Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Tendons and ligaments are both dense, regularly arranged connective tissues. The surface of the tendon is enveloped in a white, glistening, synovial-like membrane, called the epitenon , which is continuous on its inner surface with the endotenon , a thin layer of connective tissue that binds collagen fibers and contains lymphatics, blood vessels, and nerves. In some tendons, the epitenon is surrounded by a loose areolar tissue called the paratenon, which functions as an elastic sheath through which the tendon can slide. In some tendons, the paratenon is replaced by a true synovial sheath or bursa consisting of two layers lined by synovial cells, called the tenosynovium , within which the mesotendon carries important blood vessels to the tendon. In the absence of a synovial lining, the paratenon often is called a tenovagina . Together the epitenon and the paratenon compose the peritenon ( Fig. 1.1 ). The blood supply to tendons has several sources, including the perimysium, periosteal attachments, and surrounding tissues. Blood supplied through the surrounding tissues reaches the tendon through the paratenon, mesotenon, or vincula. Vascular tendons are surrounded by a paratenon and receive vessels along their borders; these vessels then coalesce within the tendon. The relatively avascular tendons are contained within tendinous sheaths, and the mesotenons within these sheaths function as vascularized conduits called vincula . The muscle-tendon and tendon-bone junctions, along with the mesotenon, are the three types of vascular supply to the tendon inside the sheath. Other sources of nutrition include diffusional pathways from the synovial fluid, which provide an important supply of nutrients for the flexor tendons of the hand, for example. The nervous supply to a tendon involves mechanoreceptors located near the musculotendinous junction, which provide proprioceptive feedback to the central nervous system.
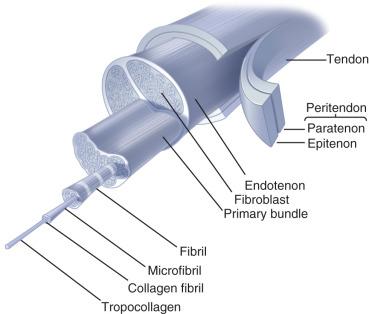
Ligaments grossly appear as firm, white fibrous bands, sheets, or thickened strips of joint capsule securely anchored to bone. They consist of a proximal bone insertion, the substance of the ligament or the capsule, and a distal bone insertion. Because most insertions are no more than 1 mm thick, they contribute only a small amount to the volume and the length of the ligament. Bundles of collagen fibrils form the bulk of the ligament substance. Some ligaments consist of more than one band of collagen fibril bundles. For example, the anterior cruciate ligament (ACL) has a continuum of fiber lengths; different fibers become taut throughout the range of motion. The alignment of collagen fiber bundles within the ligament substance generally follows the lines of tension applied to the ligament. This is in contrast to the alignment of collagen fiber bundles within the tendon, which is generally parallel to its longitudinal axis. In addition, thinner collagen fibrils extend the entire length of the tendon. Light microscopic examination has shown that the collagen bundles have a wave or crimp pattern. The crimp pattern of matrix organization may allow slight elongation of the ligament without incurring damage to the tissue. In some regions, the ligament cells align themselves in rows between collagen fiber bundles, but in other regions, the cells lack apparent orientation relative to the alignment of the matrix collagen fibers. Scattered blood vessels penetrate the ligament substance, forming small-diameter, longitudinal vascular channels that lie parallel to the collagen bundles. Nerve fibers lie next to some vessels, and, like tendon, nerve endings with the structure of mechanoreceptors have been found in some ligaments.
Tendon and ligament insertions vary in size, strength, angle of the ligament collagen fiber bundles relative to the bone, and proportion of ligament collagen fibers that penetrate directly into bone. Based on the angle between the collagen fibrils and the bone and the proportion of the collagen fibers that penetrate directly into bone, investigators group tendon and ligament insertions into two types: direct and indirect. Direction insertions typically occur at the apophysis or epiphysis of bone, often within or around a synovial joint, and consist of sharply defined regions where the collagen fibers appear to pass directly into the cortex of the bone. Although the thin layer of superficial collagen fibers of direct insertions joins the fibrous layer of the periosteum, most of the tendon or ligament insertions consist of deeper fibers that directly penetrate the cortex, often at a right angle to the bone surface. The deeper collagen fibers pass through four zones with increasing stiffness: ligament substance, fibrocartilage, mineralized fibrocartilage, and bone. This four-zone interface is known as the fibrocartilaginous enthesis. Dissipation of force is achieved effectively through this gradual transition from tendon to fibrocartilage to bone. A larger area of fibrocartilage can be found on one side of the insertion, which is thought to be an adaptation to the compressive forces experienced by the tendon or ligament on that side. Conversely, indirect or oblique insertions, such as the tibial insertion of the medial collateral ligament of the knee or the femoral insertion of the lateral collateral ligament, typically occur at the metaphysis or diaphysis of bone without an intervening fibrocartilage zone. They usually cover more bone surface area than do direct insertions, and their boundaries cannot be easily defined because the collagen fibers pass obliquely along the bone surface rather than directly into the cortex.
