Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Growth and development of the lung occurs both before and after birth and is a major determinant of respiratory health throughout postnatal life. Adequate lung growth before birth is necessary for the successful transition from fetal to postnatal life. Normal lung growth after birth is necessary to enable the lung to meet increasing metabolic demands during infancy and childhood and into adulthood. During its development, the lung must acquire an intricate system of airways allowing low-resistance airflow to and from the respiratory zone; the internal surface area of the respiratory zone must be large with a dense vascular network allowing a high level of blood flow. The distance between the distal airspaces and the surrounding vascular network must also be thin enough to enable efficient gas exchange. In terms of its mechanical properties, lung tissue must be easily expanded during inspiration but retain sufficient recoil to drive expiration, and surfactant is required to prevent collapse at the end of expiration. It is now apparent that the physiologic processes controlling lung growth before and after birth are quite different, although the molecular mechanisms are likely to be similar. This chapter presents an overview of the physiologic control of lung growth before and after birth, as well as pathophysiologic processes that perturb normal lung growth.
During fetal life, the future air spaces of the lungs are filled with a unique liquid that is produced by the lung as a result of net transepithelial ion movement into the lung that creates an osmotic gradient favoring movement of water into the lung. This osmotic gradient is primarily due to active secretion of chloride into the lung. This fetal lung liquid (FLL) plays a critical role in lung development by maintaining the future air spaces in a distended state; it also limits entry of amniotic fluid into the lungs, which can have damaging effects. The volume of FLL within the future air spaces and its flux to and from the lower airways are influenced by fetal muscular activity, as well as by fetal posture and other factors that influence fetal transpulmonary pressure. By maintaining this distended state, FLL serves as an internal “splint,” which maintains a high degree of expansion of the distal air spaces. Without this underlying expansion, the fetal lung is unable to grow and structurally mature. Experimental manipulations have clearly demonstrated that sustained reductions in FLL