recommended that very preterm infants are ventilated with starting pressures that are much lower than term infants at birth. While this may be protective for an immature lung that is well aerated, it is questionable whether this approach is relevant for an immature liquid-filled lung.
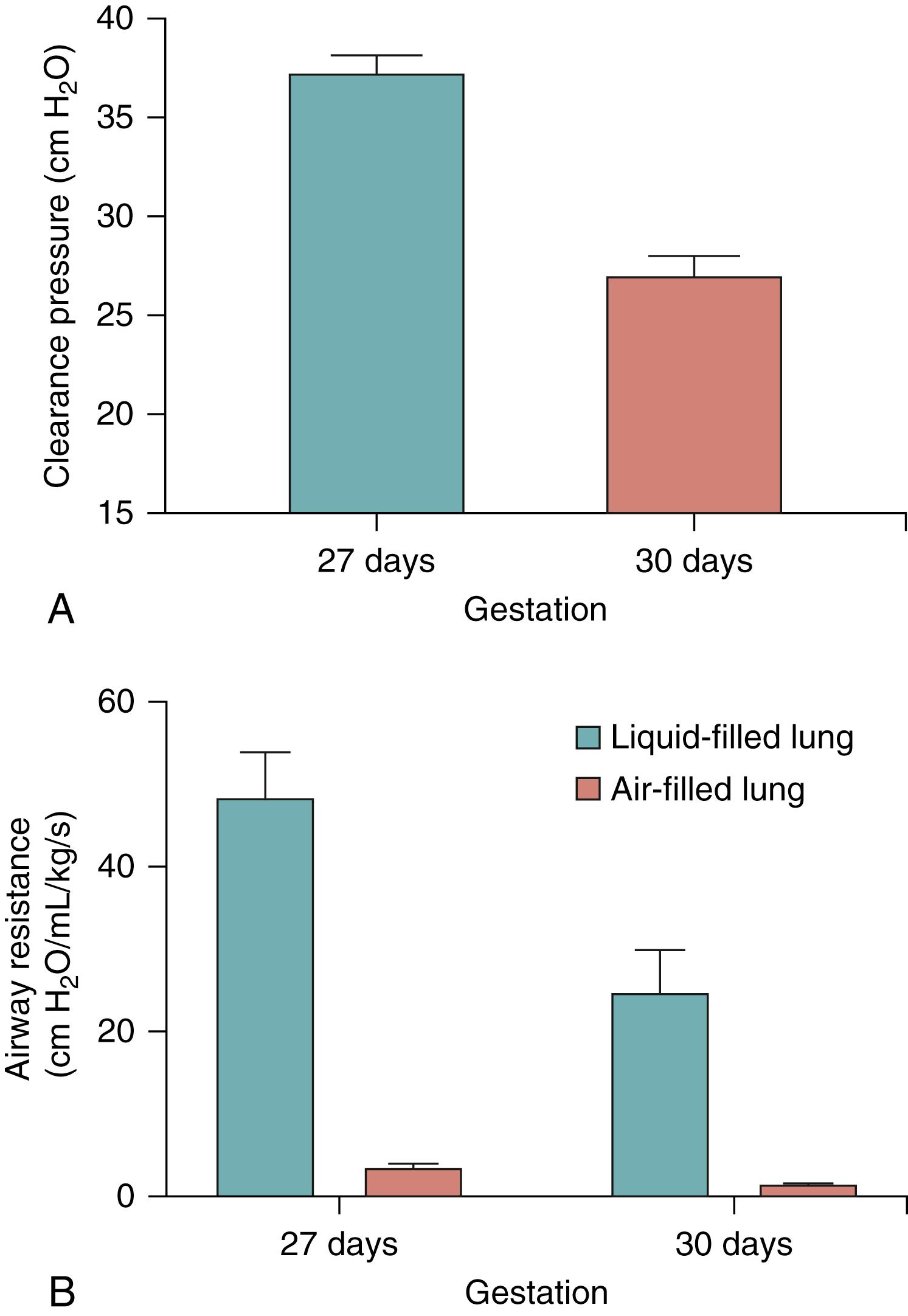
As the cross-sectional surface area of the airways increases exponentially with increasing airway generation number, airway resistance decreases exponentially as the air/liquid interface moves distally. Indeed, the huge reduction in airway resistance during lung aeration (see Fig. 150.5 ) can be very rapid (seconds) and follows an exponential function that is difficult to predict. As a result, large changes in respiratory mechanics (e.g., 10-fold increase in lung compliance) can occur on a breath-by-breath basis. Thus, use of a single set inflation pressure during intermittent positive pressure ventilation (iPPV) at birth may initially produce small tidal volume changes, but result in progressively larger tidal volumes as the lung aerates, which risk overexpanding and injuring the lung. As such, it is difficult to understand why highly sophisticated ventilators that can monitor and respond to breath-by-breath changes in respiratory mechanics are commonly used in the neonatal intensive care unit (NICU), but not in the delivery room. Indeed, respiratory mechanics have usually stabilized by the time the infant reaches the NICU, whereas in the delivery room, the changes are rapid and complex. , Despite this, ventilation in the delivery room most commonly involves the use of rudimentary devices (T-piece devices or resuscitation bags) that are incapable of automatically adjusting ventilation parameters to take into account rapidly changing respiratory mechanics. This may partly explain the large variation in tidal volumes measured in preterm infants at birth. ,
