Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lactation represents the completion of the reproductive cycle and occurs as one of the major stages of mammary gland development: (1) embryogenesis; (2) mammogenesis; (3) lactogenesis, or secretory differentiation (stage 1 lactogenesis) and secretory activation (stage II lactogenesis); (4) lactation (or stage III lactogenesis), or full milk secretion; and (5) involution.
Hormones play a central role in mammary gland development (estrogen and progesterone in particular) and in lactation (prolactin, insulin, hydrocortisone, human placental lactogen, human growth hormone, and oxytocin).
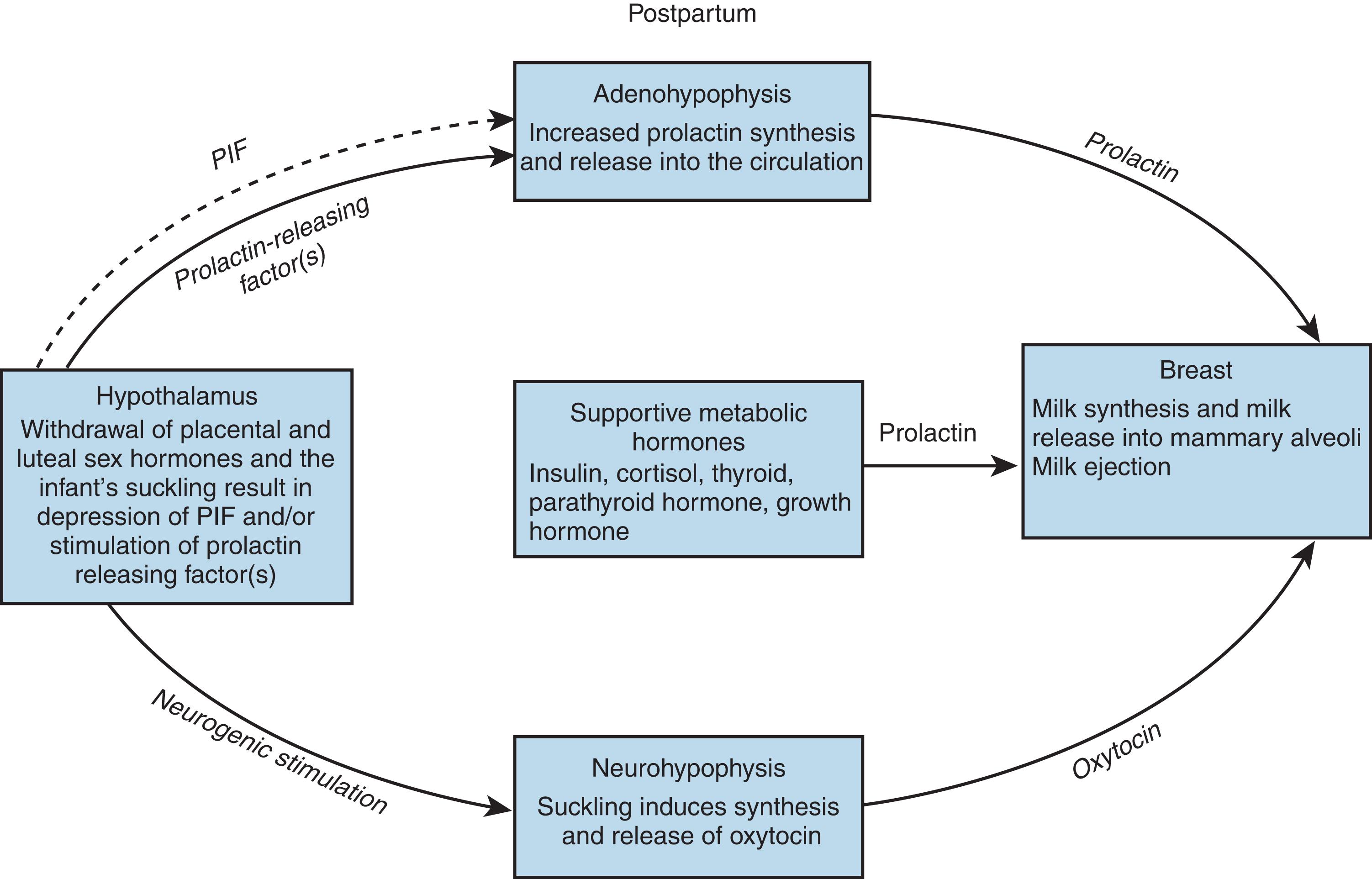
Milk ejection is both a neural and endocrinologic process, whereby suckling stimulates sensory nerve endings in the areola and nipple, which activates the afferent neural reflex pathway via the spinal cord to the mesencephalon and then to the hypothalamus, secreting and releasing prolactin and oxytocin.
Lactation changes the mother’s metabolism greatly, redistributing the blood supply and increasing the demand for nutrients, which requires an increased metabolic rate to accommodate their production.
Milk synthesis and secretion in the mammary alveolus include four major transcellular pathways and one paracellular pathway: (1) exocytosis of milk protein and lactose in Golgi-derived secretory vesicles, (2) milk-fat secretion via the milk-fat globule, (3) secretion of ions and water across the apical membrane, (4) pinocytosis–exocytosis of immunoglobulins, and (5) paracellular pathway for plasma components and leukocytes.
Lactation is the physiologic completion of the reproductive cycle. 1 , 2 , 3 Human infants at birth are the most immature and dependent of all mammals, except for marsupials. The marsupial joey is promptly attached to the teat of a mammary gland in an external pouch. The gland changes as the offspring develops, and the joey remains there until able to survive outside the pouch. In humans, throughout pregnancy, the breast develops and prepares to take over the role of fully nourishing the infant when the placenta is expelled.
There are two stages in the initiation of lactation: secretory differentiation and secretory activation. Pang and Hartmann 3 aptly describe them: “Secretory differentiation represents the stage of pregnancy when the mammary epithelial cells differentiate into lactocytes with the capacity to synthesize unique milk constituents such as lactose.” They further explain that this requires the presence of a “lactogenic hormone complex.” This complex of reproductive hormones includes estrogen, progesterone, prolactin, and some other metabolic hormones. Secretory activation, they note, is the initiation of copious milk secretion associated with major changes in the concentrations of many milk constituents. With the withdrawal of progesterone, secretory activation is triggered. This requires prolactin as well as insulin and cortisol. This terminology, introduced by Pang and Hartmann, 3 is being used more, but both this and the original terminology noted throughout the rest of this chapter are widely used within the related literature.
The breast is prepared for full lactation from 16 weeks’ gestation without any active intervention from the mother. It is kept inactive by a balance of inhibiting hormones that suppress target-cell response. In the first few hours and days postpartum, the breast responds to changes in the hormonal milieu and to the stimulus of the newborn infant’s suckling to produce and release milk. 4 The existence of mammary stem cells (MaSCs) has been speculated because the mammary gland has been regenerated by transplanting epithelial fragments in mice. Transplanted cells contributed to both luminal and myoepithelial lineages. From these, functional lobuloalveolar units during pregnancy were generated. The cells had self-renewing properties. The serial transplantations of Shackleton et al. 5 have established that single cells are multipotent and self-renewing and can generate a functional mammary gland. These cells create the potential for unlimited further understanding of the mammary gland.
Assessing the physiologic activity of the breast, accounting for both biochemical and calorimetric energy efficiencies, the human lactation process converts energy at an 80% to 85% efficiency rate. 6 Butte et al. measured and compared the energy expenditure during pregnancy and lactation. For exclusive breastfeeding, the energy cost of lactation was 2.62 MJ day −1 based on a mean milk production of 749 g day −1 and an estimated energetic efficiency of 0.80. The woman’s daily energy expenditure during lactation is significantly greater than during pregnancy.
This chapter provides a review of the physiologic adaptation of the mammary gland to its role in infant survival. Several major reviews that include substantial bibliographies for readers who need the detailed reports of the original investigators are referenced. Newer scientific techniques in the study of human lactation provide more precise, more detailed, and more integrated data on which the clinician can base a physiologic approach to lactation management.
Box 3.1 lists the abbreviations for the hormones that are involved in lactation and are discussed in this chapter. 7 , 8 , 9 , 10 , 11 , 12 , 13
| Adrenocorticotropic hormone | ACTH |
| Epidermal growth factor | EGF |
| Feedback inhibitor of lactation | FIL |
| Follicle-stimulating hormone | FSH |
| Growth hormone (human growth hormone) | GH (hGH) |
| Heregulin | HER |
| Human growth factor | hGF, HGF |
| Human placental lactogen | hPL |
| Insulin-like growth factor-1 | IGF-1 |
| Prolactin | PRL |
| Prolactin-inhibiting factor | PIF |
| Thyroid-stimulating hormone | TSH |
| Thyrotropin-releasing hormone | TRH |
| Transforming growth factor beta | TGF-β |
The mammary gland is a unique organ in human physiology, given that it is the only organ not fully developed at birth. Rather, breast development occurs through a series of steps, beginning in the embryonic phase and ending in menopause.
Epithelial apoptosis has a key role in the development and function of the mammary gland. It begins with the formation of the ducts in the embryonic phase and occurs again at puberty and within the cyclical stages of menses. Regulated apoptosis occurs at several stages of mammary development. In the embryo, epithelial buds emerge from ectoderm into mammary mesenchyme, which is the origin of the ductal tree. When the ducts later hollow out in puberty, extensive apoptosis occurs within the terminal bud. 14
Deregulated apoptosis contributes to the malignant progression in the genesis of breast cancer. Research in apoptosis continues because it may lead to new cancer treatments, but the knowledge itself related to breast development and function will be valuable.
When suckling ceases during weaning, the alveolar component of the gland involutes by both apoptosis and tissue remodeling, which rebuilds the gland to the prepregnancy state.