Tendons and ligaments consist of relatively few cells and an abundant extracellular matrix primarily containing collagen, proteoglycans, and water. Tenocytes (tendon-specialized fibroblasts) are the dominant cell of tendons, whereas fibroblasts are the dominant cells of ligaments. Tenocytes and fibroblasts form and maintain the extracellular matrix. Within ligaments, fibroblasts vary in shape, activity, and density among regions of the same tissue and with the age of the tissue. Both tenocytes and fibroblasts are spindle shaped, with fibroblasts being rounder, and extend between the collagen fibrils. Endothelial cells of small vessels and nerve cell processes are also present. Studies have shown that tendon and ligament contain a small population of resident stem cells which function to maintain tissue homeostasis during growth and repair.
Type I collagen, which is the major component of the molecular framework, composes more than 90% of the collagen content of ligaments. Type III collagen constitutes approximately 10% of the collagen, and small amounts of other collagen types also may be present. Ligaments have a higher content of type III collagen than do tendons. All types of collagen have in common a triple helical domain, which is combined differently with globular and nonhelical structural elements. The triple helix conformation of collagen is stabilized mainly by hydrogen bonds between glycine residues and between hydroxyl groups of hydroxyproline. This helical conformation is reinforced by hydroxyproline-forming and proline-forming hydrogen bonds to the other two chains. The physical properties of collagen and its resistance to enzymatic and chemical breakdown rely on covalent cross-links within and between the molecules.
Elastin is a protein that allows connective tissues to undergo large changes in geometry while expending little energy in the process. Tendons of the extremities possess small amounts of this structural protein, whereas most ligaments have little elastin (usually less than 5%), although a few, such as the nuchal ligament and the ligamentum flavum, have high concentrations (up to 75%). In most tendons, elastin is found primarily at the fascicle surface, comprising less than 1% of the tendon by dry weight, and it is responsible for the crimp pattern of the tendon when viewed by a light microscope. Elastin forms protein fibrils or sheets, but elastin fibrils lack the cross-banding pattern of fibrillar collagen and differ in amino acid composition, including two amino acids not found in collagen (desmosine and isodesmosine). In addition, unlike collagen, elastin amino acid chains form random coils when the molecules are unloaded. This conformation of the amino acid chains makes it possible for elastin to undergo some deformation without rupturing or tearing and then, when the load is removed, to return to its original size and shape.
Approximately 1% of the total dry weight of tendon and ligament is composed of ground substance, which consists of proteoglycans, glycosaminoglycans, structural glycoproteins, plasma proteins, and a variety of small molecules. Most ligaments have a higher concentration of glycosaminoglycans than do tendons, due to the functional need for more rapid adaptation. Proteoglycans and glycosaminoglycans both have important roles in organizing the extracellular matrix and control the water content of the tissue. Tendon and ligaments contain two known classes of proteoglycans. Larger proteoglycans contain long negatively charged chains of chondroitin and keratan sulfate. Smaller proteoglycans contain dermatan sulfate. Because of their long chains of negative charges, the large articular cartilage-type proteoglycans tend to expand to their maximal domain in solution until restrained by the collagen fibril network. As a result, they maintain water within the tissue and exert a swelling pressure, thereby contributing to the mechanical properties of the tissue and filling the regions between the collagen fibrils. The small leucine-rich proteoglycans usually lie directly on the surface of collagen fibrils and appear to affect formation, organization, and stability of the extracellular matrix, including collagen fibril formation and diameter. They may also control the activity of growth factors by direct association.
Although noncollagenous proteins contribute only a small percentage of the dry weight of dense fibrous tissues, they appear to help organize and maintain the macromolecular framework of the collagen matrix, aid in the adherence of cells to the framework, and possibly influence cell function. One noncollagenous protein, fibronectin, has been identified in the extracellular matrix of ligaments and may be associated with several matrix component molecules and with blood vessels. Other noncollagenous proteins undoubtedly exist within the matrix, but their identity and their functions have not yet been defined. Many of the noncollagenous proteins also contain a few monosaccharides and oligosaccharides.