volume reduce fetal lung growth and structural maturation, whereas sustained increases in FLL volume accelerate fetal lung growth and structural maturation. , Most clinical conditions that result in altered lung growth can also be explained by underlying alterations in FLL volume. Consequently, much attention has focused on understanding how the volume of FLL is controlled and the mechanisms by which it influences the growth and remodeling of fetal lung tissue. It is apparent that the fetal metabolic and endocrine environments also play important roles in lung development, especially in lung maturation, and studies in transgenic mice, genomics, transcriptomics, proteomics, and epigenetics are now elucidating the cellular and molecular mechanisms. The physical factors that are critical for normal lung development underlie many common disorders of fetal lung growth and are the major focus of this chapter.
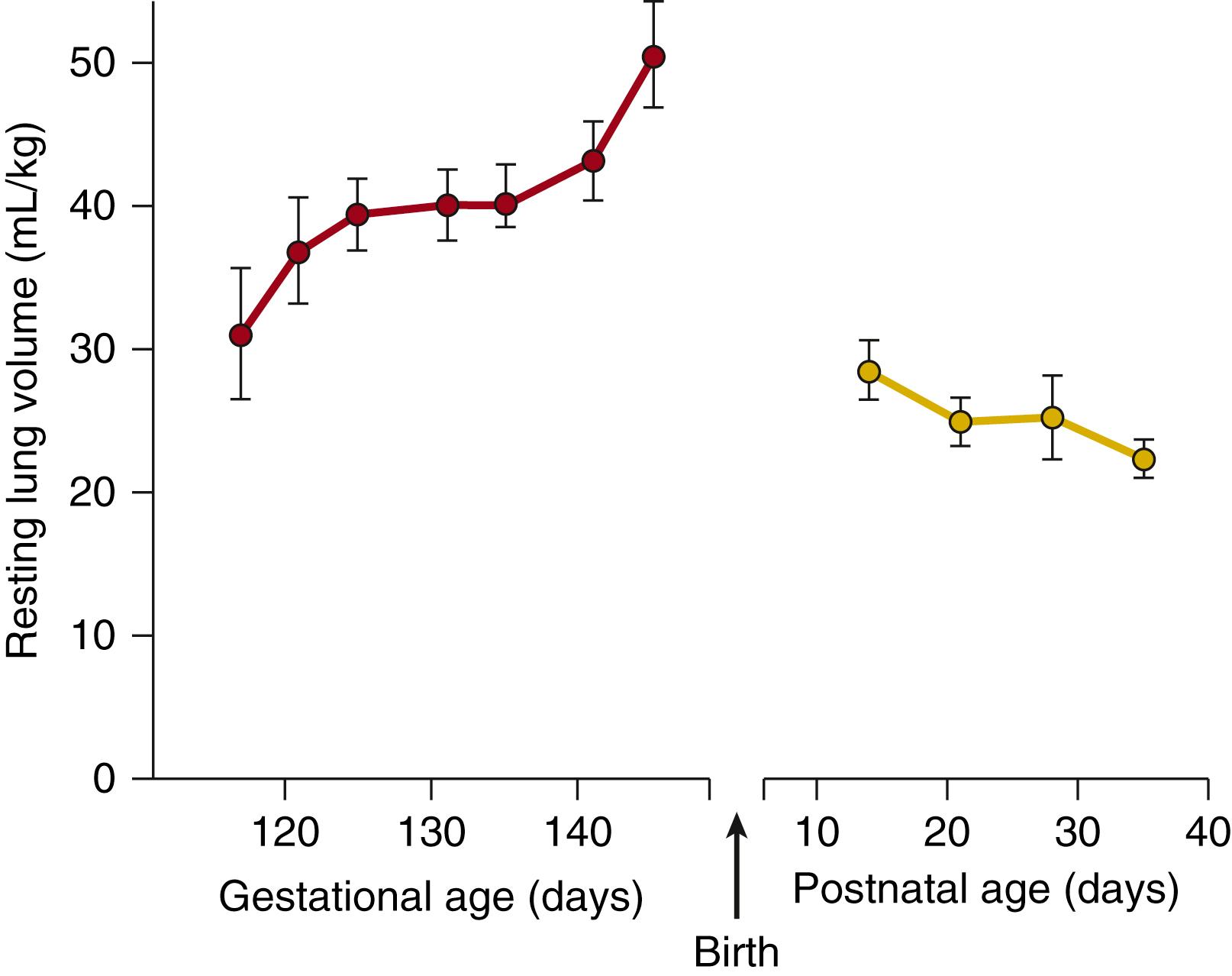
Identification of the factors that control fetal lung expansion and its fundamental role in lung growth are derived primarily from studies of chronically catheterized fetal sheep. , , During the latter half of ovine gestation, the volume of FLL within the fetal lungs progressively increases, reaching 35 to 45 mL/kg during the last weeks of gestation ; this is considerably greater than the functional residual capacity of the air-filled lung after birth (25 to 30 mL/kg) ( Fig. 57.1 ). The high level of fetal lung expansion is largely maintained by fetal muscular activity, involving fetal breathing movements (FBMs) , and laryngeal adductor muscles ( Fig. 57.2 ), but also depends on FLL secretion, resulting in the development of a transpulmonary pressure gradient. The degree to which the lungs can expand is ultimately dependent on the available intrathoracic and intrauterine space (see later). Values of lung expansion obtained from dead, anesthetized, exteriorized, or paralyzed fetuses will necessarily be underestimates, because FLL is lost under these conditions , (see Fig. 57.2 ). Similarly, measurements of FLL volume are questionable unless they were made in the absence of fetal hypoxemia or labor, and in the presence of normal amniotic fluid volumes. , Both labor and reduced amniotic fluid volume reduce FLL volume by increasing fetal transpulmonary pressure owing to increased flexion of the fetal trunk. ,
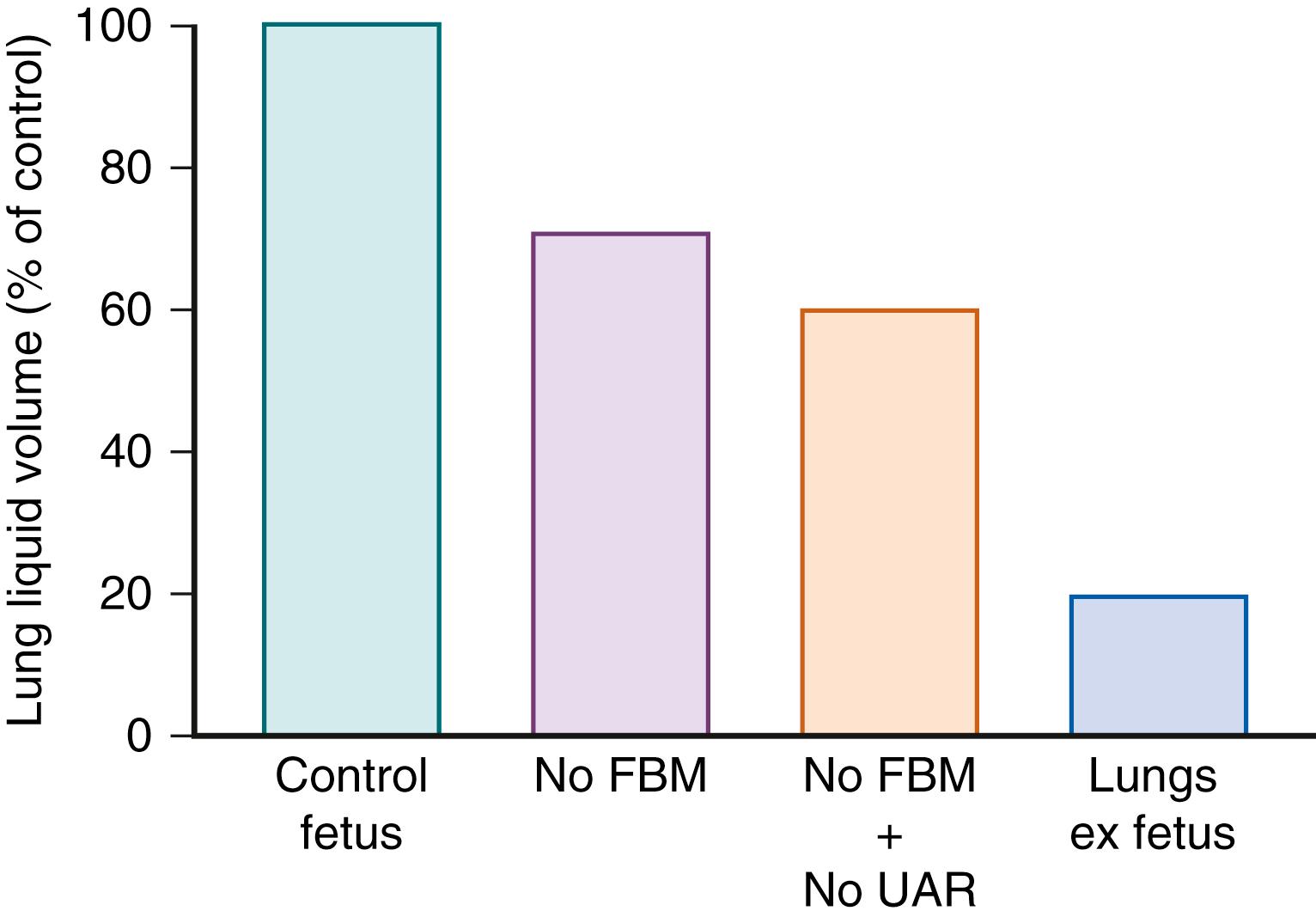
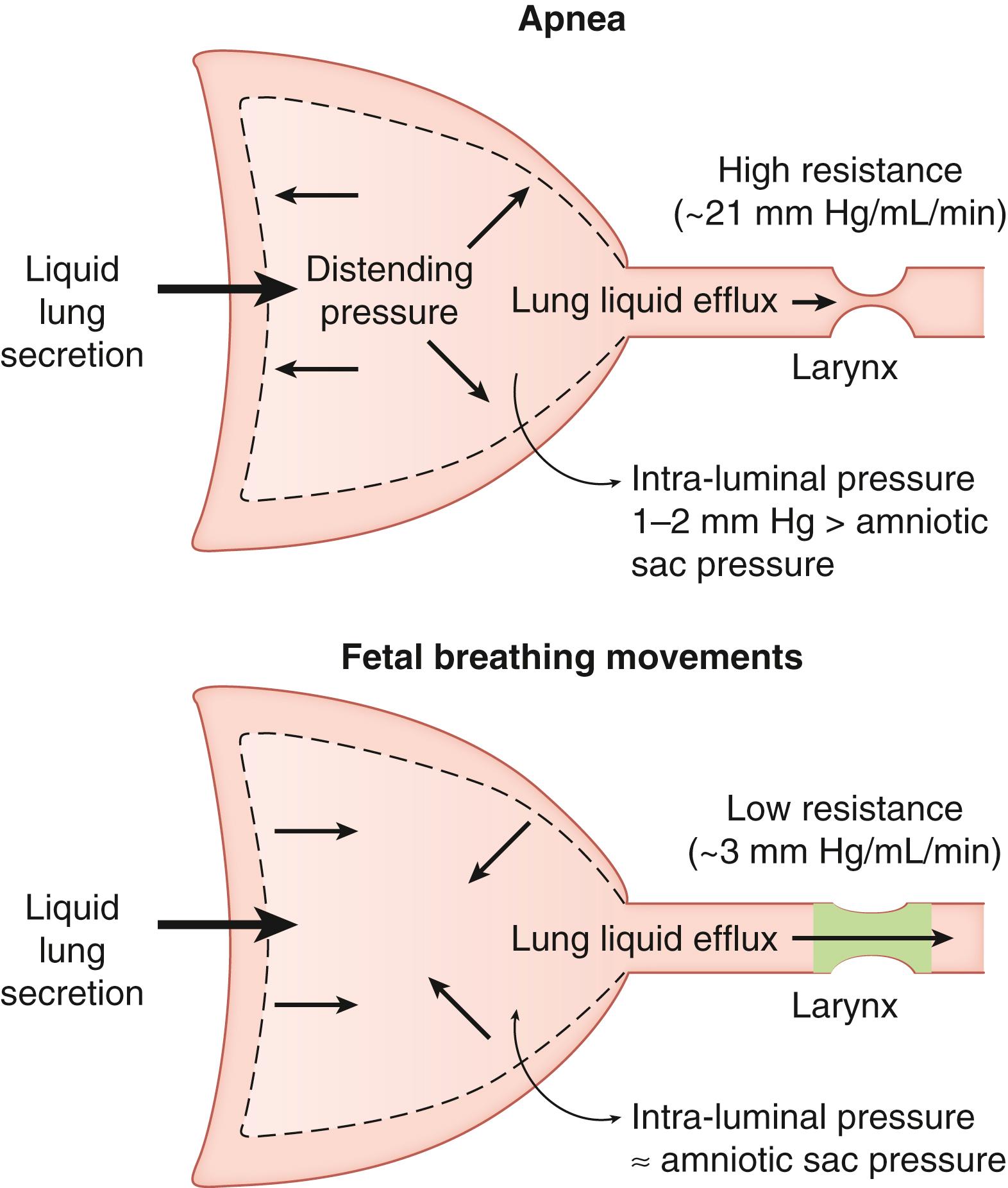