Much is being learned about mammary development and function through the intense study of the breast as an experimental system. The use of novel “knockout” mouse models has been employed to study nursing failure. The apoptosis control mechanism from the perspective of the signaling pathways has also been studied. Further work at the level of the cell is under way, including extensive genetic analysis. 14
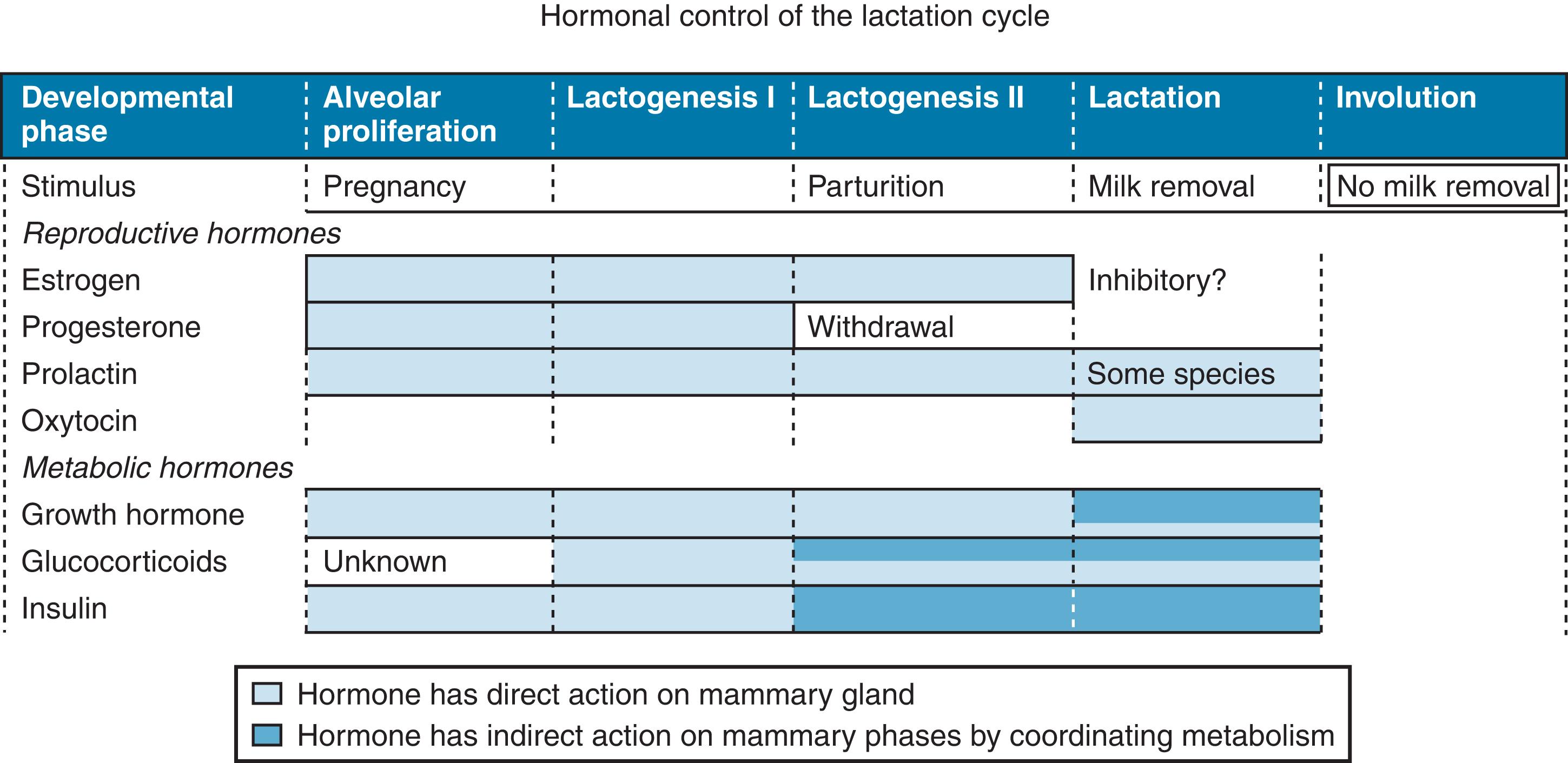
In contrast to most organs, which are fully developed at birth, the mammary gland undergoes most of its morphogenesis postnatally, in adolescence, and in adulthood. 15 Lactation is an integral part of the reproductive cycle of all mammals, including humans. The hormonal control of lactation can be described in relation to the five major stages in the development of the mammary gland: (1) embryogenesis; (2) mammogenesis, or mammary growth; (3) lactogenesis, or initiation of milk secretion; (4) lactation (stage III lactogenesis), or full milk secretion; and (5) involution ( Table 3.1 ). 15
| Developmental Stage | Hormonal Regulation | Local Factors | Description | |
|---|---|---|---|---|
| Embryogenesis | ? | Fat pad necessary for ductal extension | Epithelial bud develops in 18- to 19-week-old fetus, extending short distance into mammary fat pad with blind ducts that become canalized; some milk secretion may be present at birth | |
| Mammogenesis |
|
|
|
|
| Lactogenesis | Progesterone withdrawal, PRL, glucocorticoid | Not known | Onset of milk secretionStage I: midpregnancyStage II: parturition | |
| Lactation | PRL, oxytocin | FIL | Ongoing milk secretion | |
| Involution | PRL withdrawal | Milk stasis, ?, FIL | Alveolar epithelium undergoes apoptosis and remodeling; gland reverts to prepregnant state | |
a See Box 3.1 for abbreviations.
Current terminology divides lactogenesis into two stages. 3 Stage I, or secretory differentiation , takes place during pregnancy when the gland is sufficiently developed to actually produce milk. It begins about midpregnancy (approximately 16 weeks). It can be identified by measuring the levels of plasma lactose and α-lactalbumin. 16 Should the mother deliver at this point, milk would be produced. Some mothers can express colostrum during this time. As the pregnancy proceeds, milk production is inhibited by high levels of circulating progesterone in most mammals and estrogen as well in humans.
Stage II of lactogenesis is the onset of copious milk production at delivery. In all mammals, it is associated with the drop in progesterone levels ( Fig. 3.1 ). This drop occurs to herald delivery in some species so that milk is copious when the young are born. In humans, these levels drop during the first 4 days postpartum, which is reflected by the milk “coming in” during this time. The drop in progesterone is accompanied by the transformation of the mammary epithelium to produce increased volumes of milk, referred to by Pang and Hartmann 3 as secretion activation . This change includes a change in the permeability of the paracellular pathway and changes in secretion of protective factors (i.e., lactoferrin, immunoglobulins), as well as increases in all milk components that parallel increased glucose production.
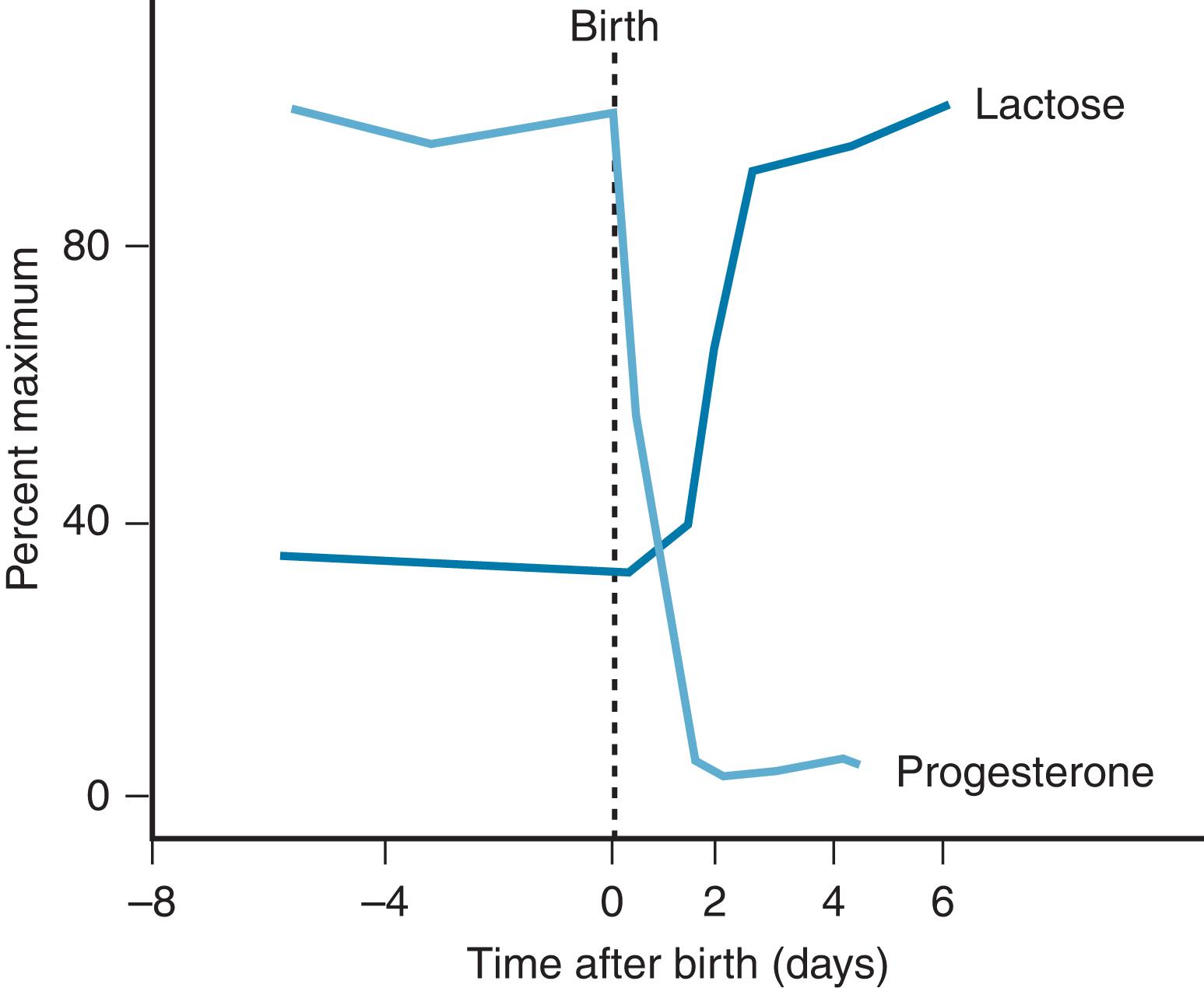
During the next 10 days, the composition of the milk slowly changes to mature milk. The composition then changes slowly over the months of full exclusive breastfeeding.
Embryogenesis begins with the mammary band, which develops around the 35th embryonic day and progresses to a bud at the 49th day (see Chapter 2 ). The ducts continue to elongate to form a mammary sprout, which invades the fat pad, branches, and canalizes, forming the rudimentary mammary ductal system present at birth. After birth, the growth of this set of small branching ducts parallels the child’s linear growth but remains limited, probably controlled by growth hormone (GH) before the onset of ovarian activity.
Under the influence of sex steroids, especially the estrogens, the mammary glandular epithelium proliferates, becoming multilayered. Buds and papillae then form. The growth of the mammary gland is a gradual process that starts during puberty. The process depends on pituitary hormones. Lobuloalveolar development and ductal proliferation also depend on an intact pituitary gland.
Box 3.2 15 , 16 , 17 , 18 , 19 , 20 presents six well-documented factors that help explain the organization of mammary growth. Much of this work has resulted since the availability of “knockout” studies in mice and associated techniques.