Acute strains and tears to tendons and ligaments disrupt the matrix, damage blood vessels, and injure or kill cells. Damage to cells, matrices, and blood vessels and the resulting hemorrhage start a response that leads to a sequential process of inflammation, repair, and remodeling. These events form a continuous sequence of cell, matrix, and vascular changes that begins with the release of inflammatory mediators and ends when remodeling ceases. As with any injury to biologic tissue, acute inflammation lasts 48 to 72 hours after the injury and then gradually resolves as repair progresses. Some of the events that occur during inflammation, including the release of cytokines or growth factors, may help to stimulate tissue repair. These mediators promote vascular dilation and increase vascular permeability, leading to exudation of fluid from vessels in the injured region, which causes tissue edema. Blood escaping from the damaged vessels forms a hematoma that temporarily fills the injured site. Fibrin accumulates within the hematoma, and platelets bind to fibrillar collagen, thereby achieving hemostasis and forming a clot consisting of fibrin, platelets, red cells, and cell and matrix debris. The clot provides a framework for vascular and fibroblast cell invasion. As they participate in clot formation, platelets release vasoactive mediators and various cytokines or growth factors (e.g., transforming growth factor-β [TGF-β] and platelet-derived growth factor). Polymorphonuclear leukocytes appear in the damaged tissue and the clot. Shortly thereafter, monocytes arrive and increase in number until they become the predominant cell type. Enzymes released from the inflammatory cells help to digest necrotic tissue, and monocytes phagocytose small particles of necrotic tissue and cell debris. Endothelial cells near the injury site begin to proliferate, creating new capillaries that grow toward the region of tissue damage. Release of chemotactic factors and cytokines from endothelial cells, monocytes, and other inflammatory cells helps to stimulate migration and proliferation of the fibroblasts that begin the repair process.
Overuse tendon injury is one of the more common forms of musculoskeletal injury and clinical causes of pain, although controversy exists in the literature about a universal classification and the responsible pathologic entities. A classification of Achilles tendon disorders provides a guide to the structural manifestations of overuse injury as follows: (1) peritendinitis, or inflammation of the peritenon; (2) tendinosis with peritendinitis; (3) tendinosis without peritendinitis; (4) partial rupture; and (5) total rupture. Other classifiers have added a sixth category, tendinitis, in which the primary site of injury is the tendon, with an associated reactive peritendinitis. The classification is not universal because some tendons lack a paratenon and instead have synovial sheaths; furthermore, it is unclear if certain histopathologic conditions are actually separate entities. For instance, human biopsy studies have been unable to show histologic evidence of acute inflammation within the tendon substance. Because of uncertainty regarding the histologic features of these conditions, several authors have suggested use of the term tendinopathy rather than tendinitis .
Studies have shown that in cases of chronic tendinosis, the pathologic lesion is typical of a degenerative process rather than an inflammatory one and that this degeneration occurs in areas of diminished blood flow. Several authors have documented the existence of areas of marked degeneration without acute or chronic inflammatory cell accumulation in most of these cases. These changes are separate and distinct from the site of rupture. A review of patients with chronic tendinitis syndrome revealed similar findings of tendon degeneration. Nirschl described the pathology of chronic tendinitis as “angiofibroblastic hyperplasia.” A characteristic pattern of fibroblasts and vascular, atypical, granulation-like tissue can be seen microscopically. Cells characteristic of acute inflammation are virtually absent. These observations suggest that factors other than mechanical overuse play an important role in the pathogenesis of these tendon lesions.
In several studies, a correlation between age and the incidence of chronic tendinopathy has been identified. In vitro studies have shown decreased proliferative and metabolic responses of aging tendon tissue. Other causative factors include the lack of blood flow in certain areas (e.g., supraspinatus and Achilles tendon) that may predispose a tendon to rupture or may result in chronic tendinopathy. Biopsy specimens of young patients with symptoms of chronic tendinopathy have revealed a change in the morphology of tenocytes adjacent to areas of collagen degeneration.
Tendons and ligaments may possess both intrinsic and extrinsic capabilities for healing, and the contribution of each of these two mechanisms probably depends on the location, extent, and mechanism of injury and the rehabilitation program used after the injury. Several studies have suggested that the inflammatory response is not essential to the healing process and that these tissues possess an intrinsic capacity for repair. Recent research has isolated intrinsic stem cells within tendon and ligament, although their in vivo identities, niche, and role in healing remain controversial. Lindsay and Thomson were the first to show that an experimental tendon suture zone can be isolated from the perisheath tissues and that healing progressed at the same rate as when the perisheath tissues were intact. Later, in isolated segments of profundus tendon in rabbits, these researchers found anabolic and catabolic enzymes, which showed that an active metabolic process existed in the isolated tendon segments.
As in other areas in the body, tendon healing proceeds in three phases: (1) an inflammatory stage, (2) a reparative or collagen-producing stage, and (3) a remodeling phase.