In the absence of labor, the volume of FLL in healthy fetuses is largely determined by fetal muscular activity and the transpulmonary pressure gradient. Although continued FLL secretion must contribute to FLL volume, the rate of secretion is relatively unimportant as alterations in the FLL secretion rate have little or no effect on the resulting volume. The rate of FLL efflux from the fetal lung via the trachea is dependent on the pressure gradient between the lung lumen and the amniotic sac (transpulmonary pressure), as well as the resistance to efflux in the upper respiratory tract. , In the absence of FBMs (i.e., during fetal apnea), the pulmonary intraluminal pressure is 1 to 2 mm Hg above amniotic sac pressure, due to the inherent recoil of lung tissue and the resistance of the upper airway. The resistance to liquid flow through the upper airway is high during apnea because laryngeal muscles are adducted. As a result, the rate of efflux of FLL through the trachea during apnea is, on average, lower than the rate of FLL production, and liquid accumulates within the lungs ( Fig. 57.3 ). During episodes of FBM, the resistance to efflux is reduced by phasic dilation of the glottis, , which together with the pressure gradient between the lungs and amniotic sac favors FLL efflux from the lungs (see Fig. 57.3 ). Thus, despite rhythmic contractions of the diaphragm, the average net flow of liquid from the lungs during FBM episodes is two to three times greater than the rate of efflux during intervening nonbreathing (apneic) periods. Although amniotic fluid can enter the fetal lungs during periods of accentuated FBM, the net flow is out of the lungs. This essentially unidirectional flux of FLL helps maintain a constant chemical environment within the future air spaces, limiting the entry of potentially harmful substances such as meconium. However, the occurrence of meconium aspiration syndrome in newborn infants suggests that there are some circumstances in which substances dissolved or suspended in amniotic fluid can enter the fetal lungs, which most likely occurs during episodes of augmented fetal breathing (or gasping) induced by fetal stress. Experimental studies injecting substances into the amniotic sac suggest that brownian motion could potentially cause some exchange of substances between lung liquid and the amniotic sac. However, the differences in ionic and protein composition between lung liquid and amniotic fluid support the notion that in healthy fetuses, the movement of lung liquid is essentially uni-directional from the lungs towards the amniotic fluid.