As indicated above, only one mechanism of airway liquid clearance (fetal postural changes) results in liquid loss from the chest as it leaves the airways via the nose and mouth. The other two mechanisms (Na + reabsorption and pressures generated by inspiration) involve liquid clearance into the lung’s interstitial tissue. As this tissue compartment has a fixed volume (see Fig. 150.6 ), the entry of liquid into the interstitial tissue increases pressures from approximately 0 to 6 cm H 2 O. This causes a form of pulmonary edema that persists for 4 to 6 hours after birth while the liquid is gradually cleared from the tissue. ,
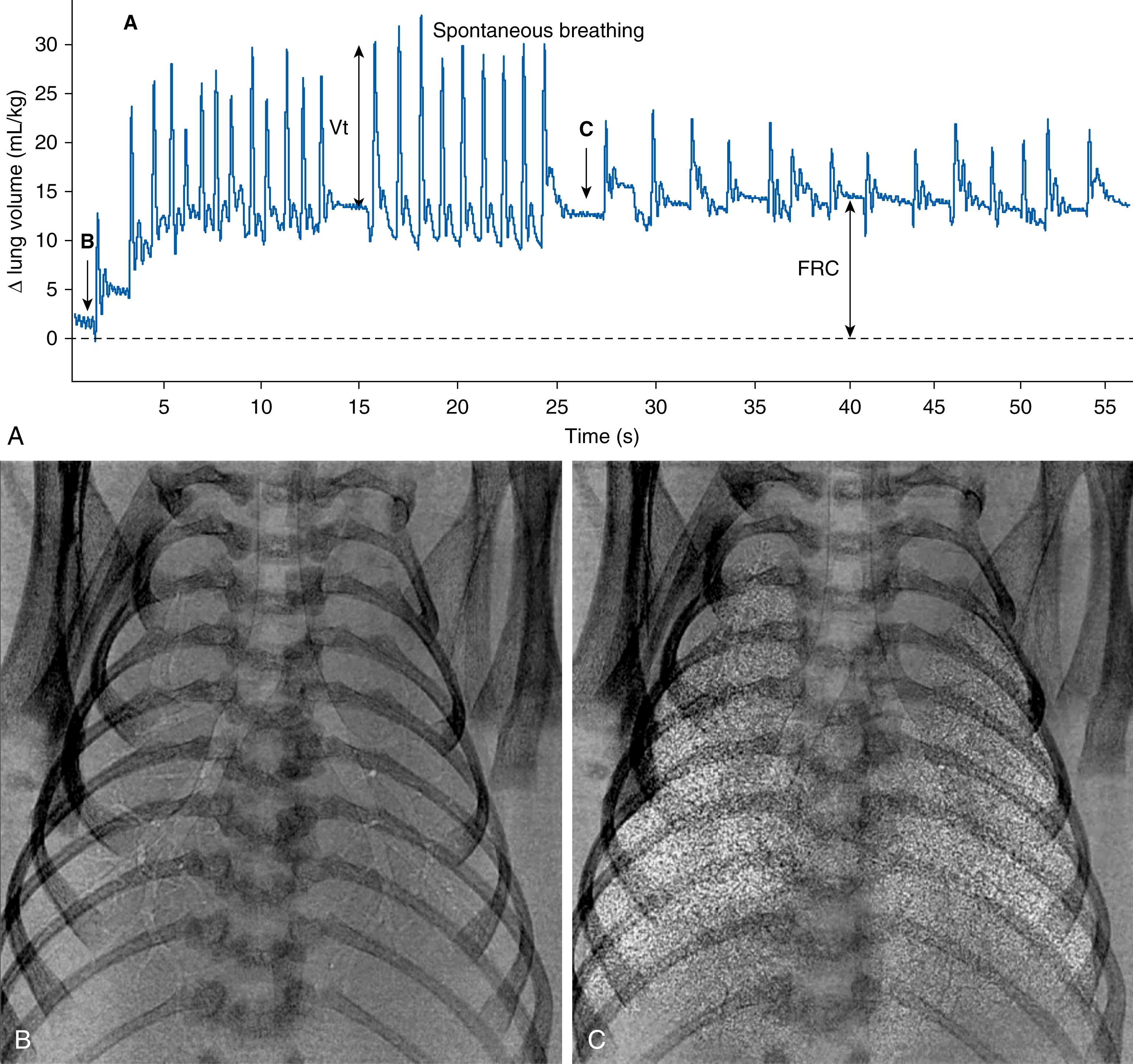
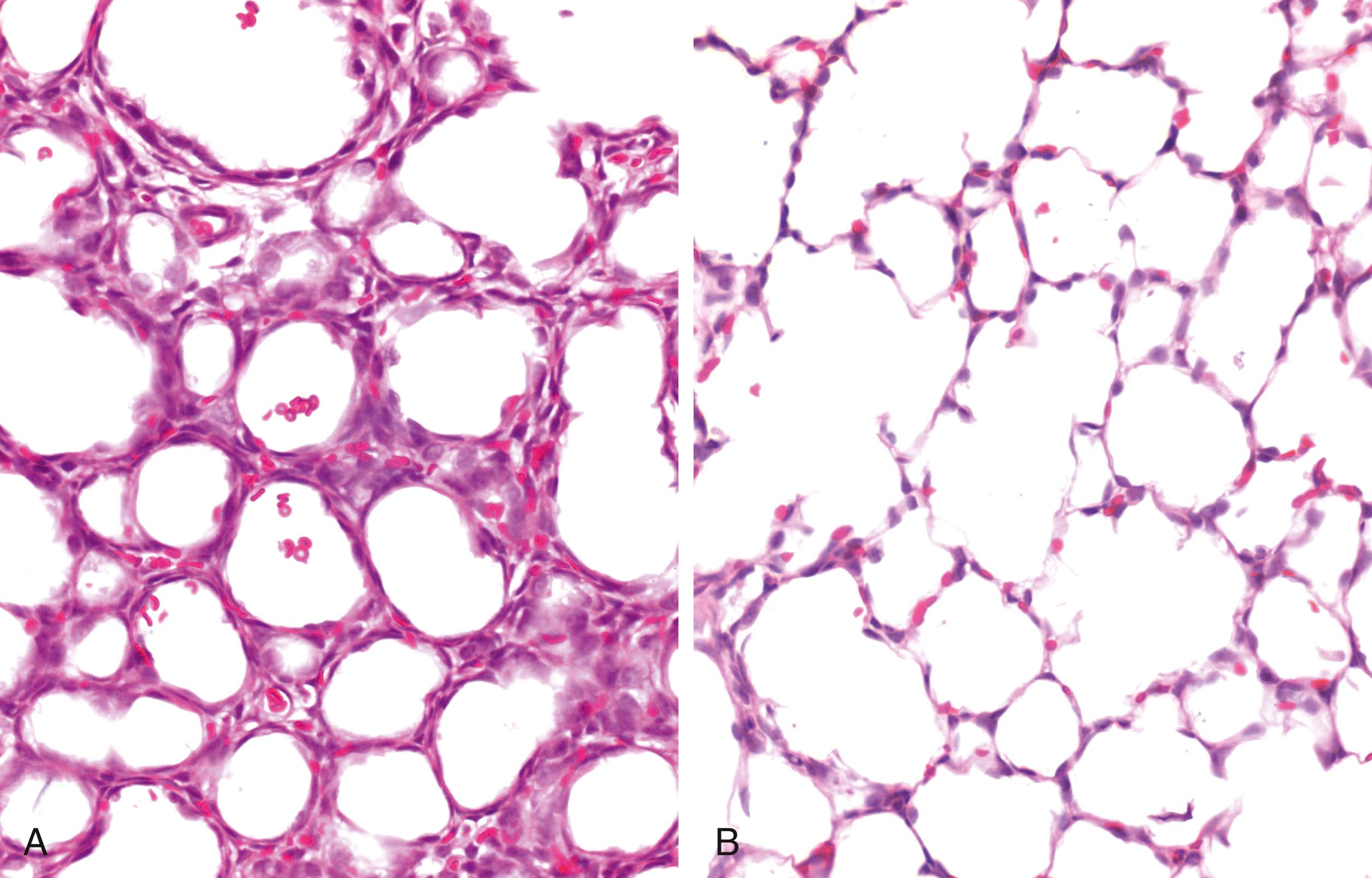
The presence of airway liquid in lung tissue potentially has major implications for the infant’s respiratory function after birth. For instance, while the liquid moves from the airways into lung tissue to allow the entry of air, it still remains within the chest. As a result, the chest wall must expand to temporarily accommodate both the liquid and the incoming volume of air, which also causes the diaphragm to flatten ( Fig. 150.7 ). , This explains why the chest wall must be highly compliant at birth, allowing it to easily expand and accommodate both the liquid and incoming air after birth. If the chest wall is stiff, its ability to expand is reduced, leading to much greater interstitial