Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
When people descend beneath the sea, the pressure around them increases progressively as they go to greater depths. To keep the lungs from collapsing, air must be supplied at very high pressure to keep them inflated. This maneuver exposes the blood in the lungs to extremely high alveolar gas pressures, a condition called hyperbarism . Beyond certain limits, these high pressures cause major alterations in body physiology and can be lethal.
A column of seawater 33 feet (10.1 meters) deep exerts the same pressure at its bottom as the pressure of the atmosphere above the sea. Therefore, a person 33 feet beneath the ocean surface is exposed to 2 atmospheres (2 atm) pressure, with 1 atm of pressure caused by the weight of the air above the water and the second atmosphere caused by the weight of the water. At 66 feet, the pressure is 3 atm, and so forth, in accord with the table in Figure 45-1 .
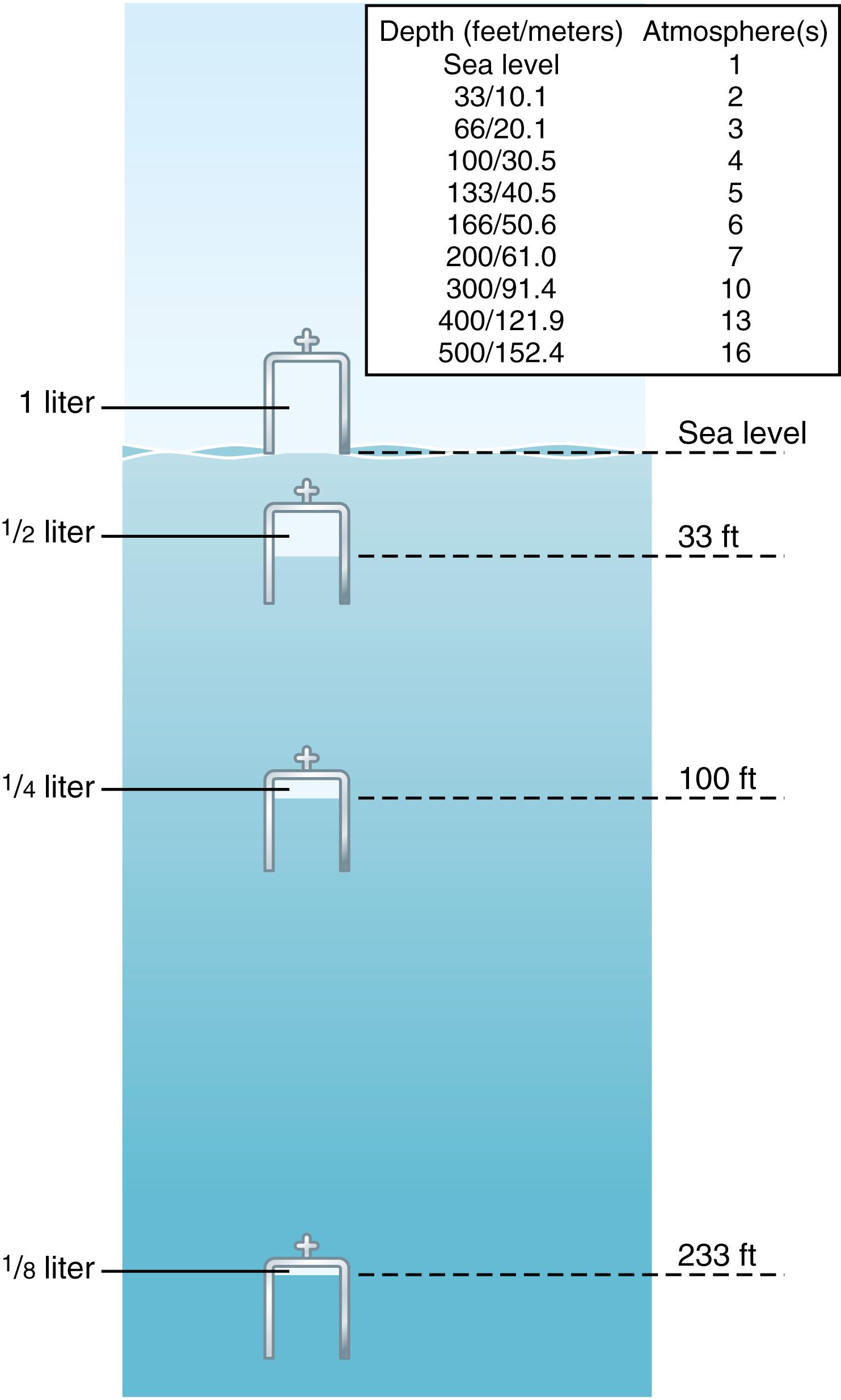
Another important effect of depth is the compression of gases to smaller and smaller volumes. The illustration in Figure 45-1 shows a bell jar at sea level containing 1 liter of air. At 33 feet beneath the sea, where the pressure is 2 atm, the volume has been compressed to only a half-liter, and at 8 atm (233 feet) it has been compressed to one-eighth liter. Thus, the volume to which a given quantity of gas is compressed is inversely proportional to the pressure. This principle of physics is called Boyle’s law , and it is extremely important in diving physiology because increased pressure can collapse the air chambers of the diver’s body, especially the lungs, and may cause serious damage.