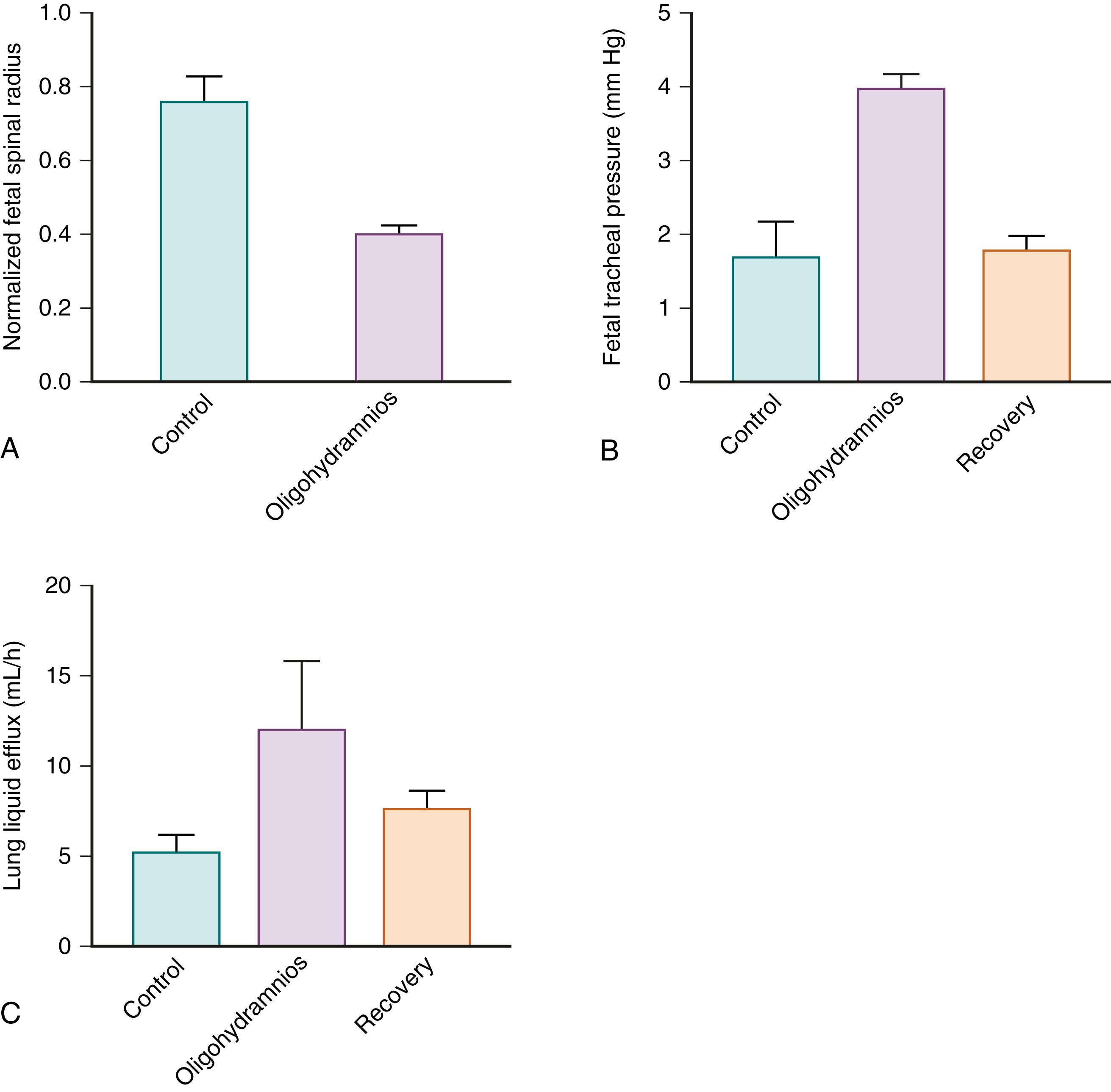
The transpulmonary pressure gradient, and hence the efflux of FLL, is also influenced by factors external to the lungs, such as fetal abdominal pressure. Changes in fetal posture (e.g., trunk flexion) that increase abdominal pressure can increase the transpulmonary pressure gradient, leading to a reduction in FLL volume. Such changes in transpulmonary pressure can be caused by uterine contractions or may develop when intrauterine space is limited as a result of oligohydramnios or by the presence of multiple fetuses. Lack of amniotic fluid forces the fetus into a more flexed posture. Increased flexion increases fetal abdominal pressure and thus transpulmonary pressure, leading to increased FLL efflux and a reduction in lung volume ( Fig. 57.4 ). This is the likely cause of lung hypoplasia associated with prolonged oligohydramnios. Because the fetal lungs and chest wall are highly compliant, even small changes in transpulmonary pressure will have marked effects on the rate of FLL efflux and consequently, on the degree of lung expansion.
Sustained reductions in fetal lung expansion are a common cause of fetal lung hypoplasia and retarded lung development, including effects on cell proliferation, extracellular matrix (ECM) deposition, alveolarization, vascular development, and alveolar epithelial cell (AEC) differentiation. The hypoplastic effects appear to depend on the degree to which lung expansion is decreased. For example, a 25% reduction in lung expansion causes lung growth to be reduced by approximately 25%, whereas total lung deflation causes lung tissue growth to effectively cease. , , The remodeling of lung parenchyma that characterizes normal lung maturation is also retarded by reduced lung expansion. In particular, perialveolar tissue volume is increased, leading to thicker septa and a reduced diffusing capacity for respiratory gases. Furthermore, alveolarization is attenuated, which is probably related to impaired elastogenesis, and there is a reduction in the growth and maturation of the pulmonary vascular bed, resulting in elevated pulmonary vascular resistance after birth. These findings indicate that lung expansion is an essential and normal stimulus for fetal lung growth. It is likely that the high degree of lung expansion during fetal life provides a direct mechanical stimulus to cells that is required to activate or suppress key regulatory gene pathways (as discussed further below).