tissue pressures. Increased interstitial tissue pressure increases the pressure gradient for liquid to re-enter the airways at end-expiration. While this liquid may be re-cleared during the next breath/inflation, the functional residual capacity (FRC) is reduced. Furthermore, the presence of this liquid in lung tissue reduces lung compliance, making the lungs more difficult to inflate. Currently, there is very little understanding of how this consequence of airway liquid clearance contributes to respiratory morbidity in newborn infants. However, an expanded chest wall and flattened diaphragm, along with a stiffer lung, will increase the work of breathing and limit tidal volumes, leading to tachypnea and labored breathing. In addition, a reduction in FRC usually induces expiratory braking (glottis closure during expiration) in the newborn, or grunting. All of these signs are evident in infants with transient tachypnea of the newborn (TTN).
![Fig. 150.7, Lung aeration increases chest wall expansion. Superimposition of two phase contrast x-ray images (contrast is inverted in one image) collected before (white ribs) and after (black ribs) lung aeration in a spontaneously breathing near-term newborn rabbit acquired between breaths (i.e., at functional residual capacity [FRC]). Following lung aeration, the chest wall significantly expands to accommodate both the air that forms the FRC after birth and the liquid that has moved from the airways into lung tissue but still remains in the chest. Chest wall expansion is also clearly evident in Fig. 150.2 . Fig. 150.7, Lung aeration increases chest wall expansion. Superimposition of two phase contrast