Often in this chapter it is necessary to refer to actual volume versus sea level volume . For example, we might speak of an actual volume of 1 liter at a depth of 300 feet; this is the same quantity of air as a sea level volume of 10 liters.
The individual gases to which a diver is exposed when breathing air are nitrogen , O 2 , and CO 2 ; each of these, at times, can cause significant physiological effects at high pressures.
About four-fifths of the air is nitrogen. At sea level pressure, the nitrogen has no significant effect on bodily function, but at high pressures, it can cause varying degrees of narcosis. When the diver remains beneath the sea for 1 hour or more and is breathing compressed air, the depth at which the first symptoms of mild narcosis appear is about 120 feet. At this level, the diver begins to exhibit joviality and loss of many of his or her cares. At 150 to 200 feet, the diver becomes drowsy. At 200 to 250 feet, the person’s strength wanes considerably, and the diver often becomes too clumsy to perform the work required. Beyond 250 feet (8.5 atm pressure), the diver usually becomes almost useless as a result of nitrogen narcosis if he or she remains at these depths too long.
Nitrogen narcosis has characteristics similar to those of alcohol intoxication, and for this reason it has frequently been called “raptures of the depths.” The mechanism of this narcotic effect is believed to be the same as that of most other gas anesthetics. That is, it dissolves in the fatty substances in neuronal membranes and, because of its physical effect on altering ionic conductance through the membranes, it reduces neuronal excitability. Ascent to a shallower depth reverses the narcosis within a few minutes, with no known long-term effects if the ascent is not too rapid.
When the P o 2 in the blood rises above 100 mm Hg, the amount of O 2 dissolved in the water of the blood increases markedly. This effect is shown in Figure 45-2 , which depicts the same O 2 -hemoglobin dissociation curve as that shown in Chapter 41 but with the alveolar P o 2 extended to more than 3000 mm Hg. Also depicted by the lowest curve in the figure is the volume of O 2 dissolved in the fluid of the blood at each P o 2 level. Note that in the normal range of alveolar P o 2 (<120 mm Hg), almost none of the total O 2 in the blood is accounted for by dissolved O 2 , but as the O 2 pressure rises into the thousands of mm Hg, a large portion of the total O 2 is then dissolved in the water of the blood, in addition to that bound with hemoglobin.