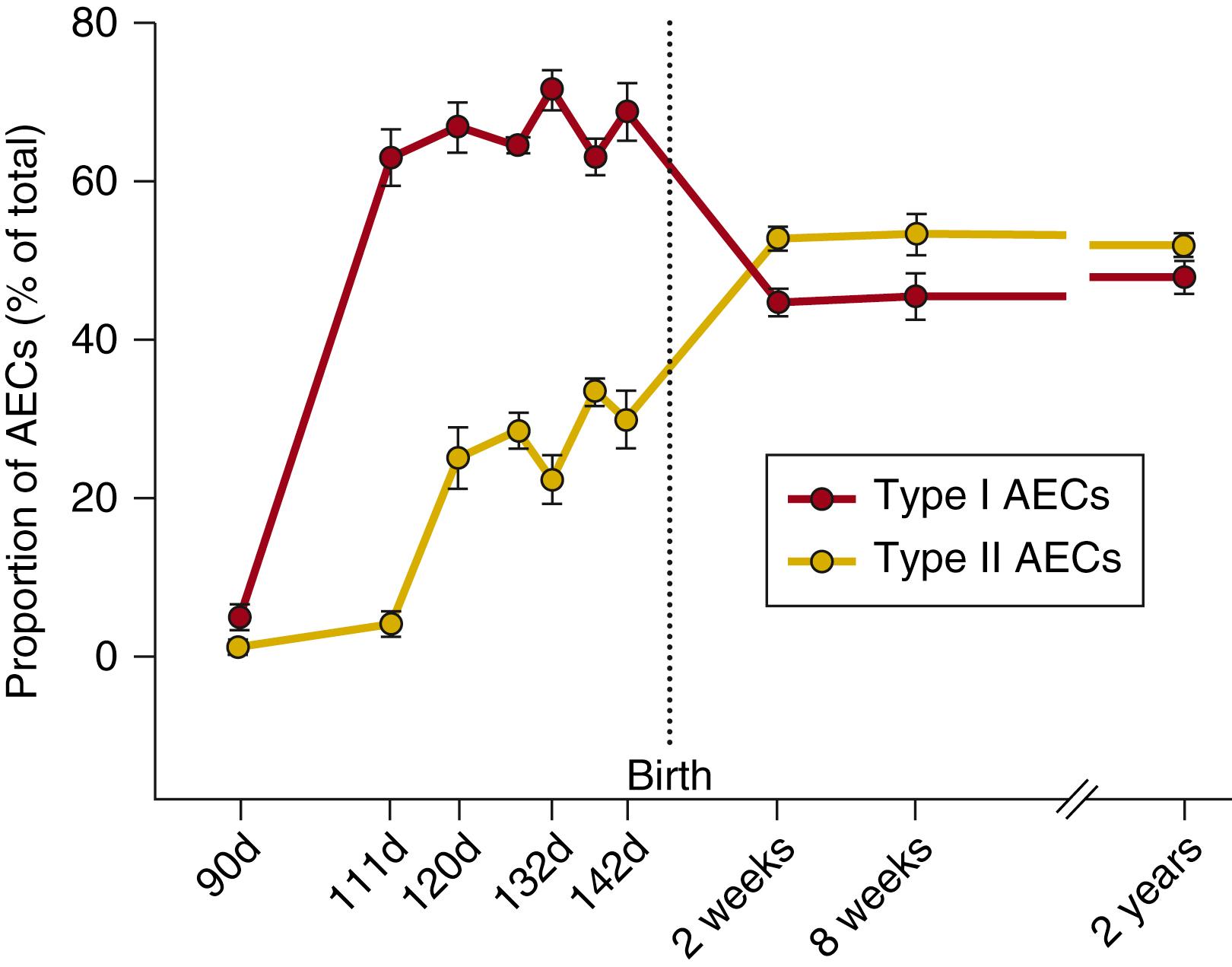
The alveolar epithelium of the fetal lung is also affected by the degree of fetal lung expansion. , Prolonged reductions in expansion promote differentiation of type I AECs into type II AECs, whereas prolonged overexpansion reduces the proportion of type II AECs to less than 2% and increases the proportion of type I AECs to greater than 90%. These findings support in vitro evidence that type I AECs are not terminally differentiated and can transdifferentiate into type II AECs. Indeed, the high degree of lung expansion during fetal life is associated with a predominance of type I AECs (60% to 70% of all AECs) relative to type II AECs (25% to 35% of all AECs). After birth, when lung expansion decreases, there is an increase in the proportion of type II AECs (to approximately 53% of all AECs) and a reduction in the type I AEC phenotype (to approximately 45% of all AECs) ( Fig. 57.5 ).
Prolonged overexpansion of the fetal lungs is a very potent stimulus for fetal lung growth and tissue remodeling. , Experimentally, this can be induced by ligation or obstruction of the bronchus or trachea. , , The increase in lung cell proliferation and cell hypertrophy in response to tracheal obstruction is time dependent and is restricted to the expanded lung tissue. This indicates that local factors, not circulating factors, must mediate the effect of lung expansion on lung development, although basal levels of growth hormone are required. , , The growth response to tracheal obstruction also differs according to the stage of fetal lung development. During the alveolar stage in fetal sheep, the acceleration in lung growth is completed within 7 days of tracheal obstruction, with proliferation of most cell types in the distal lung (fibroblasts, type II AECs, and endothelial cells) resulting in an almost doubling of lung DNA content. , The eventual cessation of accelerated growth is probably due to the physical restraint imposed by the chest wall, which prevents further lung expansion. Structural maturation is also accelerated, with a reduced proportion of tissue space and increased collagen and elastin deposition, capillary formation, , alveolar number, and luminal surface area. By contrast, during the late pseudoglandular to early canalicular stage, acceleration in lung growth in response to tracheal obstruction is slower but eventually results in a greater (∼200%) increase in lung DNA content, , primarily due to mesenchymal cell proliferation , with a large increase in perisaccular tissue volume. , Similar results have been obtained in fetal rabbits. The lower rate of accelerated lung growth in younger fetuses may be due to lower lung compliance and thus less lung expansion at the initial stages of tracheal obstruction until more significant structural remodeling has commenced. , The eventual greater increase in lung DNA content in younger fetuses is probably due to greater compliance of the chest wall, allowing the lungs to expand to a greater degree than in older fetuses. ,
The mechanisms by which lung expansion affects the rate of lung growth and structural maturation are under active investigation and are discussed below. However, it must be recognized that the mechanical stresses imposed on lung tissue by sustained alterations in lung expansion are likely to be very different depending on the location of cells within the tissue. For example, in response to increased lung expansion, some cells will experience a stretch-like stimulus, whereas others may experience compression.
FBMs begin early in fetal life and progressively become organized into discrete episodes that are largely associated with a fetal behavioral state resembling rapid eye movement sleep. During the latter half of gestation the incidence of FBMs is 40% to 50%. , , The major muscle used in FBMs is the diaphragm; respiratory activity of the intercostal muscles is largely absent, as in postnatal rapid eye movement sleep. During FBM episodes the laryngeal abductor (dilator) muscles contract in phase with the diaphragm, whereas during fetal apnea this dilator activity is absent and the glottis is closed by sustained adductor activity. , Typically, individual FBMs reduce intrathoracic pressure by up to 5 mm Hg and cause small oscillations of liquid flow within the fetal upper airway. , Much interest has focused on FBMs because they constitute an important determinant of fetal lung development, although their precise role in vivo took time to unravel. ,
Numerous techniques have been used to explore the functional role of FBMs, by eliminating or blunting their effects, including fetal paralysis, phrenic nerve sectioning, , or reversible blockade, sectioning of the fetal spinal cord above the outflow of the phrenic motoneurons, , , or replacing sections of the thoracic wall with a compliant membrane. However, the findings must be interpreted with caution owing to confounding factors associated with those procedures. For example, phrenic nerve section causes the diaphragm muscle to atrophy, whereas thoracoplasty may allow lung compression, and fetal paralysis abolishes laryngeal adductor activity and may alter fetal posture. To identify the specific role of FBMs in lung development, it is necessary to alter only FBM and then to measure FLL volume. With this investigative approach, it is apparent that the reduction in lung growth induced by the abolition of FBM can be explained by the associated decrease in the basal level of fetal lung expansion , ; indeed, the percentage decrease in lung expansion is similar to the reduction in lung growth. The decrease in lung expansion after abolition of the thoracic component of FBM can be explained by persistence of laryngeal dilator activity. As a result of this activity, the efflux of FLL is increased during centrally generated FBMs over that observed in intact fetuses. , Thus in intact fetuses, rhythmic activation of the diaphragm in FBM apparently plays a key role in restricting the loss of FLL when the resistance to FLL efflux is lowered by glottic dilation. , Collectively, these data show that fetal muscular activity, whether it is active glottic adduction during apnea or activation of the diaphragm during FBM episodes, helps to defend FLL volume and hence the degree of lung expansion (see Figs. 57.2 and 57.3 ). At present, no in vivo evidence has emerged to show that phasic stretch, per se, of the lung during FBMs is an important determinant of fetal lung growth, although in vitro evidence suggests this is possible.
