Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
By the end of this chapter the reader should:
Understand the principles of pharmacokinetics in children
Know the major pathways of drug metabolism in children and how they vary with age
Know about some of the commoner adverse drug reactions that occur in children
Understand the mechanisms by which adverse drug reactions occur in children
Know the principles of safe prescribing
Be aware of the most frequent types of medication error
Understand the pharmacology of some of the commonly used drugs in children
Understand the importance of the rational use of medicines in children
Understanding how the body handles different medicines is important for all clinicians when prescribing medications. For effective and safe prescribing, we need to make sure that we do not under-dose a medication, making it ineffective, but also that we do not give a dose that causes toxic effects. In paediatrics, significant physiological and developmental differences add to the challenges of safe prescribing. The key parameters of clinical pharmacology will be described below and the differences seen in the different paediatric age groups will be highlighted.
Pharmacokinetics (PK) describes the course of a drug within the body; this is expressed as the dose given and concentrations in different parts of the body (usually plasma). It includes how it is absorbed, distributed, metabolized and finally excreted. These will each be discussed separately, along with the common equations used. Pharmacokinetics allows us to understand the profile of a drug's concentration over time and recommend a drug dosing regimen or, when faced with a novel paediatric drug therapy, provides us with knowledge to prescribe safely and effectively. Mathematical formulae are available that describe the inter-relationship between clearance, volume of distribution and elimination half-life.
If a drug is given intravenously, 100% of the dose will enter the blood stream, but for any other route less than 100% of the dose will be absorbed. This is because it must overcome chemical, physical, mechanical and biological barriers; the percentage that enters the systemic circulation is known as its bioavailability .
Absorption is the process of drug movement from the site of administration or application into the systemic circulation. It is often reduced following oral administration in the neonatal period. Additionally, in the neonatal period, pH is elevated within the stomach. This increase in gastric pH affects the bioavailability of medicines. This higher gastric pH increases the absorption of weak base drugs such as penicillins and decreases the absorption of acidic drugs such as phenobarbital and phenytoin, which may therefore require a larger oral dose. Fortunately, in sick neonates most medicines are given intravenously and therefore absorption is not usually a clinical problem.
For children, there are other developmental changes to drug absorption that occur in different systems. Intramuscular absorption depends on skeletal muscle blood flow; neonates have poor muscle bulk and poor muscle density, reducing bioavailability. Percutaneous absorption is enhanced in childhood due to the larger surface area of the skin relative to the body weight, and better skin hydration and perfusion. Young infants and neonates also have increased absorption due to their skin being thin. This increases systemic absorption and therefore potential side effects of topical medications.
A historical catastrophic example of this is the topical disinfectant hexachlorophene in neonates, which caused neurotoxicity and death. Developmental changes in pulmonary structure and capacity in young patients may also alter the patterns of inhaled drug absorption (see also Chapter 17, Respiratory medicine ).
Which of the following is true with regards to volume of distribution (Vd)? Select ONE answer only.
A larger Vd requires a higher loading dose of a drug.
A large Vd implies a drug primarily resides in the systemic circulation.
Neonates have a higher Vd with lipophilic drugs.
Neonates have a lower Vd for hydrophilic drugs.
Vd equals the total amount of drug in the body multiplied by the concentration found in the plasma.
A. A larger Vd requires a higher loading dose of a drug.
A larger Vd implies good distribution within the tissues and subsequently will require a higher loading dose for a drug to get an adequate target concentration. Neonates have higher body water, therefore they have a lower Vd for fat-soluble drugs and higher Vd for water-soluble drugs (see below).
This is not a physiological volume, but rather an apparent volume into which the drug would have to distribute to achieve the measured concentration. The volume of distribution is usually defined in litres or litres/kg. It is calculated by dividing the amount of drug by the plasma concentration:
A small volume of distribution indicates a drug is largely retained within the systemic circulation, whereas a large volume of distribution means a drug is well distributed into other peripheral compartments. Water-soluble drugs, such as gentamicin, therefore have a volume of distribution that is similar to the extracellular fluid volume. Drugs that are highly bound to plasma proteins, such as phenytoin, have a low volume of distribution. Differences between paediatric and adult patients stem mainly from the fact that neonates and young children have a higher proportion of body water and lower concentrations of plasma proteins. Knowing the volume of distribution of a drug is useful when determining what loading dose is to be given. This is calculated from the following formula:
For example, in order to achieve a peak gentamicin concentration of 10 mg/L in a neonate weighing 1 kg, where the volume of distribution is known to be 0.5 L/kg, one would multiply 10 mg/L by 0.5 L/kg by 1 kg. This equates to 5 mg.
If some of the drug is already present in the patient, one can subtract the measured plasma concentration from the target concentration in order to calculate the dose that is required.
Total body clearance is the ability of the body to remove a drug from the plasma or blood and is the sum of drug clearances of each organ. For many drugs, this is equal to hepatic clearance plus renal clearance. Renal clearance is determined by the clearance of an unchanged drug in the urine, whereas liver clearance can occur via biotransformation to a metabolite, which is subsequently excreted via the urine, and/or excretion of the unchanged drug into the biliary tract.