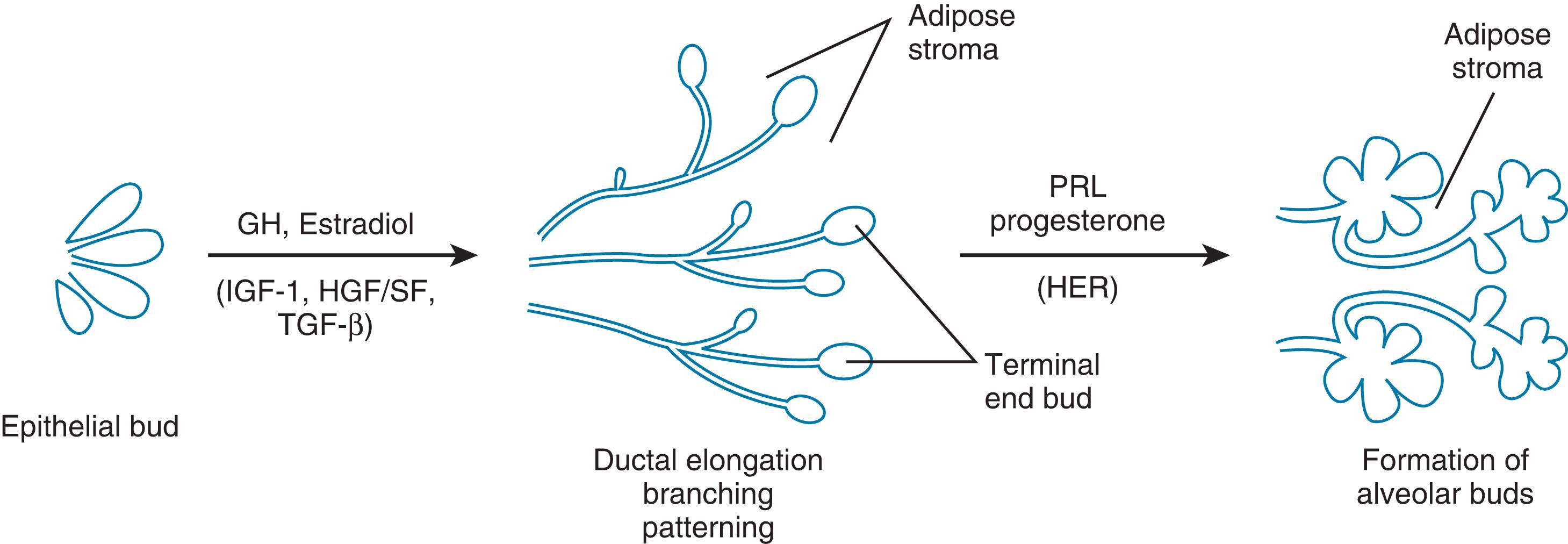
Mammary ducts must grow into an adipose tissue pad if morphogenesis is to continue. Only adipose stroma supports ductal elongation. The mammary epithelium is closely associated with the adipocyte-containing stroma in all phases of development. In midgestation during human fetal development, a fat pad is laid down as a separate condensation of mesenchyma. Rudimentary ducts expand into the fat pad but do not progress. 15 At puberty, the ducts elongate to fill the entire fat pad, terminating growth as they reach the margins of the fat pad.
Estrogen is essential to mammary growth. 3 Ductal growth does not occur in the absence of ovaries but can be stimulated when estrogen is provided. In the ovariectomized (oophorectomized) mouse, an estrogen pellet placed in the mammary tissue stimulates growth in that gland but not in the opposite gland. When the estrogen receptor is “knocked out” in the mouse, no mammary development occurs. The increase in estrogen at puberty results in mammary development. Although estrogen is essential, it is not adequate alone. 3
The exact location of the estrogen receptors in human breasts is unclear. Estrogen receptors are not in the proliferating cells and have not been located in the stroma. Cells with estrogen receptors secrete a paracrine factor that is responsible for the proliferation of ductal cells. This paracrine factor may hold the key to understanding both normal and abnormal breast development.
In addition to estrogen, the pituitary gland is necessary for breast development . Kleinberg 17 has identified growth hormone (GH) as important to pubertal development and the development of the terminal end buds in the breast. Prolactin could not replace GH in these experiments, but insulin-like growth factor-1 (IGF-1) could. It is produced in the stromal compartment of the mammary gland under stimulation by GH, and together with estradiol from the ovaries, IGF-1 brings about ductal development at puberty.
Transforming growth factor beta (TGF-β) maintains the spacing of the mammary ducts as they branch and elongate. 15 These ducts exhibit unique behavior during growth, turning away to avoid other ducts and end buds. This avoidance behavior accounts for the orderly development of the duct system in the breast and the absence of ductal entanglements. This pattern provides ample space between ducts for later development of alveoli. TGF-β has been identified as the negative regulator and is found in many tissues, including breast tissue produced by an epithelial element. The pattern formation in ductal development depends on the localized expression of TGF-β. 18
Progesterone secretion brings about the side branching of the mammary ducts. 19 The presence of progesterone receptors in the epithelial cells has been confirmed by studies in knockout mice in which mammary glands develop to the ductal stage but not to alveolar morphogenesis. Ormandy et al. 20 established that prolactin is necessary for full alveolar development through prolactin receptor studies in knockout mice in which mammary glands do not develop beyond the ductal stage. This was further confirmed in murine mammary cultures in which full development of the alveoli depends on prolactin. Further, when prolactin is withdrawn, apoptosis of the alveolar cells occurs. 19
The coordination of epithelial and stromal activity in the mammary gland is complex. Hepatocyte growth and scatter factor has been associated with the process during puberty. 20 Another growth factor, heregulin, a member of the epidermal growth factor (EGF) family, has been identified in the stroma of mammary ducts during pregnancy.
Neville 15 has diagrammed the regulation of mammary development ( Fig. 3.2 ). She notes that the concentrations of estrogen, progesterone, and lactogenic hormone in the form of prolactin or placental lactogen (PL) greatly increase, enhance alveolar development, and result in the differentiation of alveolar cells. Although many investigators have contributed pieces to the puzzle of mammogenesis, Neville succeeded in creating the current visualization. 15
Mammogenesis occurs in two phases as the gland responds to the hormones of puberty and later of pregnancy. 3 During the prepubertal phase, the primary and secondary ducts that develop in the fetus in utero continue to grow in both boys and girls in proportion to growth in general. Shortly before puberty, a more rapid expansion of the duct system begins in girls. The growth of the duct system seems to depend predominantly on estrogen and does not occur in the absence of ovaries. The complete growth of the alveoli requires stimulation by progesterone as well.
Studies of hypophysectomized animals have shown the failure of full mammary growth even with adequate estrogen and progesterone. The secretion of prolactin and somatotropin by the pituitary gland results in mammary growth. Adrenocorticotropic hormone (ACTH) and thyroid-stimulating hormone (TSH) acting on the adrenal and thyroid glands also play a minor role in the growth of the mammary gland.
Growth and development during organogenesis involve the interaction of cells with extracellular matrices and neighboring cells. 21 Necropsy breast specimens from six male and eight female infants ranging in age from 1 day to 9 months were studied to determine the process of organogenesis in humans. 22 Integrins were expressed in a pattern that correlates with morphologic and functional differentiation of the normal mammary gland. Integrins are transmembrane glycoproteins that form receptors for extracellular matrix proteins, such as fibronectin, laminin, and collagen. Integrins are widely expressed in normal tissue and are considered critical to the control of cell growth and differentiation. This suggests integrin’s involvement in the functional characterization of the adhesion molecules in the breast.
When the hypophyseal–ovarian–uterine cycle is established, a new phase of mammary growth, which includes extensive branching of the system of ducts and proliferation and canalization of the lobuloalveolar units at the distal tips of the branches, begins. 21 The organization of the stromal connective tissue forms the interlobular septa. The ducts, ductules (terminal intralobular ducts), and alveolar structures are formed by double layers of cells. One layer, the epithelial cells, circumscribes the lumen. The second layer, the myoepithelial cells, surrounds the inner epithelial cells and is bordered by a basement lamina.
The cyclic changes of the adult mammary gland can be associated with the menstrual cycle and the hormonal changes that control that cycle. Estrogens stimulate parenchymal proliferation, with the formation of epithelial sprouts. This hyperplasia continues into the secretory phase of the cycle. Anatomically, when the corpus luteum provides increased amounts of estrogens and progesterone, there is lobular edema, thickening of the epithelial basal membrane, and secretory material in the alveolar lumen. 21 Lymphoid and plasma cells infiltrate the stroma. Clinically, mammary blood flow increases in this luteal phase. This increased flow is experienced by women as fullness, heaviness, and turgescence. The breast may become nodular because of interlobular edema and ductular-acinar growth.
After the onset of menstruation and the reduction of sex steroid levels, milk-secretory prolactin action is limited. Postmenstrual changes occur rapidly, with degeneration of glandular cells and proliferation tissue, loss of edema, and a decrease in breast size. The ovulatory cycle actually enhances mammary growth in the early years of menstruation (until about age 30 to 35 years) because the postmenstrual regression of the glandular-alveolar growth after each cycle is not complete. 21 These changes of ductal and lobular proliferation, which occur during the follicular phase before ovulation, continue in the luteal phase and regress after the menstrual phase, exemplifying the sensitivity of this target organ to variations in the balance of hormones.
Fowler et al. 23 measured cyclic changes in the composition and volume of the breast during the menstrual cycle using nuclear magnetic resonance T1-weighted imaging. The T1 relaxation time (spin-lattice T1 relaxation) is a measure of the rate of energy loss from tissues after T1 excitation. This energy loss depends on the biophysical environment of the excited protons. A short T1, therefore, indicates the presence of lipids and organic structures that bind water tightly. A longer T1 occurs with greater hydration and with the greatest amount of cellular water. This study revealed that the lowest total breast volume and parenchymal volume of T1 and water content occurred between days 6 and 15 of the cycle. Between days 16 and 28, T1 rose sharply, and it peaked on the 25th day. The rise in parenchymal volume in the second half of the cycle resulted from not only increased tissue water but also from growth and increased tissue fluid, according to Fowler et al. 23
Hormonal influences on the breast cause profound changes during pregnancy ( Figs. 3.3 and 3.4 ). Early in pregnancy, a marked increase in ductular sprouting, branching, and lobular formation is evoked by luteal and placental hormones. 21 PL, prolactin, and chorionic gonadotropin have been identified as contributors to the accelerated growth (see Fig. 3.4 ). The dichorionic ductular sprouting has been attributed to estrogen, and lobular formation to progesterone.