Numerous in vitro studies have used phasic stretch of fetal lung cells in culture in an attempt to simulate FBMs in vivo. Such studies usually expose isolated fetal lung cells to 5% phasic distension, either constantly at approximately 60 cycles/minute, or intermittently (e.g., for 15 minutes in each hour), in either two-dimensional or three-dimensional cultures. Other studies use much higher levels of distension in culture (20% phasic stretch), but those studies are designed to mimic overdistension injury in ventilated patients, which leads to abnormal, not normal lung development, and are not therefore, included as part of this discussion.
The 5% distension regimen causes an increase in fetal lung cell proliferation and differentiation of distal lung epithelial cells, implying that phasic distension as a result of FBM may be important for lung development in a manner similar to basal distension. However, the stimulus used (approximately 5% stretch) probably exceeds that induced by FBMs in vivo. In vivo, individual FBMs are essentially isovolumic, so the percent length change experienced by a lung cell with each FBM will be negligible. This is because the fetal chest wall is very compliant, and FLL is very viscous compared with air and, owing to its mass, has a large inertia. Thus although activation of the diaphragm causes a reduction in intrathoracic pressure, very little liquid is inhaled with each inspiratory effort, because other sections of the chest wall are simultaneously drawn in and free liquid must be present within the pharynx before any liquid can be inhaled. As a result, the tidal volume in the fetus is very small. In late-gestation fetal sheep, the tidal volume is usually less than 0.5 mL at FBM rates of up to 3/second. In human fetuses during the last trimester, the mean FBM frequency is approximately 1/second, although respiratory cycle times of up to 1.5 to 2.0 seconds have been reported. Color Doppler ultrasound imaging has been used to measure liquid flow velocity waveforms in the trachea and nasopharynx of human fetuses, although the contribution of this liquid movement to lung volume changes is unclear. Thus, at least in sheep, fetal tidal volume is less than 1% of resting lung luminal volume but markedly increases immediately after birth to approximately 20% of resting lung volume (functional residual capacity). Nevertheless, in vitro studies investigating the effects of phasic distension on lung cells have provided important information about the cellular mechanisms by which the fetal lung responds to physical forces. These experiments are described in more detail in the next section.
All cells, tissues, and even whole organs are subjected to physical forces in vivo, including shear stress, stretch, and compression. These forces can result from gravity, osmotic pressures, fluid flow, intracellular tensile forces, body movements, and changes in the internal volume of hollow organs such as the lung. It has been proposed that cells exist in a state of isometric tension that is generated by the intracellular contractile filaments of the cell. In cultured AECs this tension is 0.1 to 0.2 kPa. Thus, externally applied forces are imposed on a preexisting equilibrium of force, causing changes in cell shape and intracellular structural fiber alignment until the force equilibrium is reestablished. ,
Physical forces play an important role in cell growth and differentiation, are critical regulators of three-dimensional tissue structure, particularly in the lung, , and constitute an important means by which cells interact with and adapt to changes in their environment. The transduction pathways by which physical forces are translated into chemical stimuli, and lead to changes in cell function, are still being explored. Transmembrane cell surface receptors, such as integrins, are ideally placed to detect and respond to alterations in the physical environment. Integrins bind to ECM proteins via their extracellular domain, whereas their intracellular domains are bound to fibrillar-actin bundles of the cell cytoskeleton via cytoskeleton-associated proteins (e.g., talin, vinculin, paxillin), and are associated with other signaling proteins, including phosphatidylinositol 3-kinase, pp60src, growth-factor-receptor-bound protein 2, and p130Cas. The cell cytoskeleton is in turn coupled to the nuclear skeleton via the LINC complex composed of nesprins, SUN proteins, and lamins. Nesprin on the outer nuclear membrane binds intermediate and microfilaments of the cell cytoskeleton and connects with SUN proteins on the inner nuclear membrane. SUN proteins bind to the internal nuclear scaffold and to nuclear proteins via A-type lamins. Together these complexes form a structural continuum from the ECM to the nucleus. It is through these physical couplings that mechanical forces can be detected and translated into intracellular chemical signals. , , The chemical signaling pathways are less well defined, but they are likely to include stretch-activated ion channels, activation of intracellular second messenger systems, direct activation of RNA polymerases and DNA-synthesis enzymes, and recruitment of messenger RNA and protein translation machinery, as well as alteration of chromatin structure allowing access of transcription factors to gene promoters. , , Together, these pathways likely result in the synthesis or release of ECM proteins, growth factors, cytokines, and other factors that can act in an autocrine or paracrine manner to induce cell proliferation, differentiation, migration, or other alterations in cell function ( Fig. 57.6 ) that lead to marked changes in the size, structure, and function of the lung.