Tendon and ligament healing begins with hematoma formation and an inflammatory reaction that includes an accumulation of fibrin and inflammatory cells. A clot forms between the two ends and is invaded by cells resembling fibroblasts and migratory capillary buds. Within 2 to 3 days of the injury, fibroblasts within the wound begin to proliferate rapidly and synthesize new matrix. They replace the clot and the necrotic tissue with a soft, loose fibrous matrix containing high concentrations of water, glycosaminoglycans, and type III collagen. Inflammatory cells and fibroblasts fill this initial repair tissue. Within 3 to 4 days, vascular buds from the surrounding tissue grow into the repair tissue and then canalize to allow blood flow to the injured tissue and across small tissue defects. This vascular granulation tissue fills the tissue defect and extends for a short distance into the surrounding tissue but has little tensile strength. The inflammatory phase is evident until the 8th to 10th day after injury.
As the repair progresses during the next several weeks, proliferating fibroblasts continue to produce fibrous tissue containing a high proportion of type III collagen. Collagen synthesis reaches its maximal level after approximately 4 weeks, and at 3 months, collagen synthesis continues at a rate 3 to 4 times that of normal tissue. Over time, water, glycosaminoglycan, and type III collagen concentrations decline, the inflammatory cells disappear, and the concentration of type I collagen increases. Newly synthesized collagen fibrils increase in size and begin to form tightly packed bundles, and the density of fibroblasts decreases. Matrix organization increases as the fibrils begin to align along the lines of stress, the number of blood vessels decreases, and small amounts of elastin may appear within the site of injury. The tensile strength of the repair tissue increases as the collagen concentration increases.
Repair of many tendon and ligament injuries results in an excessive volume of highly cellular tissue with limited mechanical properties and a poorly organized matrix. Remodeling reshapes and strengthens this tissue by removing, reorganizing, and replacing cells and matrix. In most tendon and ligament injuries, evidence of remodeling appears within several weeks of injury as fibroblasts and macrophages decrease, fibroblast synthetic activity decreases, and fibroblasts and collagen fibrils assume a more organized appearance. As these changes occur in the repair tissue, collagen fibrils grow in diameter, the concentration of collagen and the ratio of type I to type III collagen increase, and the water and proteoglycan concentrations decline. During the months after the injury occurs, the matrix continues to align, presumably in response to loads applied to the repair tissue. The most apparent signs of remodeling disappear within 4 to 6 months of injury. However, removal, replacement, and reorganization of repair tissue continue to some extent for years. The mechanical strength of the healing tendon and ligament increases as the collagen becomes stabilized by cross-links and the fibrils assemble into fibers.
Among the most important variables that affect healing of tendon and ligament are the type of tendon or ligament, the size of the tissue defect, and the amount of load applied to the repair tissue. For example, injuries to capsular and extra-articular ligaments stimulate production of repair tissue that will fill most defects, but injuries to intra-articular ligaments, such as the ACL, often fail to produce a successful repair response. Treatments that achieve or maintain apposition of torn tissue and that stabilize the injury site decrease the volume of repair tissue necessary to heal the injury, which can benefit the healing process. Such treatments may also minimize scarring and help to provide near-normal tissue length. For these reasons, avoidance of wide separation of ruptured tendon or ligament ends and selection of treatments that maintain some stability at the injured site during the initial stages of repair are generally desirable.
Early excessive loading in the immediate postoperative period may have a deleterious effect on tendon and ligament healing by disrupting the repair tissue, leading to gap formation and ischemia, adverse changes in tendon matrix, and possible rupture. However, controlled loading of tendon and ligament repair tissue can promote healing and enhance the mechanical and biologic characteristics of tendon-to-bone healing. The optimal amount of tension necessary to promote an acceptable clinical response is currently not well understood and depends on the type of tissue and healing environment, but it is clear that remodeling of collagen scar tissue into mature tendon tissue depends on the presence of tensile forces. The concept of immediate passive mobilization after flexor tendon repair in the hand was introduced by Kleinert and coworkers, who showed that, during limited active extension, reciprocal relaxation of the flexor tendons occurs, allowing passive extension of the repaired tendon. This controlled passive motion was found to be effective experimentally and clinically in decreasing the tethering effect of adhesions and in improving the rates of tendon repair, gliding function, and strength of the tendon.