x-ray images (contrast is inverted in one image) collected before (white ribs) and after (black ribs) lung aeration in a spontaneously breathing near-term newborn rabbit acquired between breaths (i.e., at functional residual capacity [FRC]). Following lung aeration, the chest wall significantly expands to accommodate both the air that forms the FRC after birth and the liquid that has moved from the airways into lung tissue but still remains in the chest. Chest wall expansion is also clearly evident in Fig. 150.2 .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PhysiologyofNeonatalResuscitation/6_3s20B9780323712842001506.jpg)
The fetal circulation is considerably more complex than that of the adult (see Fig. 150.1 ). Like adults, fetuses have both a pulmonary and a systemic circulation, but also have a placental circulation connected in parallel across the lower body. Furthermore, unlike the adult, all of these circulations are interconnected due to the presence of multiple shunts, which include the DV, FO, and the DA (see Fig. 150.1 ). The DV allows approximately 50% of umbilical venous blood to flow directly into the inferior vena cava (IVC, in sheep) or right atrium (in humans). The other 50% passes into the hepatic portal system and eventually back into the IVC. Much of the blood passing through the DV also passes through the FO to directly enter the left atrium, thereby bypassing the right atrium, right ventricle, and pulmonary circulation. Of the blood that does enter the right atrium and right ventricle, the majority (80% to 90%) bypasses the lungs and flows from the pulmonary artery into the descending aorta via the DA.