It is defined as the volume (usually of plasma) that is completely cleared of drug in a given time period. In adults, clearance is therefore described in relation to volume/time (L/hour). In paediatric patients, clearance is also described in relation to body weight (either as L/hour/kg or mL/min/kg). Clearance can be used in conjunction with the target steady state concentration (C SS ) to calculate the rate of administration of a drug given intravenously. This is shown in the following equation:
Or for children, where a dose and clearance are expressed in relation to body weight:
This formula is appropriate for the administration of drugs given intravenously.
For example, the maintenance dose of an intravenous aminophylline infusion to achieve a theophylline level of 10 mg/L in a 20 kg child where clearance is 0.087 mg/kg/hour is 10 mg/L × (0.087 × 20) = 17.4 mg/hour.
For drugs that are given orally, one needs to take account of the bioavailability as well as the dosage interval between different doses. If a drug is given via regular bolus intervals, the ‘average’ target C SS is used as steady state fluctuates between the peak and trough and the dosing interval (π) is also added into the equation. The maintenance dose can therefore be calculated by the following formula:
Maturation of renal function occurs during childhood. The maturation process of kidney structure and function is associated with prolongation and maturation of the tubules, increase in renal blood flow, and improvement of filtration efficiency. This knowledge allows us to provide a rational dose schedule for drugs exclusively eliminated via the kidneys. In general, the neonate will need longer dose intervals than the infant to maintain target concentrations.
For example, the dose of benzylpenicillin changes from 25–50 mg/kg 12 hourly in a neonate <7 days to 8 hourly in a 7–28-day neonate and 4–6 hourly in children over a month old.
A new antibiotic, X, has been developed. It has exceptional antimicrobial activity but is toxic in higher doses. The half-life is 2 hours in children.
After how long will 97% of this new antibiotic, X, be eliminated from the child? Select ONE answer only.
4 hours
6 hours
8 hours
10 hours
12 hours
D. 10 hours.
See below for discussion.
A new agent has come to the market as a treatment for severe pain. It is intended to be given as a single dose rescue treatment. There have been no large studies in children but in the literature its plasma half-life is 12 hours.
What percentage of the dose is still in the body after one day? Select ONE answer only.
87.5%
75%
50%
25%
10%
D. 25%
50% of the drug is eliminated every 12 hours, therefore 25% left in the body after two half-lives.
Half life is a secondary pharmacokinetic parameter and is the time taken for the drug concentration (usually in the plasma) to decrease by half. Therefore, 50% of the dose will be eliminated in one half-life. It is inversely related to the clearance and can be calculated using a drug's volume of distribution and clearance with the following equation:
(Note: the value 0.693 is the natural logarithm of 2)
Half-life can be used to determine the time it takes to achieve steady state and the time for a drug to be completely eliminated. It takes around 3–5 times the drug's half-life to reach steady state and the same for it to be completely eliminated in constant dosing. Five half-lives is the time required for 97% of the drug to be eliminated ( Fig. 36.1 ). For instance, the t 1/2 of intravenous midazolam is of the order of 1.1 hours in 3–10-year-olds, therefore it takes around 3.3 to 5.5 hours to reach steady state.
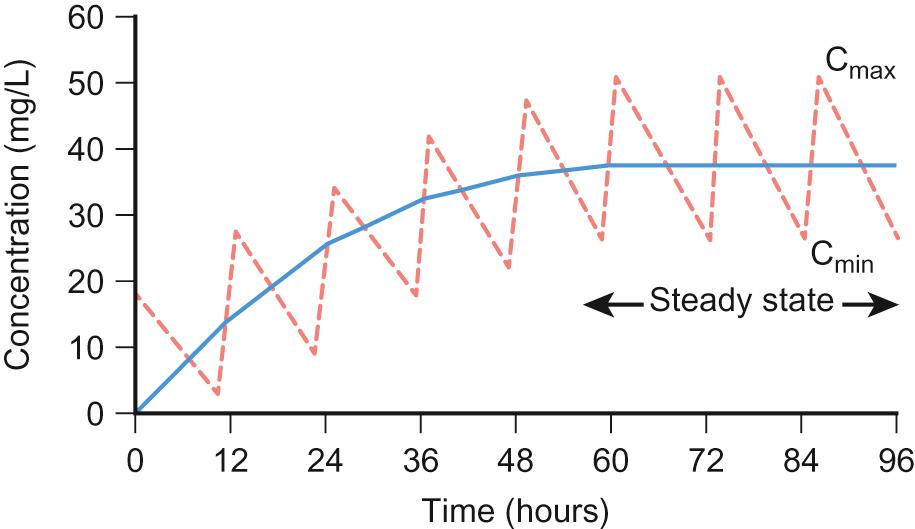
The half-life helps the clinician to establish an appropriate drug dosing interval. When a medication is given every half-life, the plasma concentration will have a twofold fluctuation over the dosing interval (see Fig. 36.1 ). For drugs with a half-life <6 hours, it is sometimes not practical to give frequent doses, so sustained release formulations are given (e.g. theophylline). In drugs with a very long half-life (e.g. amiodarone), a daily treatment may be appropriate. A loading dose helps to reach the steady state more quickly. The t 1/2 of phenobarbital in neonates is 67–99 hours, so without a loading dose it could take 8–20 days to reach a steady state. Although drug half-lives are quoted in the literature, they represent average values mainly in adults and should be used cautiously.