A large body of research has demonstrated the potential for growth factors to improve tendon and ligament tissue healing by stimulation of cell proliferation, chemotaxis, matrix synthesis, and cell differentiation (summarized in Table 1.1 ). In addition to multifunctional cytokines such as TGF-β and platelet-derived growth factor, work has focused on recapitulating the cellular and molecular signals that are expressed during embryonic tendon development, such as scleraxis and TGF-β3. However, challenges in the delivery of these growth factors, specifically regarding the optimal carrier vehicles and proper dosing regimen, to the desired site still remain.
| Biologic Factor | Functions | Reference |
|---|---|---|
| TGF-β |
|
|
| GDF 5/6/7 |
|
|
| IGF-1 |
|
|
| PDGF-B |
|
|
| bFGF |
|
|
| HGF |
|
|
| PRP |
|
|
| VEGF |
|
|
| BMP-12 |
|
|
Platelet-rich plasma (PRP), an autologous blood concentrate, can be used to locally deliver a high concentration “cocktail” of cytokines and has gained popularity as a treatment modality for tendon and ligament injuries. Recent studies have reported potentially promising results with the use of PRP to augment healing of rotator cuff repair and patellar tendinopathy. However, the results of PRP for augmentation of tendon and ligament healing have been variable, which can partially be attributed to the lack of understanding of the optimal PRP formulation for different tissues and pathologies, as well as the tremendous variability in the methods of PRP production among commercial systems. To complicate matters further, within a given separation technique, there is a high degree of intersubject and intrasubject variability in the composition of PRP produced.
Cell-based approaches appear promising for tendon and ligament tissue engineering and improvement of healing. Therapies using mesenchymal stem cells (MSCs) derived from adipose and bone marrow to augment tendon and ligament healing have garnered the most attention due to their multipotent potential and ability to exert a paracrine effect to modulate and control inflammation, stimulate endogenous cell repair and proliferation, inhibit apoptosis, and improve blood flow. However, like PRP augmentation therapy, continued research is needed to identify the optimal cell source and the ideal treatment protocol needed to drive differentiation of these or neighboring cells into mature tenocytes and fibroblasts. Recent studies have identified resident tissue-specific stem cells in the perivascular regions of native tendon and ligament that detach from vessels in response to injury, migrate into the interstitial space, and deposit extracellular matrix, although their precise potential for use in augmenting tendon and ligament healing remains to be elucidated.
Research has also investigated scaffold materials to augment tendon repair and ligament reconstruction. Porcine-derived small intestine submucosa has been used as a collagen scaffold to augment Achilles tendon and rotator cuff tendon repair. However, negative clinical results have been reported, including inflammatory/immunologic response to the small intestine submucosa material believed to be due to residual porcine DNA in the implant. Various other allografts and xenografts, such as collagen allograft matrices and porcine dermal xenografts, are commercially available and differ from porcine small intestine submucosa in both biologic and mechanical composition. Nanomaterials are promising for tendon and ligament tissue engineering because the microstructure of the material mimics native extracellular matrix. Multiphasic scaffolds are being used to create bone-ligament composites. In addition to various scaffold materials and cell types, it has become clear that mechanical stimulation of the neotissue is also critical to optimize the structure and composition of the tissue. The specific scaffold can be modified in vitro by seeding marrow stromal cells on the scaffold and applying cyclic stretching to increase the alignment of cells, as well as to improve the production and orientation of collagen. When applied in vivo, such a tissue-engineered scaffold could serve to accelerate the healing process, ultimately helping to make a better neoligament or tendon.
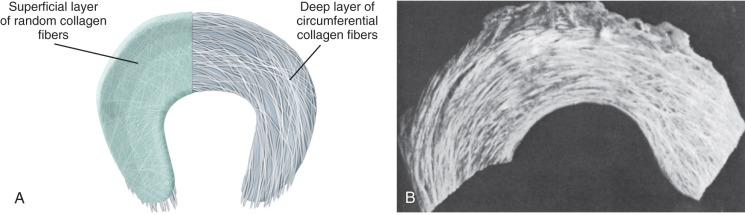
Human menisci are semilunar in shape and consist of a sparse distribution of cells surrounded by an abundant extracellular matrix. The meniscus functions to optimize force transmission and provide stability to the knee. The medial meniscus is the dominant secondary stabilizer in an ACL-deficient knee during the Lachman maneuver, whereas the lateral meniscus is the dominant secondary stabilizer in an ACL-deficient knee during the pivot shift maneuver. Within the meniscus lies an anisotropic, inhomogeneous, and highly ordered arrangement of collagen fibrils. The meniscal surface is composed of a randomly woven mesh of fine collagen type II fibrils that lie parallel to the surface. Below this surface layer, large, circumferentially arranged collagen fiber bundles (mostly type I) spread through the body of the tissue ( Fig. 1.2 ). These circumferential collagen bundles give menisci great tensile stiffness and strength parallel to their orientation. The collagen bundles insert into the anterior and the posterior meniscal attachment sites on the tibial plateau, providing for rigid and strong attachment sites. Fig. 1.2A illustrates these large fiber bundles and the thin superficial surface layer. Fig. 1.2B is a photograph of a bovine medial meniscus with the surface layer removed, showing the large collagen bundles of the deep zone.