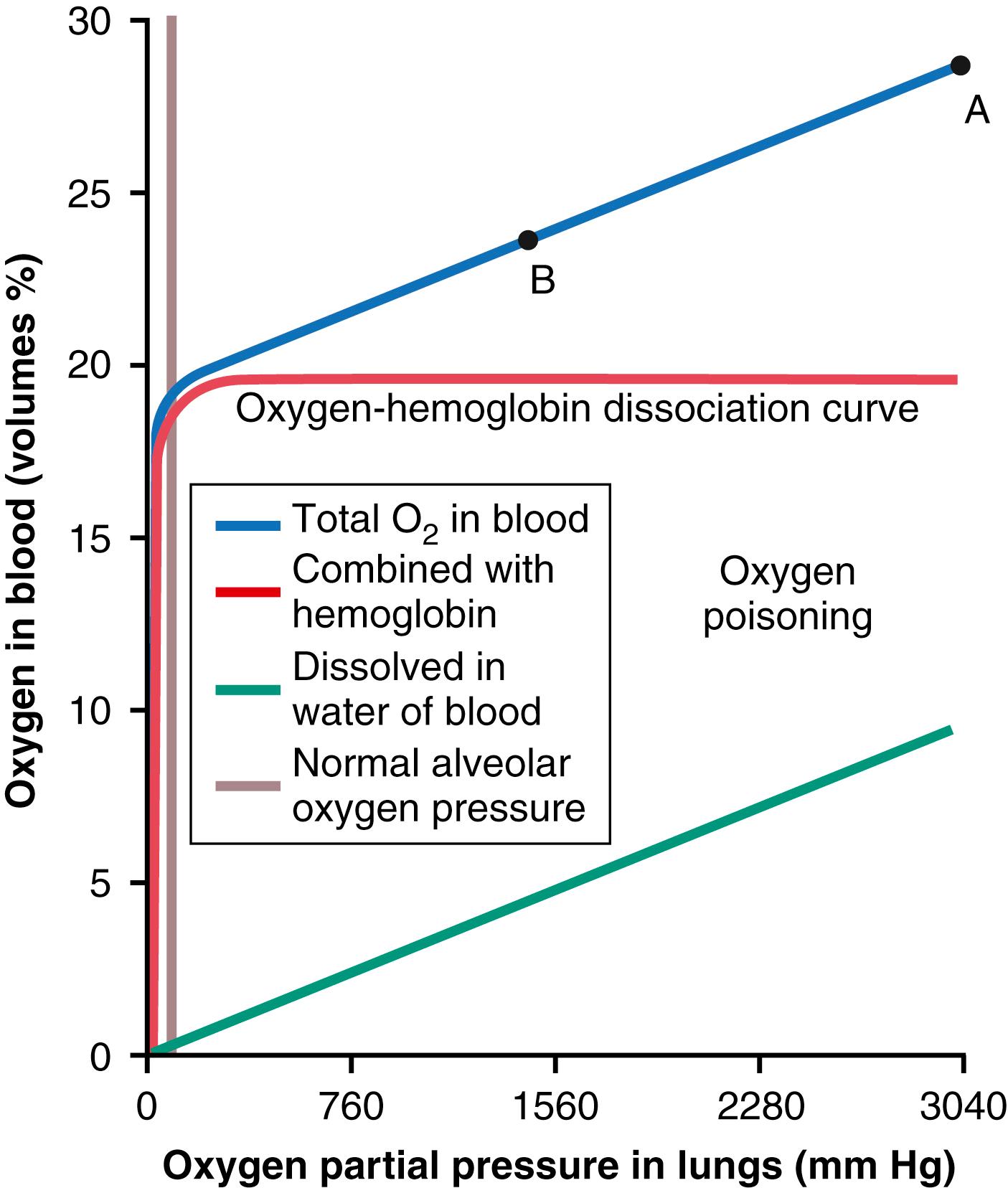
Let us assume that the P o 2 in the lungs is about 3000 mm Hg (4 atm pressure). Referring to Figure 45-2 , one finds that this pressure represents a total O 2 content in each 100 ml of blood of about 29 volumes percent, as demonstrated by point A in the figure, which means 20 volumes percent bound with hemoglobin and 9 volumes percent dissolved in the blood water. As this blood passes through the tissue capillaries, and the tissues use their normal amount of O 2 , about 5 ml from each 100 ml of blood, the O 2 content on leaving the tissue capillaries is still 24 volumes percent (point B in the figure). At this point, the P o 2 is approximately 1200 mm Hg, which means that O 2 is delivered to the tissues at this extremely high pressure instead of at the normal value of 40 mm Hg. Thus, once the alveolar P o 2 rises above a critical level, the hemoglobin-O 2 buffer mechanism (discussed in Chapter 41 ) is no longer capable of keeping the tissue P o 2 in the normal safe range, between 20 and 60 mm Hg.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here