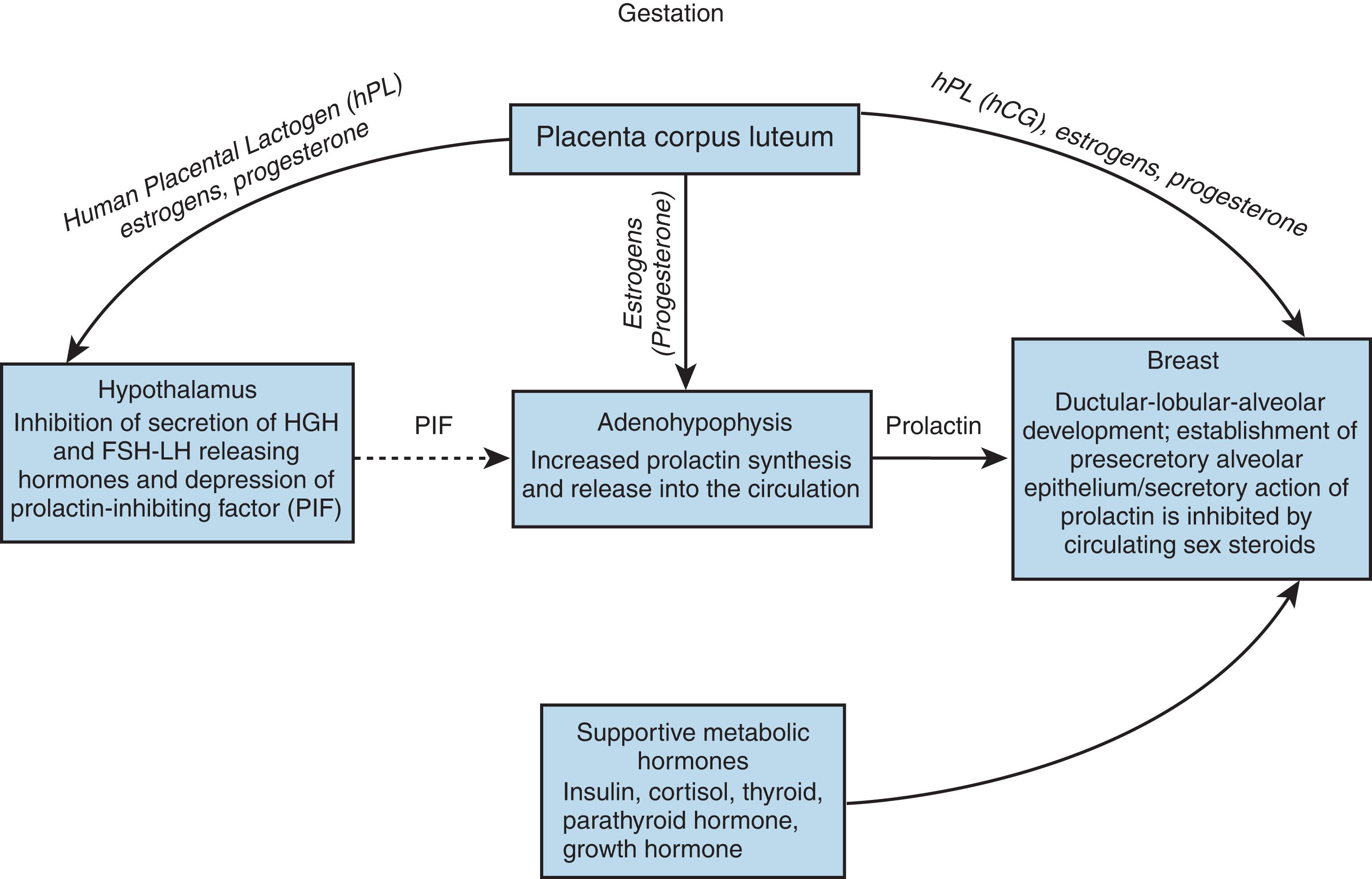
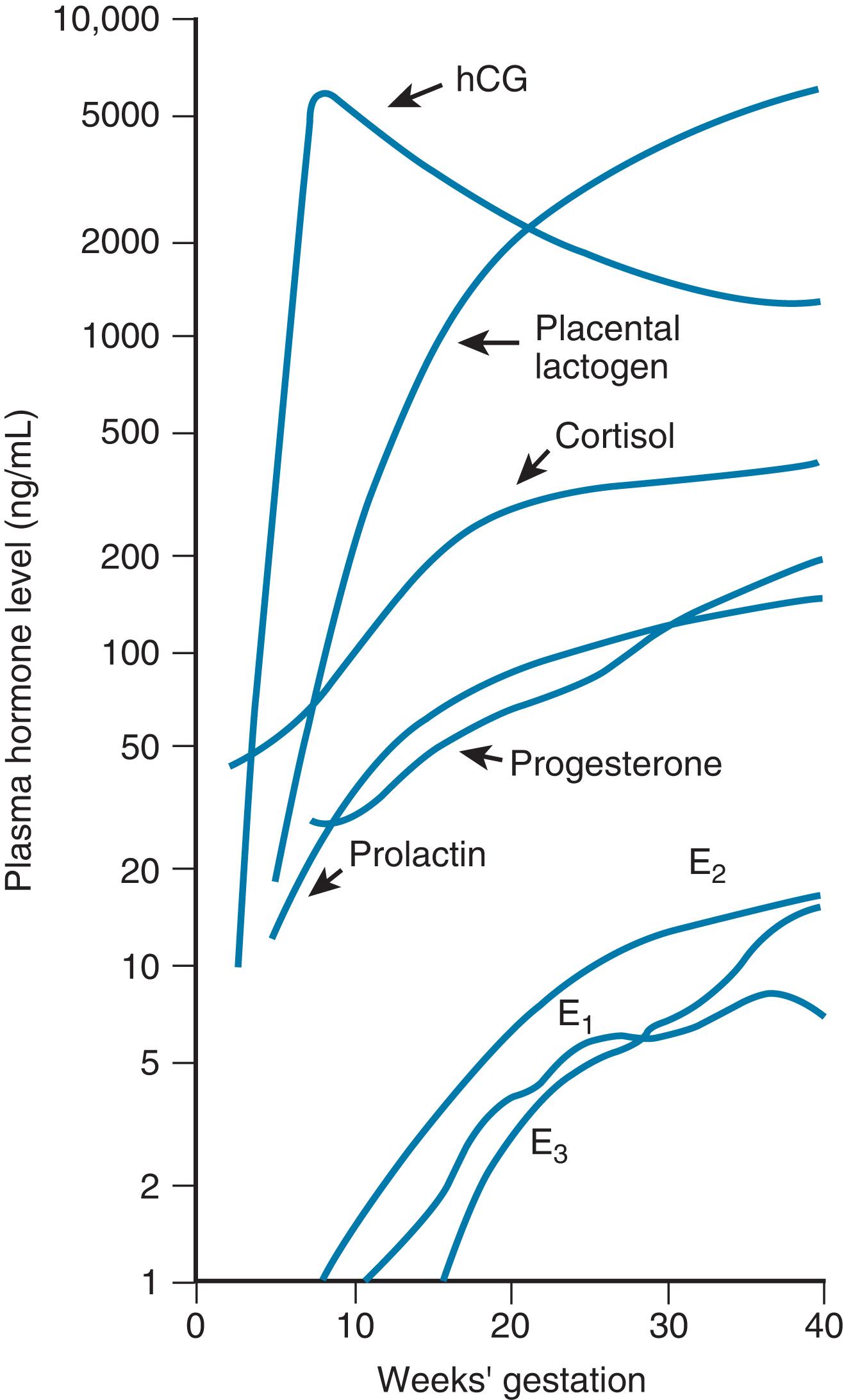
Prolactin is essential for complete lobular-alveolar development of the gland. Growth of the mammary lobular-alveolar system requires the “lactogenic hormone complex” of estrogen, progesterone, prolactin, and certain metabolic hormones. 24 Prolactin, as with other protein hormones, exerts its effect through receptors for the initiation of milk secretion located on the alveolar cell surfaces. The induction of milk synthesis requires insulin-induced cell division and the presence of cortisol.
From the third month of gestation, secretory material that resembles colostrum appears in the acini. Prolactin from the anterior pituitary gland stimulates the glandular production of colostrum. By the second trimester, PL begins to stimulate the secretion of colostrum. A mother who delivers after 16 weeks’ gestation will secrete colostrum, even though she has had a nonviable infant. This demonstrates the effectiveness of hormonal stimulation on lactation.
An estrogen-mediated increase in prolactin secretion in pregnancy may produce as much as a 10- to 20-fold increase in plasma prolactin. This effect may be partially controlled by lactogen from the placenta, which inhibits the production of prolactin. 3 Hormonal regulation of the growth and proliferation of the mammary gland cells has been carefully studied in many species.
Studies of mice in which receptors for each of the hormones have been ablated demonstrate that progesterone and prolactin (or possibly placenta lactogen) are key to alveolar development in pregnancy. The major inhibitor of milk production during pregnancy has been shown to be progesterone, whereas estrogen also contributes to secretion inhibition. 21
A complex sequence of events, governed by hormonal action, prepares the breast for lactation (see Fig. 3.3 ). During pregnancy, 17β-estradiol stimulates the ductal system of epithelial cells to elongate. In contrast to puberty, however, when estrogens appear to directly and indirectly stimulate breast development, estrogens have no indispensable role in mammary development during pregnancy except as a prolactin potentiator: according to Neville, 12 when estrogen levels are low in pregnancy, the breast still develops. Estrogen levels are normally high in pregnancy, but not for mammogenesis. Induced lactation in the cow is dependably reproduced with 7 days of estrogen and progesterone treatment. Progesterone, in turn, induces the specific epithelial cells of the tubular invaginations to produce distinct ducts, which branch from the main tubules. 3 , 21
The end result of the combined actions of estrogen and progesterone is a richly branched arborization of the gland. Highly differentiated secretory alveolar cells develop at the ends of these ducts under the influence of prolactin ( Fig. 3.5 ).
Serum growth factor, which is present in normal human serum, and insulin can stimulate the stem cells of the gland to proliferate. These dividing cells are further directed to the formation of alveoli by corticosteroid hormones. At least two types of cells are identified in the epithelial layer of the gland: stem cells and secretory alveolar cells. At this point in the pregnancy, prolactin influences the production of the constituents of milk.
Transforming growth factor-β (TGF-β) influences pattern formation in the developing mammary gland and may negatively regulate ductal growth as well. 18 The pattern of mammary ductal development varies widely among species and is a function of both genotype and hormonal status. Normal human breast cells secrete TGF-β and are themselves inhibited by it, suggesting an autoregulatory feedback circuit that may be modulated by estradiol. Growth and patterning of the ductal tree are regulated in part by TGF-β operating through an autocrine feedback mechanism and by paracrine circuits associated with epithelial-stromal interactions. 24
The high circulating levels of prolactin in pregnancy are not associated with milk production partly because of the progesterone antagonism of the stimulatory action of prolactin on casein messenger ribonucleic acid (mRNA) synthesis. During late pregnancy, the lactogenic receptors, which have similar affinities for both prolactin and human placental lactogen (hPL), are predominantly occupied by hPL. High doses of estradiol impair the incorporation of prolactin into milk secretory cells.