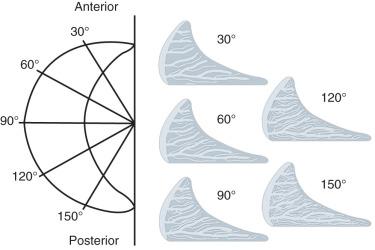
Radial sections of meniscus ( Fig. 1.3 ) show radially oriented bundles of collagen fibrils, or “radial tie fibers,” among the circumferential collagen fibril bundles, weaving from the periphery of the meniscus to the inner region. These tie fibers help to increase the stiffness and the strength of the tissue in a radial direction, thereby resisting longitudinal splitting of the collagen framework. In cross section, these radial tie fibers appear to be more abundant in the posterior sections than in the anterior sections of the meniscus.
Unlike articular cartilage, the peripheral 25% to 30% of the lateral meniscus and the peripheral 30% of the medial meniscus have a blood supply, and the peripheral regions of the meniscus, especially the meniscal horns, have a nerve supply as well. Branches from the geniculate arteries form a capillary plexus along the peripheral borders of the menisci, with the medial inferior geniculate artery supplying the peripheral medial meniscus and the lateral inferior geniculate artery supplying the peripheral lateral meniscus. Small radial branches project from these circumferential parameniscal vessels into the meniscal substance. The central aspects of the meniscus do not have a direct arterial supply and instead receive nutrients primarily through synovial fluid diffusion.
The mechanical functions of the menisci depend on a highly organized extracellular matrix consisting of fluid and a macromolecular framework formed of collagen (types I, II, III, V, and VI), proteoglycans, elastin, and noncollagenous proteins, along with the cells that maintain this matrix.
Based on morphologic characteristics, two major types of meniscal cells exist. Near the surface, the cells have flattened ellipsoid or fusiform shapes and are considered more fibroblastic; in the deep zone, the cells are spherical or polygonal and considered more chondrocytic. The superficial and the deep meniscal cells appear to have different metabolic functions and perhaps different responses to loading. Like most other mesenchymal cells, these cells lack cell-to-cell contacts. Because most of the cells lie at a distance from blood vessels, they rely on diffusion through the matrix for transport of nutrients and metabolites. The membranes of meniscal cells attach to matrix macromolecules through adhesion proteins (e.g., fibronectin, thrombospondin, and type VI collagen). The matrix, particularly the pericellular region, protects the cells from damage due to physiologic loading of the tissue. Deformation of the macromolecular framework of the matrix causes fluid flow through the matrix and influences meniscal cell function. Because meniscal tissue is more fibrous than is hyaline cartilage, some authors have proposed that meniscal cells be called fibrochondrocytes.
Water comprises 65% to 75% of the total weight of the meniscus. Some portion of this water may reside within the intrafibrillar space of the collagen fibers. Most of the water is retained within the tissue in the solvent domains of the proteoglycans due to both their strong hydrophilic tendencies and the Donnan osmotic pressure exerted by the counter ions associated with the negative charge groups on the proteoglycans. Because the pore size of the tissue is extremely small (<60 nm), very large hydraulic pressures are required to overcome the impact of frictional resistance when forcing fluid flow through the tissue. These interactions between water and the macromolecular framework of the matrix significantly influence the viscoelastic properties of the tissue.
Some meniscal regions have a proteoglycan concentration of up to 3% of their dry weight. Like proteoglycans from other dense fibrous tissues, meniscal proteoglycans can be divided into two general types. The large, aggregating proteoglycans expand to fill large volumes of matrix and contribute to tissue hydration and the mechanical properties of the tissue. The smaller, nonaggregating proteoglycans usually have a close relationship with fibrillar collagen. The large aggregating meniscal proteoglycans have the same structure as the large aggregating proteoglycans from articular cartilage. The concentration of large aggregating proteoglycans suggests that they probably contribute less to the properties of meniscus than to the properties of articular cartilage. As with the quantitatively minor collagens, the smaller nonaggregating meniscal proteoglycans may help to organize and stabilize the matrix, but currently their exact function remains unknown.
Noncollagenous proteins also form part of the macromolecular framework of the meniscus and may contribute as much as 10% of the dry weight of the tissue in some regions. Two specific noncollagenous proteins, link protein and fibronectin, have been identified in the meniscus. Link protein is required for the formation of the stable proteoglycan aggregates that are capable of forming strong networks. Fibronectin serves as an attachment protein for cells in the extracellular matrix. Other noncollagenous proteins such as thrombospondin may serve as adhesive proteins in the tissue, thus contributing to the structure and the mechanical strength of the matrix; however, the exact details of their composition and function in the meniscus remain largely unknown.