While anatomically distinct (see Fig. 150.1 ), the fetal and adult circulations are functionally similar, differing only because the placenta, rather than the lung, is the fetal organ of gas exchange. As the DA shunts blood from the pulmonary artery into the aorta, the right ventricle provides the majority of blood flow to the organ of gas exchange (placenta) in the fetus, just like it does in the adult. Similarly, due to the presence of the DV and FO, highly oxygenated umbilical venous blood passes directly into the left atrium and left ventricle. Thus, blood returning from the gas exchange organ provides the majority of preload for the left ventricle in the fetus, just like it does in the adult. , , As the lungs must take over the role of the gas exchange at birth, the circulation has to undergo a massive re-organization so that the lungs can (1) become the sole recipient of blood exiting the right ventricle and (2) become the sole provider of preload (venous return) for left ventricular output. It is very important not to overlook this second role, as it is critical for cardiac function after birth.
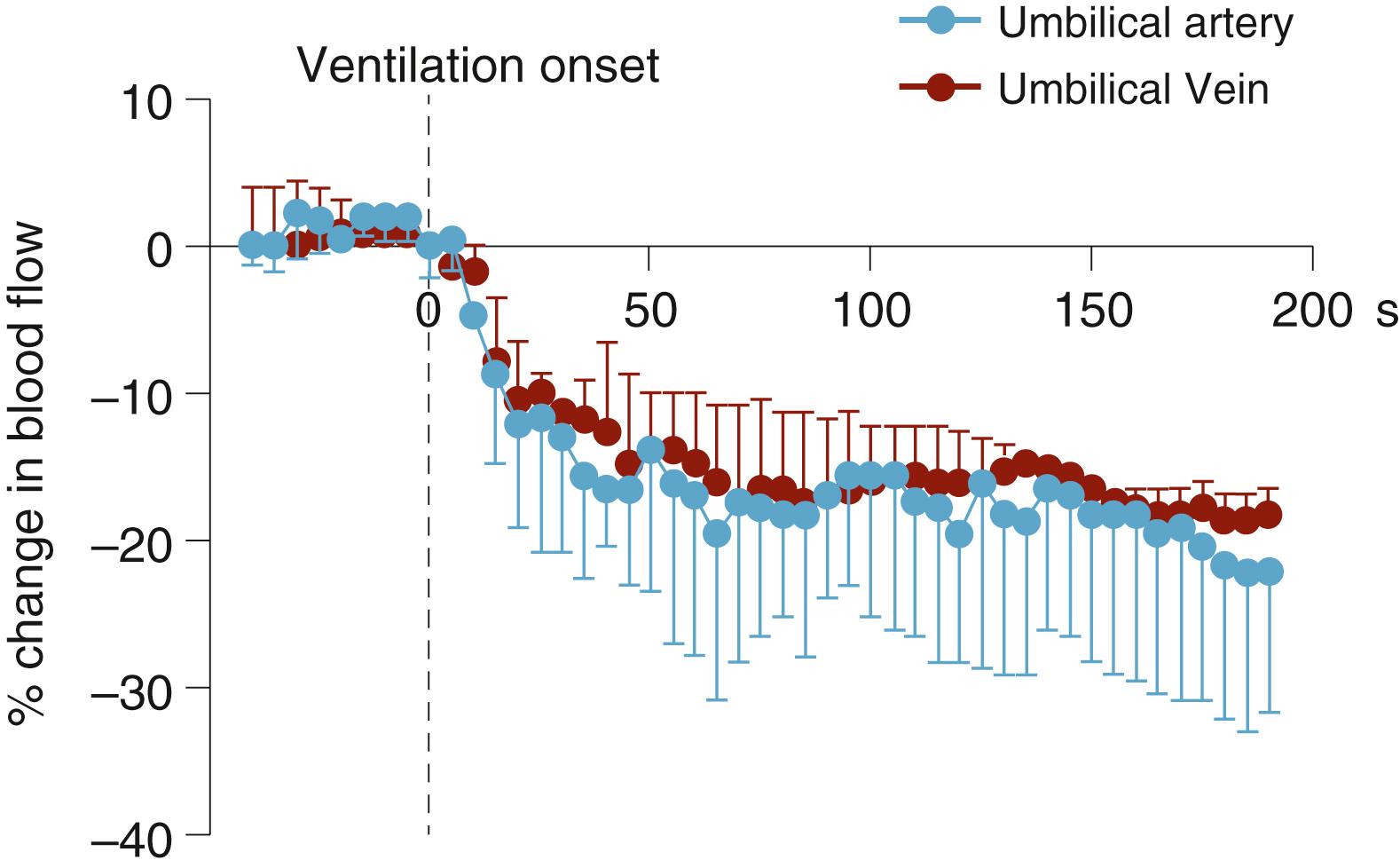
At birth, lung aeration decreases pulmonary vascular resistance (PVR), which increases PBF and redirects right ventricular output through the lungs. As a result, right-to-left shunting of blood through the DA decreases and flow in the umbilical artery also decreases, if the cord remains unclamped ( Fig. 150.8 ; see below). In effect, the lung and placenta compete with one another for both right and left ventricular output due to the ongoing presence of the