Prolactin is prevented from exerting its effect on milk excretion by the elevated levels of progesterone. After the drop in progesterone and estrogen at delivery, copious milk secretion begins. The key hormone requirements for lactation to begin are prolactin, insulin, and hydrocortisone. A high level of plasma prolactin is essential to lactogenesis in humans as well. There is a question as to whether it is a surge in prolactin that is necessary for lactogenesis at parturition. Prolactin levels are now described as biphasic in humans for the initiation of lactogenesis at birth. 25 Prolactin stabilizes and promotes transcription of casein mRNA and stimulates the synthesis of a lactalbumin that is the regulatory protein of the lactose–synthetase enzyme system. 26 Prolactin further increases the lipoprotein lipase activity in the mammary gland. Prolactin exists in three heterogenic forms of varying biologic activity. The monomer is in the greatest quantity and is the most active form.
Stage I lactogenesis starts approximately 12 weeks before parturition and is heralded by significant increases in lactose, total proteins, and immunoglobulin; decreases in sodium and chloride; and the gathering of substrate for milk production. The composition of prepartum secretion is fairly constant until delivery, as monitored by the milk protein α-lactalbumin.
Lactogenesis is initiated in the postpartum period by a fall in plasma progesterone, but prolactin levels remain high ( Fig. 3.6 ). The initiation of the process does not depend on suckling by the infant until the third or fourth day, when the secretion declines if milk is not removed from the breast. 27
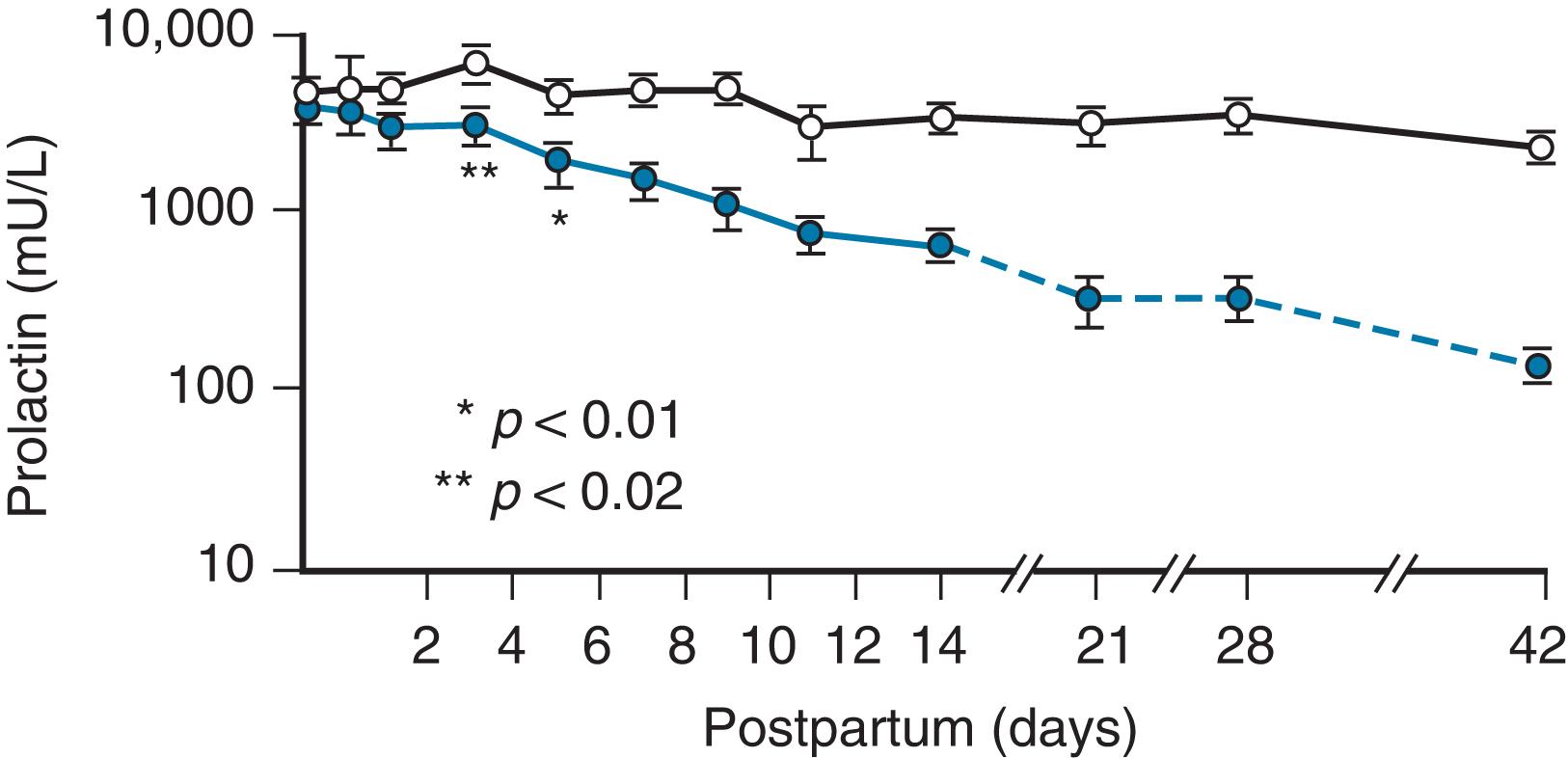
Stage II lactogenesis includes an increase in blood flow and oxygen and glucose uptake as well as a sharp increase in the citrate concentration, considered a reliable marker for lactogenesis stage II. Stage II at 2 to 3 days postpartum begins clinically when the secretion of milk is copious and biochemically when plasma α-lactalbumin levels peak (paralleling the period when “the milk comes in”). The major changes in milk composition continue for 10 days, when “mature milk” is established. The establishment of the mature milk supply, once called galactopoiesis , is now referred to as stage III of lactogenesis.
The profound changes in milk composition have been established for the period of transition to mature milk in relation to an increase in milk volume. Detailed studies of successfully lactating women were performed by Neville et al., 28 who report that a significant fall in sodium, chloride, and protein and a rise in lactose precede the major increase in milk volume during early lactogenesis. At 46 to 96 hours postpartum, copious milk production is accompanied by an increase in citrate, glucose, free phosphate, and calcium concentrations and a decrease in pH.
The breast, one of the most complex endocrine target organs, has been prepared during pregnancy and responds to the release of prolactin by producing the constituents of milk (see Fig. 3.5 ). The lactogenic effects of prolactin are modulated by the complex interplay of pituitary, ovarian, thyroid, adrenal, and pancreatic hormones ( Fig. 3.7 ).
The ability to lactate is characteristic of all mammals, from the most primitive to the most advanced, and the instinct to feed through suckling occurs spontaneously in infants postpartum. Normal, alert newborns have been observed to “crawl” to the nipple and latch on unassisted when placed on the maternal abdomen following a normal delivery and the clamping and severing of the umbilical cord. 29 The divergence of suckling patterns, however, makes it urgent that human patterns be studied specifically. Some aquatic mammals, such as whales, nurse under water; others, such as the seal and sea lion, nurse on land. Various erect or recumbent postures are assumed by different terrestrial mammals. 30 Nursing may be continuous, as in the joey attached to a marsupial teat, or at widely different intervals characteristic of the species and parallel to the nutrient concentrations of the milk. The intervals may be a half hour for dolphins, an hour for pigs, a day for rabbits, 2 days for tree shrews, or a week for northern fur seals.
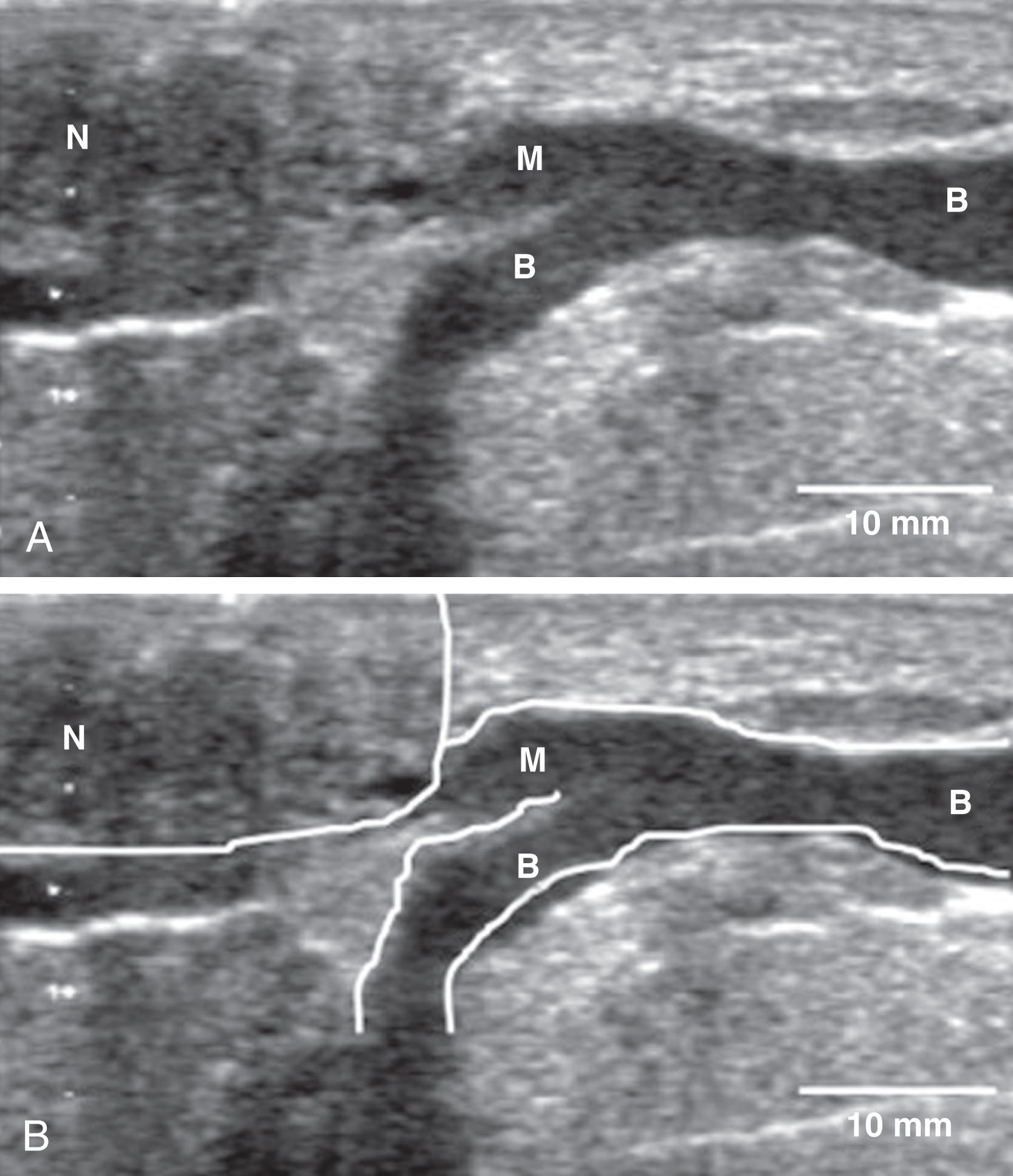
New anatomy research, gathered for the first time in 160 years since the brilliant work with dissections by Sir Ashley Cooper, has been generated in the laboratory of Peter Hartmann in Australia and his eclectic team of scientists. They have had access to the latest digital technology and have shown that the milk ducts of the breast are small ( Fig. 3.8 ), compressible, superficial, and closely intertwined. 31 There are no “dilated sinuses” that store large amounts of milk. The amount of adipose tissue in the breast is very variable and not a measure of the amount of glandular tissue; there is twice as much glandular tissue as fat. 32 Magnetic resonance imaging has identified some central ducts in the breasts of lactating women. The anatomy of the lactating breast was redefined with ultrasound imaging in Hartmann’s laboratory. 33 Ducts were found to number four to eight, and branches drain glandular tissue directly beneath the nipple and merge into a collecting duct very close to the nipple. They do increase in diameter during milk ejection. Milk production is not dependent on neural stimulation but is hormonal. Milk ejection is critical to successful lactation. A failure to remove milk results in decreased milk production. Multiple milk ejections occur during breastfeeding, even though a woman usually only senses the first milk ejection.