Finally, elastin contributes less than 1% of the dry weight of the meniscus. The contribution of elastin to the mechanical properties of meniscal tissue is not well understood because the sparsely distributed elastic fibers are unlikely to play a significant role in the organization of the matrix or in determining the mechanical properties of the tissue.
Traumatic meniscal tears occur most frequently in young, active people. Tension, compression, or shear forces that exceed the strength of the meniscal matrix in any direction can lead to tissue failure. Acute traumatic injuries of normal meniscal substance usually produce longitudinal or transverse tears, although the morphology of these tears can be highly variable, including oblique, radial horizontal, bucket-handle, and complex tears. The configuration of tears due to overloading of normal meniscal tissue depends strongly on the direction of the load and the rate of stretch. Unlike acute traumatic tears through apparently normal meniscal tissue, degenerative meniscal tears occur as a result of age-related changes in the tissue. These degenerative tears are most common in persons older than 40 years. Often, these persons do not recall a specific injury, or they recall only a minor load applied to the knee. Degenerative tears often have complex shapes or may appear as horizontal clefts or flaps, as though they were produced by shear failure. Multiple degenerative tears often occur within the same meniscus. These features of degenerative meniscal tears suggest that they result more from age-related changes in the collagen-proteoglycan solid matrix than from specific acute trauma.
The response of meniscal tissue to tears depends on whether the tear occurs through a vascular or an avascular portion of the meniscus. The peripheral, vascular regions respond to injury as other vascularized, dense fibrous tissues do. The tissue damage initiates a sequence of cellular and vascular events including inflammation, repair, and remodeling that can result in healing and restoration of tissue structure and function. Although tears through the vascular regions of the meniscus can typically heal, tears through the avascular regions do not typically heal spontaneously, resulting in tissue deficiency. Therefore strategies for meniscal repair in the avascular zone are continuously being explored.
Partial meniscal resection through the peripheral vascularized region or complete meniscal resection initiates production of repair tissue that can extend from the remaining peripheral tissue into the joint. Although the repair cells usually fail to replicate normal meniscal tissue, many authors have referred to this phenomenon as meniscal regeneration . Some repaired menisci grossly resemble normal menisci, but the functional capabilities and mechanical properties of this “regenerated” meniscal tissue have not been comprehensively studied. Surgeons have reported meniscal regeneration in many clinical situations. Investigators have also examined the tissue produced by meniscal regeneration in animals. Meniscal regeneration can occur repeatedly in the same knee and occasionally occurs after total knee replacement. In rabbits, meniscal regeneration occurs more frequently on the medial side of the knee than on the lateral side, and development of degenerative changes in articular cartilage after a meniscectomy is inversely correlated with the extent of meniscal regeneration. Synovectomy appears to prevent meniscal regeneration, which suggests that synovial cells contribute to the formation of meniscal repair tissue. The mechanisms and conditions that promote this type of repair, its functional importance, and the factors related to the predictability and frequency of meniscal regeneration remain unknown.
The response of meniscal tissue to tears in the avascular portion resembles the response of articular cartilage to lacerations in many respects. Experimental studies show that a penetrating injury to the avascular region of the meniscus causes no apparent repair or inflammatory reaction. Meniscal cells in the injured region, like chondrocytes in the region of an injury limited to the articular cartilage, may proliferate and synthesize new matrix, but they appear to be incapable of migrating to the site of the defect or producing enough new matrix to fill it. The ineffective response of meniscal cells in the avascular region of the meniscus has led investigators to develop novel methods to stimulate repair. Some promising approaches include creation of a vascular access channel to the injury site and stimulation of cell migration to the avascular region using implantation of a fibrin clot, an artificial matrix, or growth factors. Synovial abrasion has also been shown to stimulate proliferation of the synovial fringe into the meniscus and allows blood vessels to enter the avascular regions. Although early results appear promising, the quality of the repair tissue, its biomechanical properties, and the long-term results of these methods have not been evaluated.
Given the well-established poor intrinsic healing potential of the meniscus, particularly in avascular regions, intense interest exists regarding methods to augment healing using cytokines, exogenous cells, and scaffolds. Fibroblast growth factor-2 and connective tissue growth factor have been evaluated in rabbit models, and vascular endothelial growth factor has been tested in a sheep meniscus tear model. Although these cytokines appear to have a positive effect on basic meniscal fibrochondrocyte biology, the challenge at this time is to identify the optimal carrier vehicles and dosage to translate these preclinical data to clinical trials. Although some studies have suggested that PRP, as a source of cytokines, may confer some benefit in meniscus healing, other studies have demonstrated no differences in outcomes or reoperation rates. In addition, in an animal model of meniscus injury, PRP treatment increased hypertrophic fibrous tissue rather than meniscal cartilage. Thus further investigation is necessary to better elucidate the role of growth factors and PRP in meniscal healing.