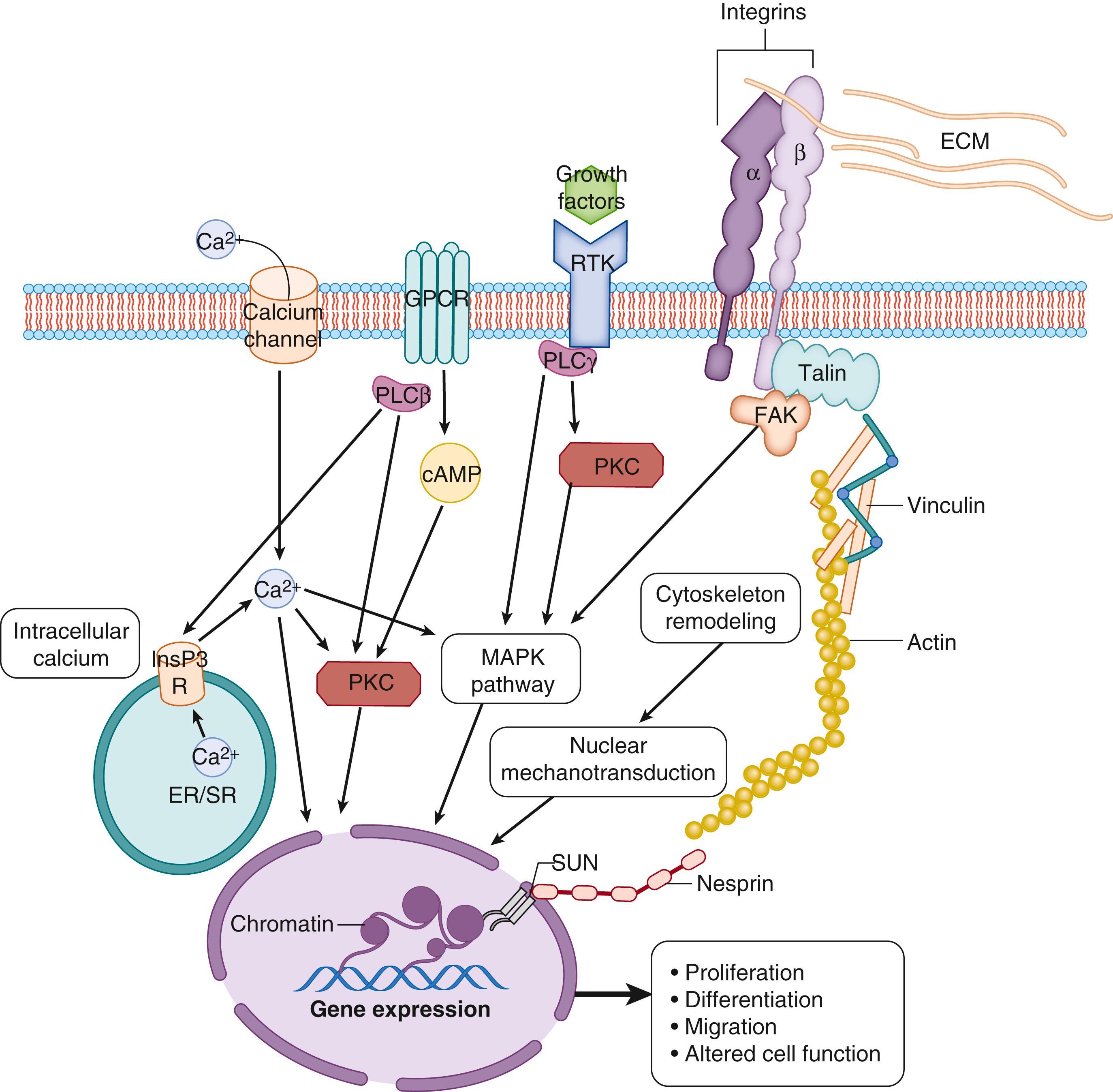
The importance of signaling through mechanotransduction pathways has been highlighted by transgenic mice and isolated fetal lung cells exposed to cyclic stretch. Integrin α3–null mice have abnormal branching morphogenesis, whereas double α3/α6 integrin null–mice have left lung agenesis and severe right lung hypoplasia. The lungs of integrin α8–null mice have defective alveolarization and abnormal elastin deposition. Epithelial cell differentiation also appears to require integrin signaling, as blocking antibodies against integrins β1, α3, and α6 reduce the increases in SP-C mRNA levels induced by 5% phasic stretch of cultured fetal lung epithelial cells. Exposing fetal lung cells to cyclic stretch also increases pp60src activity and its translocation to the cytoskeleton, which activates phospholipase Cγ1, leading to increases in diacylglycerol and inositol 1,4,5-trisphosphate (InsP3) content. InsP3 releases calcium from intracellular stores via the InsP3 receptor (InsP3R), which, via diacylglycerol, activates protein kinase C and its downstream mitogenic effects. Activation of phospholipase Cγ1 and protein kinase C is required for cyclic stretch–induced proliferation of fetal lung cells in culture (see Fig. 57.6 ).
Mechanical forces can also be converted into biochemical signals by activation of stretch-activated ion channels, which alter intracellular ion concentrations. Calcium, in particular, is an important cofactor for the activation of many signaling pathways. Blocking strain-induced calcium channels or chelating intracellular calcium stores abolishes the proliferation of lung cells in culture, suggesting that calcium entry into cells, via stretch-activated ion channels, is vital for fetal lung growth. Blockade of voltage-dependent calcium channels also causes hypoplasia in lung explants. Inhibiting calmodulin, a major calcium-signaling molecule, in type II AECs of transgenic mice disrupts lung development, and postnatal rats treated with a calmodulin antagonist have impaired lung growth. This is consistent with the finding that calmodulin mRNA levels are increased in response to an increase in fetal lung expansion, when lung cells are proliferating at rates approximately 800% above that in control fetuses. Hemi-pneumonectomy in young rats induces compensatory lung growth in the remaining lung, which like fetal lung growth, is thought to be expansion mediated. , The time course for the increase in lung growth after postnatal hemi-pneumonectomy in rats is very similar to that induced by tracheal obstruction in fetal sheep, with a maximal increase in both cell proliferation and calmodulin expression within 2 days. , , The calmodulin inhibitor trifluoperazine reduces both calmodulin activity and the increase in lung growth induced by hemi-pneumonectomy. These findings indicate that calcium signaling is critical for enabling expansion-induced lung growth both before and after birth.
Calcium may also mediate the effects of lung stretch on AEC differentiation. As noted above, 5% phasic stretch of cultured fetal lung epithelial cells increases SP-C mRNA levels; this effect is increased in the presence of an agonist for the calcium-permeable cation channel TRPV4, while a TRPV4 antagonist completely abolishes the effect of phasic stretch on SP-C expression.