DA (see Fig. 150.8 ). If the decrease in PVR precedes umbilical cord clamping (UCC), UCC greatly increases PBF due to a large increase in left-to-right shunting through the DA. , , This results from the loss of the low-resistance placental circulation, which increases systemic vascular resistance and promotes blood flow from the systemic into the pulmonary circulation. This reversal in DA blood flow ( Fig. 150.9 ) is a normal feature of the cardiovascular transition at birth, irrespective of the timing of cord clamping (see below) but is transient (1 to 2 hours) in normal healthy infants. Nevertheless, the DA blood flow profile after birth is very dynamic and bidirectional, depending upon the phase of the cardiac cycle. While it is mostly left-to-right (see Fig. 150.9 ), during early systole the flow is right-to-left but becomes left-to-right during late systole and throughout diastole. During early systole, the pressure wave emanating from the right ventricle reaches the pulmonary artery end of the DA before the pressure wave emanating from the left ventricle reaches the aortic end (see Fig. 150.1 ). As a result, the pressure gradient across the DA is initially higher at the pulmonary end, resulting in right-to-left flow, but rapidly reverses as the pressure wave from the left ventricle reaches the aortic end of the DA (see Fig. 150.9 ). The continued left-to-right flow during diastole reflects the lower resistance to blood flow through the pulmonary circulation, compared with the systemic circulation, and significantly contributes to PBF (up to 50% of flow) in the immediate newborn period.