Although many anatomic distinctions exist as well, the principal mechanism of milk removal common to all mammals is the contractile response of the mammary myoepithelium under the hormonal influence of oxytocin released from the neurohypophysis. 34
The key function in all species is effective control of milk delivery to the young in the right amount and at the appropriate intervals, which requires a production system, exit channels, a prehensile appendage, an expulsion mechanism, and a retention mechanism. The primary, secondary, and tertiary ducts form an uninterrupted channel for the passage of milk from the milk-producing alveoli to the prehensile appendage. A process of erection of the areolar region facilitates prehension by the young during suckling. The principal object of the suction produced by the facial musculature of the young is to draw the nipple into the mouth and retain it there. Positive pressure is used to expel milk from the gland by the contractile changes in the mammary gland provided by the myoepithelial cells. The sympathetic nervous stimuli can oppose milk ejection by increasing vasoconstrictor tone, thereby reducing the access of circulating oxytocin to the mammary myoepithelium. Sympathetic activity also can occur during conditions of apprehension or muscular exertion. The milk-ejection reflex can be blocked by emotional disturbance or reflex excitation of the neurohypophysis. The central nervous system control of milk ejection indeed suggests that restraining mechanisms exist to ensure that milk ejection can only occur under circumstances wholly conducive to the effective removal of milk by the suckling young.
In all species that have been studied, a rise in intramammary pressure and flow of milk occurs as a reflex event in suckling. The excitation of the neurohypophysis results in the release of oxytocin, which is conveyed via the bloodstream to mammary capillaries, where it evokes contraction of the myoepithelium. The successive ejection-pressure peaks, demonstrated in lactating women, can be duplicated more accurately by a series of separate oxytocin injections than by the same total dose as a single injection or by a continuous infusion of the hormone. This strongly suggests that oxytocin is released from the neurohypophysis in spurts. The study of suckling patterns in all species shows a high degree of ritualization, which in turn suggests a close neural connection between cognitive or behavioral and hormonal responses.
Attention has focused on the mechanisms that control suckling behavior, on its incidence, on events that precipitate and terminate it, on the effects of stress, and on how development modifies it. Suckling is characteristic of each species and is vital for survival. Suckling means to take nourishment at the breast and specifically refers to “breastfeeding” in all species. Sucking , however, means to draw into the mouth by means of a partial vacuum, which is the process employed when bottle-feeding. Sucking also means to consume by licking.
Although suckling has been studied in the young and mothers in other species, a large portion of human data has been collected using a rubber nipple and bottle. Other mammals suckle only in the nutritive mode, whether receiving milk from the nipple or not. Human infants were noted to have two distinct patterns with rubber nipples: a nutritive mode and a nonnutritive mode. 33 , 35 When this work was repeated using the breastfeeding model, no difference between nutritive and nonnutritive suckling rates was seen but, rather, a continuous variation of the suckling rate in response to the milk-flow rate. 36 Suckling rates in other species correlate with milk composition and species-specific feeding schedules (one suck per second in great apes and four to five sucks per second in sheep and goats).
In further experiments, an inverse linear relationship was found between milk flow and the suckling rate. Thus, the higher the milk flow, the lower the suckling rate. In human infants younger than 12 weeks of age, suckling will terminate with sleep and be reinstated on awakening, a pattern that is well described in other species. In infants older than 12 weeks, suckling is not always terminated by sleep. At 12 to 24 weeks, infants will play with the nipple, explore the mother, and not always elicit nipple attachment. Continuous measurement of milk intake during a given feeding from one breast showed a progressive reduction in intake volume per suck and an increase in the proportion of time spent pausing between bursts of sucking.