It is also possible that increased expansion of the fetal lung stimulates growth factors and/or an increase in their receptors that act locally, in an autocrine or paracrine manner, to stimulate cellular proliferation. The fetal lung produces a number of growth factors and their receptors, including platelet-derived growth factor, , vascular endothelial growth factor, insulin-like growth factors 1 and 2, fibroblast growth factors, and transforming growth factor β, which have been shown to be critical for fetal lung development. Furthermore, phasic distension of cells in culture induces the expression of many of these growth factors, , , , suggesting that they may mediate the effects of lung expansion on lung development.
Insulin-like growth factor-2 expression is decreased by reductions and enhanced by sustained increases in lung expansion, , but the increase in insulin-like growth factor-2 expression occurs only after the maximal increase in expansion-induced DNA synthesis rates has already occurred. Similarly, expression of platelet-derived growth factor subunit B is reduced in response to tracheal obstruction, when DNA synthesis rates are elevated, suggesting that expansion-induced lung growth is unlikely to be mediated by those growth factors. Surprisingly, increased fetal lung expansion does not activate extracellular signal–regulated kinase 1 or extracellular signal–regulated kinase 2 of the mitogen-activated protein kinase pathway, suggesting that growth factor receptor activation, at least via the extracellular signal–regulated kinase pathway, is unlikely to be a major mediator of expansion-induced fetal lung growth. However, basal levels of genes in the ERK pathway are critical for lung growth, as transgenic manipulations of these genes in a variety of lung cell types cause either lung agenesis or lung hypoplasia. However, vascular endothelial growth factor expression is reduced in lung hypoplasia and transiently increased after tracheal obstruction, indicating that vascular endothelial growth factor may mediate impaired vascular development in lung hypoplasia and may regulate endothelial cell proliferation in growth-accelerated lungs, , , potentially via activation of c-Jun N-terminal kinase instead of extracellular signal–regulated kinase 1.
Several studies have used transcriptomic or proteomic approaches to identify larger-scale changes in gene expression and protein levels in response to decreases or increases in lung fetal lung expansion. , , Numerous genes likely to be involved in expansion-induced lung growth, epithelial cell differentiation, or alveolarization were identified by Sozo and colleagues, using subtraction hybridization in fetal sheep, and by Seaborn and colleagues, using serial analysis of gene expression in fetal mice. In the fetal rabbit model of surgically induced congenital diaphragmatic hernia (which induces lung hypoplasia) and restoration of growth using tracheal obstruction, targeted gene screening and mRNA sequencing has been used to identify candidate genes associated with expansion-induced alveolarization and angiogenesis. , More recently, Peiro and colleagues, used a proteomic approach to identify proteins in tracheal liquid from fetal sheep with congenital diaphragmatic hernia (CDH)-induced lung hypoplasia and tracheal obstruction. Many of the genes and proteins identified in the above studies have strong correlations with cell proliferation or differentiation in vivo, and cell culture or transgenic mouse studies often support the likelihood of those factors being involved. , , , , However, proving the involvement of the identified genes and proteins in mechano-transduction-mediated lung development is much more challenging. This is due to the difficulty of performing transgenic manipulations and the cost of performing siRNA and CRISPR technologies in the large animals commonly used to manipulate lung expansion and the difficulties of altering lung expansion in small animals used for transgenic studies.
However, where specific inhibitors are available, these studies are possible. For example, studies in fetal mice with reduced fetal lung expansion induced by oligohydramnios, and increased fetal lung expansion induced by tracheal occlusion, have demonstrated that the Rho/Rho-associated kinase (ROCK) pathway, which regulates actin cytoskeleton assembly, is a likely mechanosensory pathway critical for mediating the effects of lung expansion on the generation of distal airways.
Recent studies have focused on identifying the molecular mechanisms that regulate lung development more broadly (not specifically in relation to effect of physical forces on the lung). The rapidly evolving fields of genomics, transcriptomics, proteomics, metabolomics, lipidomics, and epigenomics techniques are identifying novel genes and proteins and regulatory mechanisms, in addition to the molecules already known to have roles in cell growth, cell survival, cell migration, cell differentiation, and remodeling of the ECM. These studies are generating information regarding entire protein pathways and interactions between pathways, that likely play important roles in stimulating and coordinating normal and abnormal fetal lung growth and maturation. ,
Those studies are dramatically enhancing our understanding of the molecular mechanisms that regulate lung development and publicly available databases like LungMAP (the Molecular Atlas of Lung Development Program; https://lungmap.net/ ) , , and Jackson Laboratory lung development database ( http://lungdevelopment.jax.org/ ), enable data sharing and interrogation by other investigators. Many of the genes, proteins and pathways identified in such studies are discussed in more detail in Chapter 55, Chapter 56, Chapter 58 and have been the subject of numerous reviews. , Studies in transgenic mice, particularly those that use inducible, cell-specific transgenic techniques, are also rapidly improving our understanding of how individual genes regulate lung development, and improved imaging and molecular techniques in mice and cell culture are improving our knowledge of the cell types, structural context, stages of lung development, and species in which these genes are expressed. However, the exact roles of these genes in mediating the effect of lung expansion on lung development are less well understood.
Another exciting area of investigation is the role that extracellular vesicles (EV) are likely to play in cell-to-cell communication in the lung. EV are small membrane vesicles that are released from many cell types and can carry microRNA (miRNA), proteins, and lipids from one cell to another. , Numerous studies have identified miRNAs that may regulate the expression of genes that are critical for lung development. A recent study by Najrana and colleagues demonstrated the presence of EV in lung liquid from late gestion and newborn mouse lungs and in cultured lung cells exposed to stretch. Cells exposed to 10% cyclic stretch for 24 hours produced twice as many EVs as unstretched controls, and nine of the miRNAs isolated from the EVs were differentially expressed. In contrast, cells exposed to 5% continuous stretch had a similar number of EVs to unstretched controls, but contained nearly four times as many differentially expressed miRNAs, including several miRNA species known to regulate T1α. T1α is a marker of type-I epithelial cells in mice and sustained increases in lung expansion promote the differentiation of alveolar type II cells into alveolar type-I cells. , EVs and associated miRNA molecules may mediate the effect of expansion by cell-cell transmission and also offer an exciting potential route for the targeted delivery of new therapeutics to the lungs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here