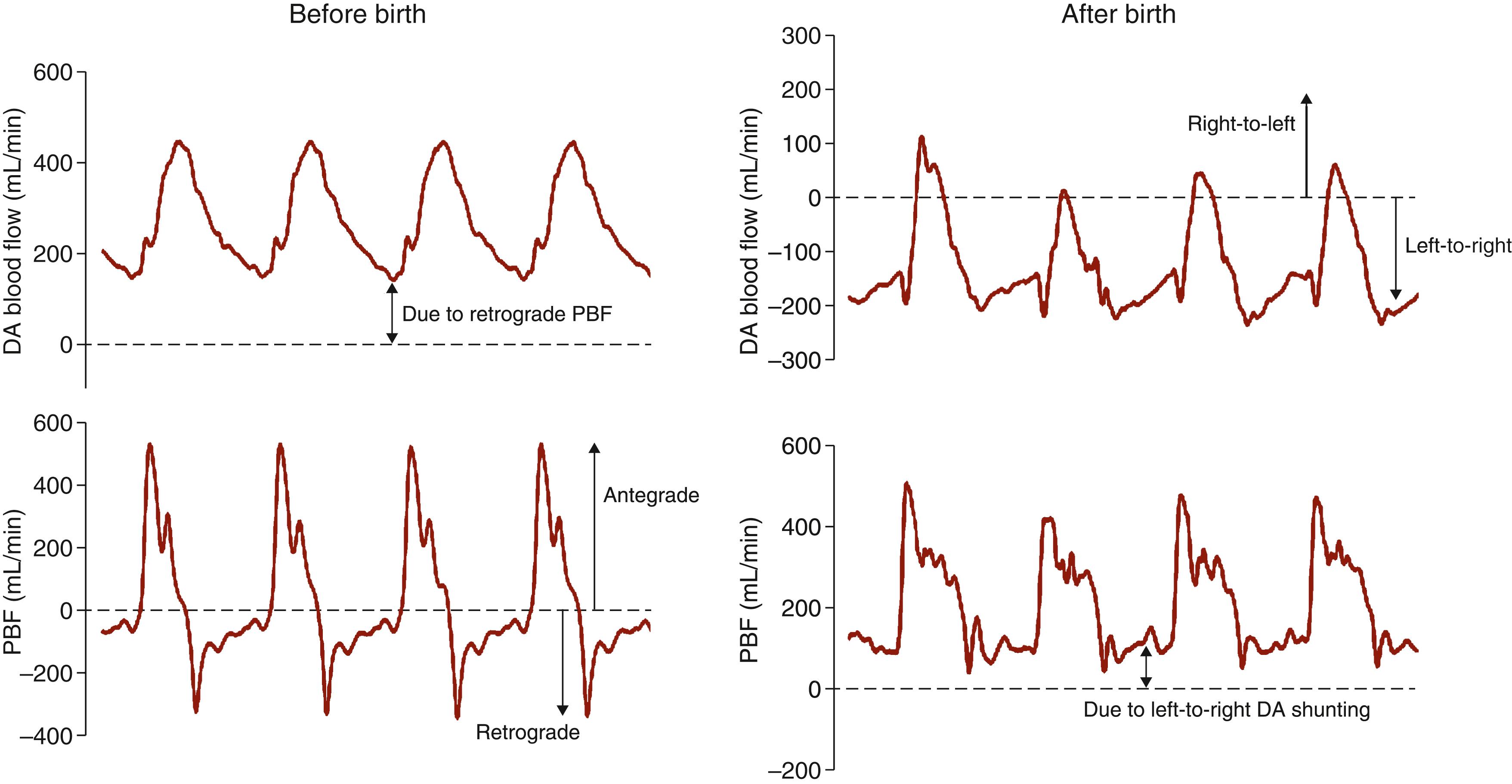
Umbilical venous return is lost following UCC and so the left ventricle becomes solely dependent upon pulmonary venous return for preload. While this has implications for the timing of UCC in relation to lung aeration (see below), the large contribution of left-to-right shunting through the DA to PBF immediately after birth (see Fig. 150.9 ) indicates that the left ventricle contributes to its own preload. That is, shortly after birth, a left ventricle, DA, lung, left ventricle short circuit develops (see Fig. 150.1 ). While this is commonly referred to as “ductal steal” when the DA persists into the newborn period, it is a normal feature of the fetal to neonatal cardiovascular transition immediately after birth. The significance of this is unclear, but as the “short circuit” includes all preductal arteries (see Fig. 150.1 ), it would appear that these vessels retain a privileged position within the newborn circulation. It may also offer an opportunity for the output of both ventricles to gradually come into balance before the DA closes and the circulations separate.