Cell-based approaches have also been evaluated for augmentation of biologic healing mechanisms. Various sources of both autogenous and allogeneic cells have been evaluated using different carrier materials. Both differentiated cells, such as chondrocytes, and undifferentiated cells, such as MSCs, have been tested in animal models. Few human studies investigating the role of MSCs for meniscal repair have been performed. Although the authors suggest that MSCs may be effective in repairing meniscal tears, these studies are limited, and more rigorous, placebo-controlled trials are necessary.
The use of scaffold materials to replace a portion of the damaged meniscus or to replace the entire structure is an appealing option and has the theoretical benefit of providing mechanical stability to the injured site while allowing for cell attachment and proliferation. A collagen-based scaffold (Collagen Meniscus Implant, Menaflex, ReGen Biologics, Glen Rock, NJ) and a resorbable porous polyurethane-based scaffold (Actifit, Orteq Sport Medicine, London, United Kingdom) have demonstrated satisfactory clinical outcome in up to 80% of cases at up to 10 years and 2 years of follow-up, respectively. Both of these devices are designed for partial meniscus replacement. Although it remains unclear whether the use of such scaffolds can affect the long-term sequelae of meniscectomy, early results are promising and may represent a new horizon in the treatment of these complex injuries. Further optimization of these materials may occur by incorporating undifferentiated cells into the scaffold.
Synovial joints allow the rapid controlled movements necessary to support joint motion and to participate in sports. Normal function of these complex diarthrodial structures depends on the structural integrity and macromolecular composition of articular cartilage. Sports-related traumatic disruptions of cartilage structure and alterations in the macromolecular composition or organization change the biomechanical properties of the tissue, compromise joint function, and can lead to progressive pain and disability.
The specialized composition and organization of hyaline articular cartilage impart its unique biomechanical properties that permit normal synovial joint function. In the joint, cartilage distributes the loads of articulation, thereby minimizing peak stresses acting on the subchondral bone. The tensile strength of the tissue provides its structural integrity under such loads. Alterations in the mechanical properties of cartilage due to injury, disease, or increasing age have not been well defined, but the available information shows that these properties change with age and loss of structural integrity. Cartilage from skeletally immature joints (open growth plates) is much stiffer than cartilage from skeletally mature joints (closed growth plates). Older cartilage and fibrillated cartilage have much lower tensile stiffness and strength. Participation in sports often subjects the articular cartilage to intense repetitive, compressive high-energy impact forces that can cause tissue injury. These abnormally large forces generate high shear stresses at the cartilage-subchondral bone junction, causing matrix disruption and death of the articular chondrocytes that may lead to early osteoarthritis. Because cartilage is aneural, patients with pure chondral injuries can remain asymptomatic.
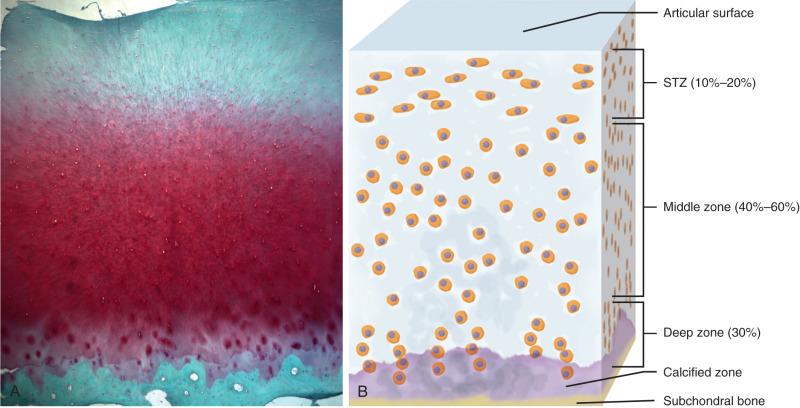
Like the dense fibrous tissues and meniscus, articular cartilage consists of cells, matrix water, and a matrix macromolecular framework. Unlike the most dense fibrous tissues, cartilage lacks nerves, blood vessels, and a lymphatic system. The composition, organization, and mechanical properties of the matrix of articular cartilage and the cell morphology and function vary according to the depth from the articular surface ( Fig. 1.4 ). Matrix composition, organization, and function also vary with distance from the cell. Morphologic changes in articular cartilage cells and matrix from the articular surface to the subchondral bone make it possible to identify four zones or layers of articular cartilage.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here