Using the miniature Doppler 37 ultrasound flow transducer, Woolridge and Baum 38 studied 32 normal mother–baby pairs from 5 to 9 days postpartum. Intakes during trials averaged 34.2 g (±3.7 g) on the first breast and 26.2 g (±3.5 g) on the second breast. At the start of feeds, the average suck volume was approximately 0.14 mL/suck, which decreased to approximately 0.10 mL/suck or less. The mean latency for the release of milk was 2.2 minutes after the infant began to suckle. The researchers also noted that on the first breast, the flow increased and stabilized after 2 minutes, with concomitant slowing and stabilizing of the sucking pattern during the remainder of the feed. On the second breast, the suck volume fell off dramatically toward the end of the feed (50% reduction from peak to end of feed; Fig. 3.9 ).
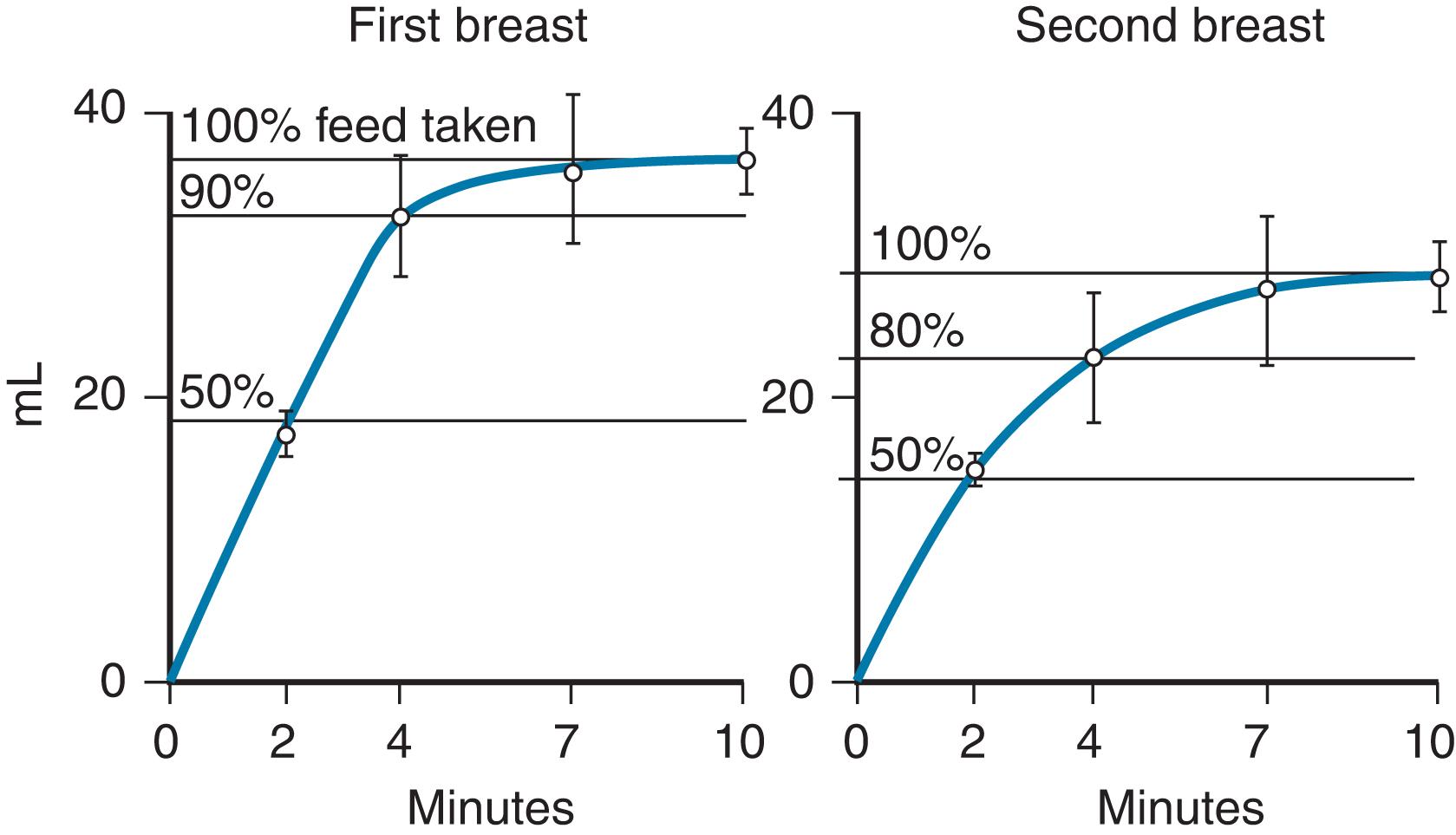
These observations support the theory that infants become satiated at the breast, and milk remains unconsumed in the breast. During the first month of life, infants consume a given amount of fluid with decreasing investment of time. 37 The amount of fluid per suck increases over time. The control of intake appears to come under intrinsic control of the infant during the first month of life. 39
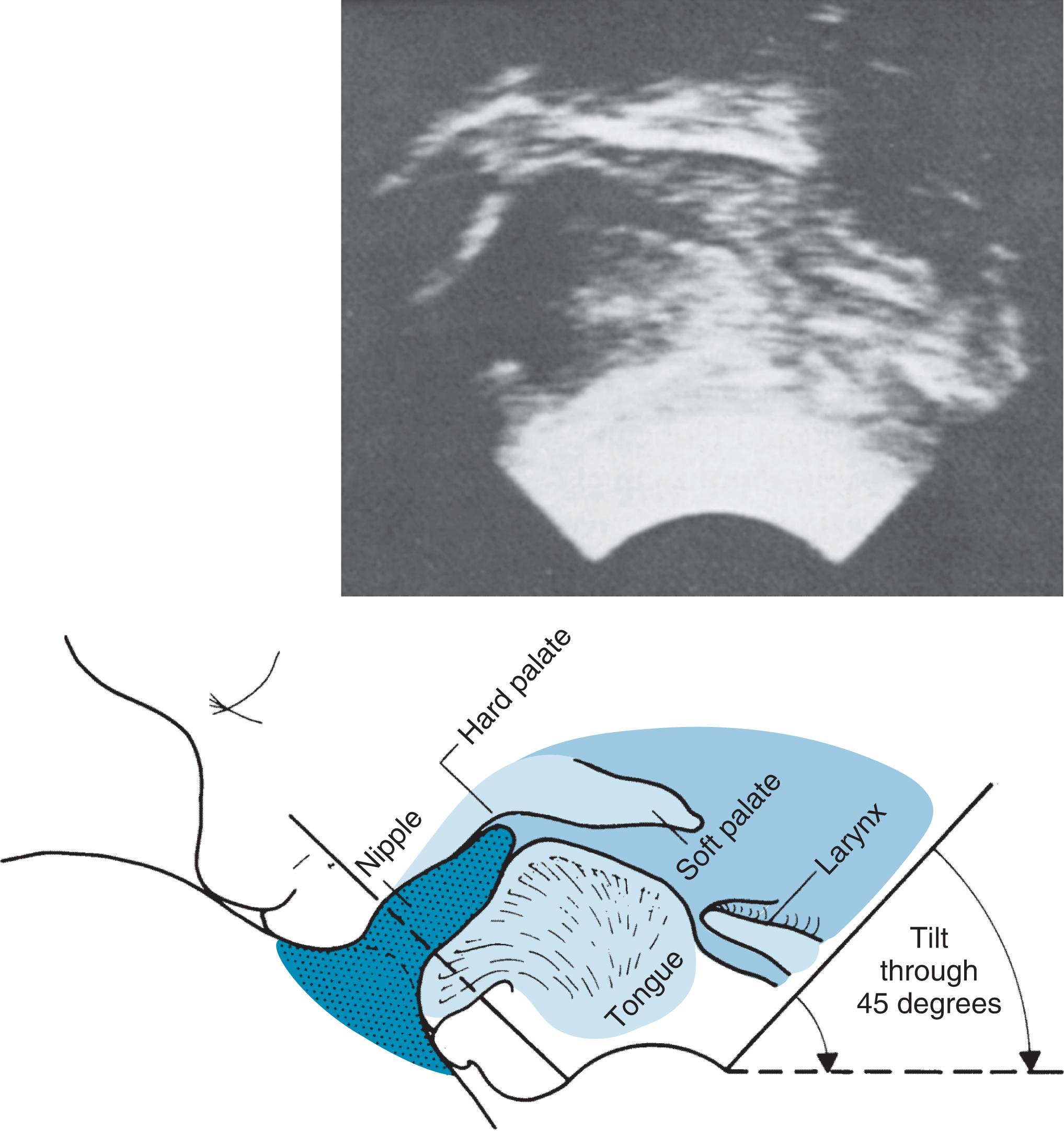
A cineradiographic study of breastfeeding was done by Ardran et al. 40 in 1957 and compared with a similar study of bottle-feeding. 41 The nipples and areolae of 41 breastfeeding mothers were coated with a paste of barium sulfate in lanolin, and cineradiographic films were taken with the infant at the breast. These were then reviewed meticulously. Box 3.3 lists the authors’ conclusions in their original description. These observations are of historical interest, but newer techniques in imagery have more accurately described the understanding of human suckling.
The nipple is sucked to the back of the baby’s mouth, and a teat is formed from the nipple and the adjacent areola and underlying tissues.
When the jaw is raised, this teat is compressed between the upper gum and the tip of the tongue resting on the lower gum. The tongue is applied to the lower surface of the teat from the front backward, pressing it against the hard palate; the teat is reduced to approximately half its former width. As the tongue moves toward the posterior edge of the hard palate, the teat shortens and becomes thicker.
When the jaw is lowered, the teat is again sucked to the back of the mouth and restored to its previous size.
Each cycle of jaw and tongue movement takes place in approximately 1.5 seconds. The pharyngeal cavity becomes airless, and the larynx closes every time the upward movement of the tongue against the teat and hard palate is completed.
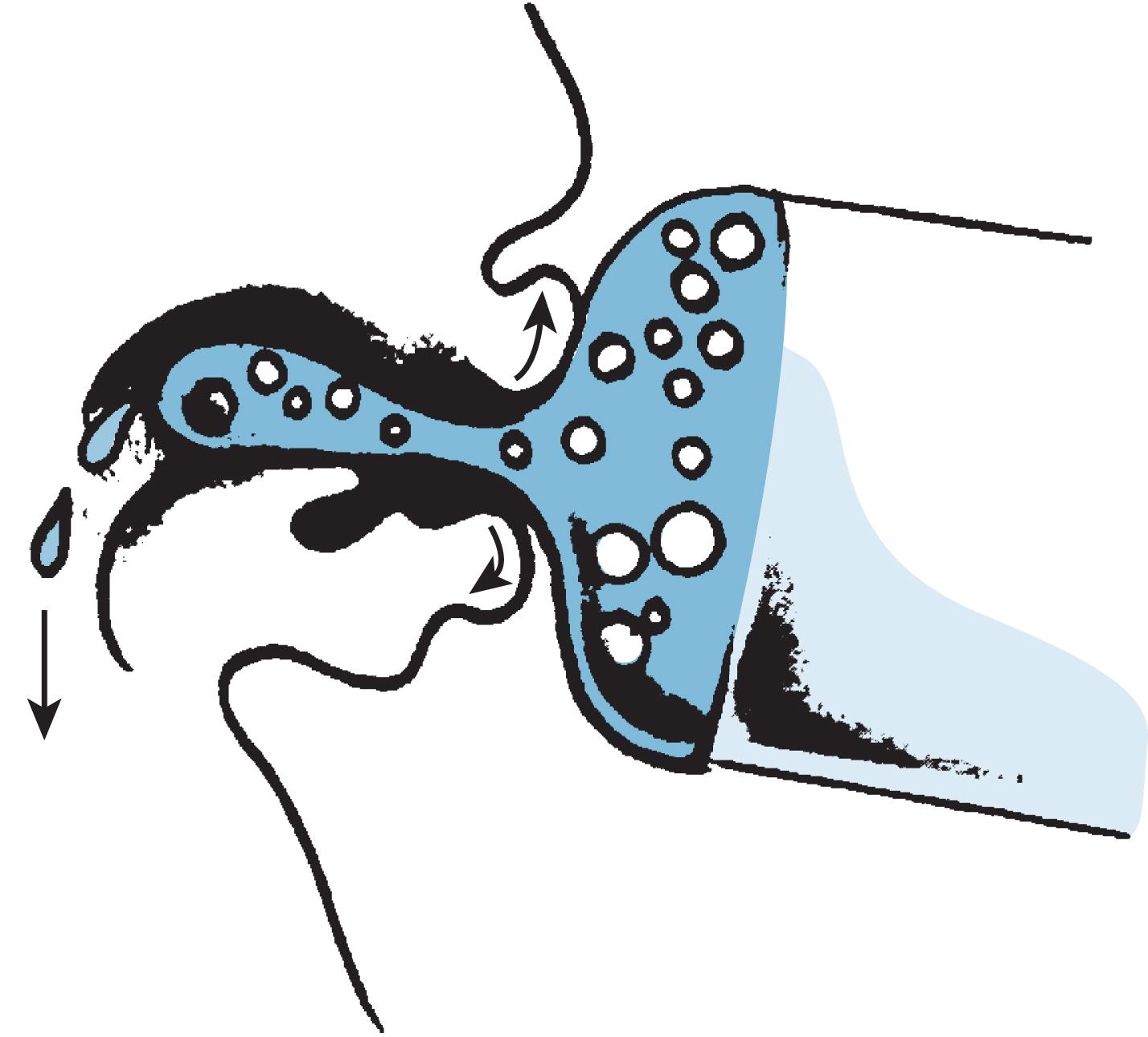
The development of real-time ultrasound improved the definition of images. Several studies have been published using this noninvasive technique to observe the action of the infant’s tongue and buccal mucosa and the maternal nipple areola. Using a video recorder in the 1980s that allowed frame-by-frame analysis and recorded simultaneous respiration, the pattern of suck, swallow, and breathing was documented during a period of active suckling at the breast. A suck was defined by Weber et al. 42 as the beginning of one indentation of the nipple by the tongue to the beginning of the next. Weber et al. had examined six breastfed and six bottle-fed infants between 1 and 6 days of life. Not all sucks were associated with a swallow. Box 3.4 summarizes the process.
The lateral margins of the tongue cup around the nipple, creating a central trough.
The suck is initiated by the tip of the tongue against the nipple, followed by pressure from the lower gum.
There is peristaltic action of the tongue toward the back of the mouth.
The tongue elevation continues to move the bolus of milk into the pharynx.
Observations of suckling using improved techniques from 2 to 26 weeks showed that suckling starts with a series of fast sucking movements and then stabilizes. In a 2-week-old breastfeeding infant, sucking and breathing pattern proportions alternated smoothly at about two sucks to one breath, with swallowing occurring with every suck. Bottle-feeding patterns were variable and sometimes asynchronous with sucking and breathing.
The process of suckling has been described as a pulsating process similar to peristalsis along the rest of the gastrointestinal (GI) tract. This undulating motion, as described by cineradiography, did not involve stroking or friction, as was clearly pointed out by Woolridge. 37 The nipple should not move in and out of the infant’s mouth if the breast is positioned correctly. The tip of the tongue does not move along the nipple. The positive pressure of the tongue against the teat (areola and nipple), coupled with ejection of the milk from increased intraductal pressure, evacuates the milk, not suction. The negative pressure created in the mouth holds the nipple and breast in place and reduces the “work” to refill the ducts. Visual observations and videotapes made in our laboratory to study suckling show the undulating motion of the external buccal surfaces even in newborns. Ultrasound confirms the molding of the buccal mucosa and tongue around the teat, leaving no space.