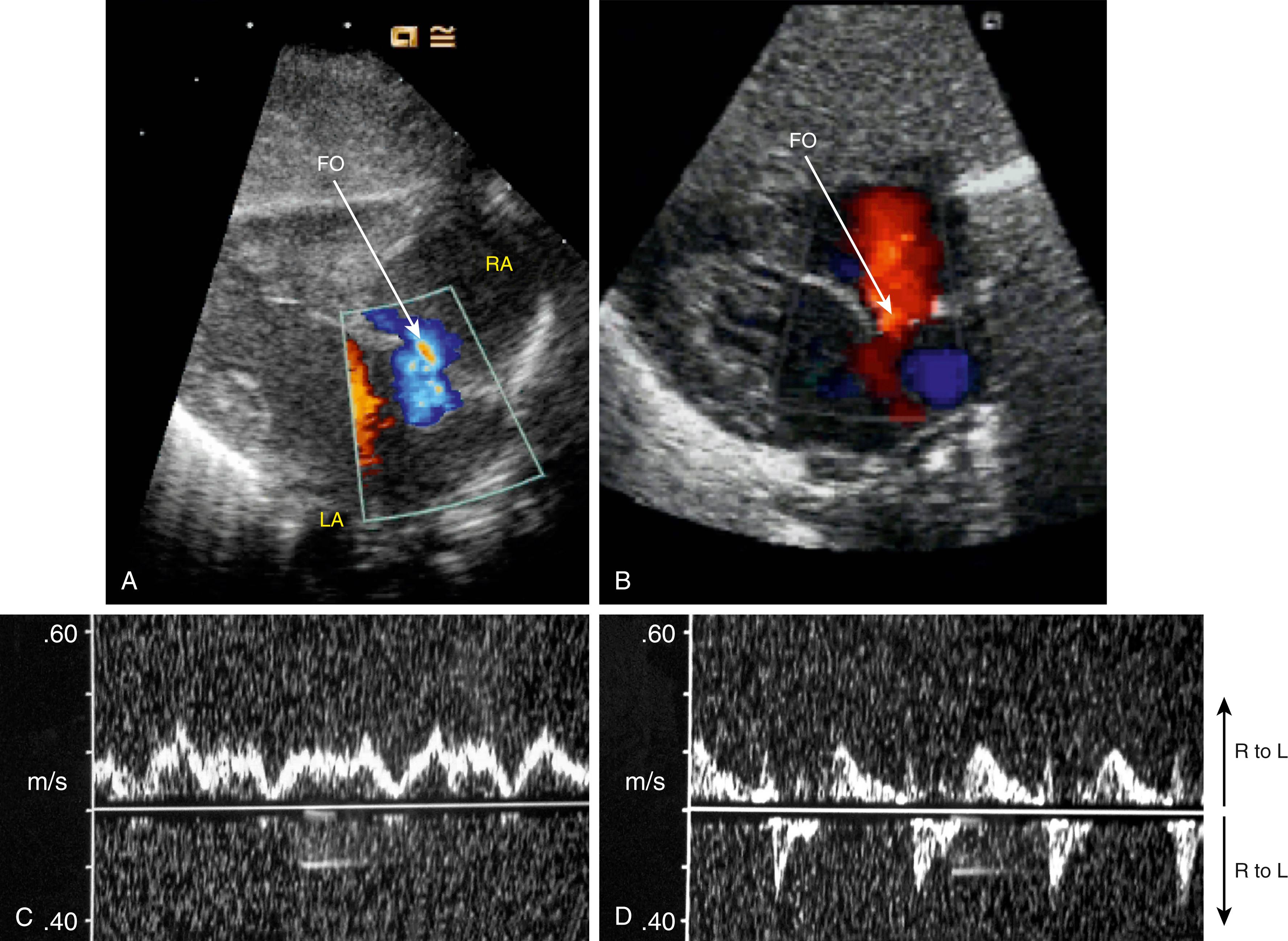
In view of the large amount of left-to-right shunting in the DA (see Fig. 150.9 ), venous return to the right ventricle must decrease, which may induce left-to-right shunting through the FO. While it is widely assumed that flow through the FO is unidirectional, bidirectional flow has been observed in fetal sheep following cord clamping, and left-to-right shunting has been observed in premature infants ( Fig. 150.10 ). This may also help balance preload and output from both ventricles before the two circulations eventually separate. On the other hand, left-to-right shunting through the FO can only occur if left atrial pressure exceeds right atrial pressure, which is thought to be the primary mechanism driving closure of the FO. As such, the contribution of the left ventricle and left-to-right shunting through the DA to PBF (see Fig. 150.9 ) and pulmonary venous return would help to increase left atrial pressure above right atrial pressure and facilitate closure of the FO.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here