In breastfeeding, the tongue action is a “rolling,” or peristaltic, action from the tip of the tongue to the base, not side to side. In bottle-feeding, the tongue action is more piston-like or squeezing. When the infant rests between sucks, the human nipple is indented by the tongue, and the latex teat is expanded in bottle-feeding ( Figs. 3.10 and 3.11 ).
The change in nipple dimensions during suckling is detailed by Smith et al., 43 who also used ultrasound and examined 16 term infants ages 60 to 120 days and their mothers. They demonstrated that human nipples are highly elastic and elongate during active feeding, including approximately 2 cm of areola, to form a teat approximately twice its resting length. They also showed that infants’ cheeks (buccal membranes), with their thick layer of fatty tissue, known as sucking fat pads , act to make a passive seal to create a vacuum (as opposed to the concept that the cheeks are sucked in by the negative pressure). Milk ejection was noted to occur after maximal compression of the nipple.
The ability to swallow is developed in utero during the second trimester and has been well demonstrated by fetal ultrasound. Fetal swallowing of amniotic fluid is an important part of the complex regulation of amniotic fluid. The suck is actually part of the oral phase of the swallow. Little was done to examine the role of swallowing on the suckling rate until Burke 44 studied the role of swallowing in the organization of suckling behavior, although with a bottle and solutions of 5% and 10% sucrose solution. The author reported two major observations: “First, the frequency of swallowing in newborns increased significantly as a function of increasing concentration and amount of sucrose solution given per criterion suck. Second, there was a significant difference in the duration of the sucking interresponse times that immediately followed the onset of swallowing and the duration of interresponse times was not associated with swallowing.” These observations explain those of previous investigators regarding nutritive and nonnutritive sucking.
The coordination of sucking and swallowing was observed by ultrasound by Weber et al. 42 as a movement of the larynx. By 4 days of age, both breastfed and bottle-fed infants were swallowing with every suck. Later in the feeding, the ratio of sucks to swallows changed to 2:1 or more until sucking stopped. Swallowing occurred in the end-expiratory pause between expiration and inspiration (see Fig. 3.10 ). The change in the suck/swallow ratio seemed to be a function of the availability of milk.
As one manages infants with difficulty feeding, a number of rituals are often initiated to enhance infant behavior. Only a few of these have been evaluated for their effect. 45 The effect of the infant’s position, that is, supine or supported upright to a 90-degree angle, was found to have no influence on the sucking pattern or pressure. The effect of temperature, however, was found to be significant. Sucking pressure decreased as environmental temperature increased from 80°F to 90°F (26.6°C to 32.2°C), which may have applications in encouraging an infant to nurse. This effect was shown to increase from the third to the fifth day of life. Higher sucking pressures have been recorded in the morning than in the afternoon.
When the size of latex nipples was studied, the large nipple elicited fewer sucks and a slower sucking rate than smaller nipples, although the volume of milk delivered was the same, in this study, with all nipple sizes. Although human nipple size cannot be altered, this knowledge may help in assessing the response of a newborn in specific situations. Increasing nipple size and decreasing sucking rate may be significant in considering using an adult finger for finger feeding.
The volume of each swallow was calculated during breastfeeding in 1905 by Süsswein, 46 who counted swallows and made test weighings. His observations were later confirmed with elaborate electronic equipment. 37 The average swallow of a newborn is 0.6 mL, which is also the exact amount drawn from a bottle equipped with an electromagnetic flowmeter transducer and a valve that responds to negative pressure at each suck in modern studies, even though the sucking mechanism between breast and bottle is different. 47 The size of the hole in the nipple influences the volume of the suck only in the valved bottle. When breastfed infants were compared with a group fed by cup from birth and a group fed by bottle, the breastfed infants had a stronger suck than either of the other two groups, who did not differ from each other in sucking skill. 29 , 48
Patterns of milk intake using electronic weighings in interrupted feeds were studied. Fifty percent of a feed from each breast was consumed in 2 minutes and 80% to 90% by 4 minutes, with minimal feeding from each breast in the last 5 minutes. Bottle-fed infants, evaluated with the same technique of test weighings, took 84% of the feeding in the first 4 minutes. Bottle-feeding patterns were linear, whereas the breastfed infant had a biphasic pattern when nursed on both breasts. The total intake of the two types of feeds was similar in volume in the same 25 minutes of total time.
The high concentration of fat in breast milk toward the end of a feed was hypothesized as a satiety signal to terminate the feeding. When this was studied using high- and low-fat formulas, it was found that high-fat milk did not act to cue babies to slow or stop feeding. 49 In fact, babies appeared to feed more actively on high-fat milk, sucking in longer bursts and with less resting. When human milk of low- and high-fat content was fed from bottles, switching the baby from low-fat breast milk to high-fat breast milk, the babies did not alter either the milk-intake rate or sucking patterns.
To test the hypothesis fully, a study carefully observed infants switching from the first to the second breast and back to the first breast. Infants were 2 months old and well established at exclusive breastfeeding. No significant difference was seen in the time taken to attach to the new breast and the time taken to reattach to the previously suckled breast. Mean milk intake from the first breast was 91.7 g (range 58 to 208 g), higher than that from the second breast (mean 52.5 g, range 8 to 75 g). The mean fat contents before and after nursing on the first breast were 23 and 52 g/L, whereas on the second breast, they were 24 and 48 g/L. This shows that infants will nurse when fat content is higher, contrary to the theory that increasing fat causes satiation. 48
Studies of 3-day-old bottle-fed infants fed sucrose and glucose solutions show that they manifest tongue movements of greater amplitude when fed stronger concentrations of carbohydrate, even though they do not respond to fat content in formula. The sensory apparatus responsible for assessing sweetness is apparently competent in the newborn.
Breathing and sucking during feeding were studied in normal full-term infants from 1 to 10 days of age, measuring breathing, sucking, and flow of fluid from a feeding bottle with a flow meter. No infant aspirated water, but 8 of 18 infants inhaled saline. Even from a bottle, breast milk was associated with more regular breathing than was formula feeding. It has been demonstrated in other species that newborns will become apneic when fed milk from species other than their own. The coordination of breathing and swallowing improves with an increase in milk availability and with the maturity of the infant. 41
The behavior of an infant at birth is the first opportunity to observe the infant’s adeptness at suckling. In a careful analysis of videotapes of newborns in the first 90 minutes of life, Widström and Thingström-Paulsson 50 observed a consistent pattern. Licking movements preceded and followed the rooting reflex in alert infants. The tongue was placed in the bottom of the mouth cavity during distinct rooting. The authors suggest that forcing the infant to the breast might disturb reflex action and tongue position. They further observed that a healthy infant should be given the opportunity to show hunger and optimal reflexes and attach to the mother’s nipple by itself. 49
Righard and Alade 29 observed that an infant placed on the mother’s abdomen will self-attach to the breast and suckle correctly in less than 50 minutes. They further reported that when the infants were separated from their mothers for delivery room procedures, the initial suckling attempts were disturbed, and many infants were too drowsy to suckle at all. 29
Righard and Alade 51 also investigated the prognostic value of suckling technique (faulty vs. correct) during the first week after birth in relation to the long-term success of breastfeeding. For assessment of breastfeeding technique, 82 healthy mother–infant pairs were observed before discharge. The authors defined correct sucking as the infant’s mouth being wide open, the tongue under the areola, and the milk expressed in slow and deep sucks. Incorrect sucking was defined as the infant positioned as if bottle-feeding, using the nipple as a teat. The oral searching reflex was defined as the infant opening the mouth wide in response to proximity of the nipple to the lips and thrusting the tongue forward in preparation to take the breast. This reflex is a part of the normal response to a circumoral stimulus, resulting in rooting by the infant, who comes forward, opens the mouth wide, and extends the tongue when stimulated centrally on the lower lip and even the upper lip. A stimulus on the side of the mouth or cheeks elicits turning to that side.
Stricker and Grueter 52 discovered the pituitary hormone prolactin in 1928. They observed that extracts of the pituitary gland induced lactation in rabbits.
Human prolactin is a significant hormone in pregnancy and lactation. 53 Prolactin also has a range of actions in various species that is greater than any other known hormone. Prolactin has been identified in many animal species, whether they nurse their young or not. Because of the original association with lactation, the term describes its action, “support or stimulation of lactation.” Prolactin, however, has been shown to control nonlactating responses in other species and has been identified with more than 300 different physiologic processes, unrelated to lactation. The study of prolactin was hampered until 1970, when it became possible to separate prolactin from human growth hormone (hGH) and isolate and characterize prolactin from human pituitary glands.
Before 1971, hGH and prolactin in humans were considered the same hormone. Until 1971, in fact, it was thought that prolactin did not exist in humans. 3 However, hGH is present in the human pituitary gland in an amount 100 times that of prolactin. 54
Although prolactin is secreted by the anterior pituitary gland, the brain is exposed to it. Prolactin is found in the cerebrospinal fluid and may even be produced by neurons in the portal vessels of the hypothalamus. Prolactin increases the activity of tuberoinfundibular neurons, which control dopamine. 55
Prolactin, the lactogenic hormone, is essential for glucocorticoid stimulation of the milk-protein genes. 3
Synthesis and secretion are not restricted to the anterior pituitary gland but include multiple sites in the brain (cerebral cortex, hippocampus, amygdala, cerebellum, brainstem, and spinal cord). It is also produced in the placenta, amnion, decidua, and uterus. Evidence suggests that lymphocytes from the immune system, thymus, and spleen release bioactive prolactin. Prolactin is found in the epithelial cells of the lactating mammary gland and the milk itself. Prolactin reaches the milk by crossing the mammary epithelial cell basement membrane, attaches to a specific prolactin-binding protein, and ultimately moves by exostosis through the apical membranes into the alveolar lumen. Prolactin mRNA in milk contains more prolactin variants than serum. Milk prolactin participates in the maturation of the neuroendocrine and immune systems.
The information generated by the use of knockout mice with prolactin knockouts or prolactin-receptor knockouts has refined the understanding of mammary morphogenesis and subsequent lactogenesis. 10 It has been confirmed that prolactin does not operate alone but depends on estrogen, progesterone, and glucocorticoids, as well as insulin, thyroid hormone, parathyroid hormone, and even oxytocin (see Fig. 3.7 ). Prolactin also stimulates the uptake of some amino acids, the uptake of glucose, and the synthesis of milk sugar and milk fats. 1
Plasma prolactin varies in relation to psychosocial stress. Utilizing four different real-life stress studies in a longitudinal design, Theorell 56 found that changing situations associated with passive coping are accompanied by increased plasma prolactin levels. Changing situations associated with active coping are associated with unchanged or even lowered prolactin levels. The regulation of plasma prolactin is part of a dopaminergic system (see the list of pharmacologic suppressors in the next section).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here