The pharmacokinetic principles outlined above assume that the drug follows first-order or linear pharmacokinetic characteristics. This means that the steady-state concentration changes in direct proportion to a drug dose alteration. However, for some drugs, the relationship is more complex. For example, phenytoin saturates the metabolizing enzymes at clinical doses. Subsequent increases in dosing cause a disproportionate elevation of the steady-state concentration. This is known as zero order or saturated kinetics.
Interactions between food and drugs can unintentionally reduce or increase the effect of an oral medicine, resulting in potential therapeutic failure or toxicity. Firstly, food intake also impacts on drug absorption by stimulating gastrointestinal secretions, pancreatic hormones, and bile salts (which lower gastric pH), as well as delaying stomach emptying and increasing gastrointestinal transit time. The size and content of a meal, especially those with a high fat content, also play a role and can reduce a drug's rate of absorption.
Secondly, food has the ability to affect a drug's bioavailability by interaction with the food constituents. A good example is the reduction in bioavailability of tetracyclines following dietary calcium caused by chelation. Food–drug interactions affecting metabolism, distribution or elimination are not very common, apart from interactions with grapefruit juice. Grapefruit juice contains potent inhibitors of the cytochrome P450. CYP3A4, a P450 enzyme, may markedly increase the bioavailability of drugs that it metabolizes, including ciclosporin, midazolam and carbamazepine.
Therapeutic drug monitoring (TDM) consists of measuring plasma concentrations of the drug in order to improve its efficacy whilst reducing its toxicity ( Box 36.1 outlines criteria for use). TDM is recommended for certain antibiotics, including the aminoglycosides and glycopeptides, in order to reduce potential toxicity. It may be beneficial in patients with poorly controlled epilepsy who are receiving carbamazepine, phenytoin or phenobarbital. Interpretation of the plasma concentration of a drug requires details of the time of administration of the drug and time of collection of the blood sample, as well as an understanding of why TDM has been requested.
Good correlation between serum concentrations and pharmacological effect.
A narrow margin between serum concentrations that cause toxic effects and those that produce therapeutic effects.
Marked pharmacokinetic intra- and inter-individual variability.
The pharmacological effects of the drug are not readily measurable.
It provides a rapid and reliable method for the analysis of the drug.
Drug levels should only be taken once the drug has reached its steady state, unless there are concerns regarding toxicity. In general, trough levels measured just prior to drug administration provide accurate interpretation of drug concentrations. Peak levels are less accurate due to individual variability and are reserved for treatments with short half-lives where peak levels are associated with efficacy or toxicity, e.g. gentamicin.
This class of drug is bactericidal and works by irreversibly binding the 30S subunit of the bacterial ribosome, and interfering with bacterial protein synthesis. Aminoglycosides are mainly used for the treatment of severe Gram-negative infections, with tobramycin and gentamicin having some activity against Pseudomonas infections. Tobramycin is used frequently in children with cystic fibrosis. Gentamicin also works synergistically with β-lactams for the treatment of Gram-positive Staphylococci infections. This is why benzylpenicillin and gentamicin are used in combination for the treatment of group B streptococcal neonatal infections.
TDM is essential when using aminoglycosides because of the significant oto- and nephrotoxicity that can occur with these agents. It is thought that toxicity is associated with high trough concentrations. Studies in adults suggest that ototoxicity is more frequent than nephrotoxicity. Ototoxicity has been described following single doses and is thought to occur in 5–10% of adults who receive aminoglycosides. Both prolonged and repeated courses are thought to be risk factors for toxicity. Aminoglycosides used to be given three times daily but current practice is to give them once daily. This larger daily dose produces a higher peak level than the standard regime, which in turn increases the rate and extent of bacterial cell death. It also lengthens the post-antibiotic effect (suppression of bacterial regrowth) without increasing the risk of any drug toxicity. When multiple daily dose regimens are used, as well as a pre-dose (trough concentration), one should measure a one-hour (peak) post-dose concentration. Most hospitals in the UK provide a clinical pharmacy service to help interpret the plasma concentration and give advice regarding dose adjustment. The beneficial effect of discussing management with a clinical pharmacist has been demonstrated. In addition to minimizing toxicity, one needs to ensure that the individual patient receives a dose that is effective in treating the significant bacterial infection that the patient is likely to be suffering from.
Glycopeptides are another group of antibiotics that require therapeutic drug monitoring and include vancomycin and teicoplanin. They act by interfering with the bacterial cell wall synthesis in Gram-positive bacteria. They bind to the end of the pentapeptide chains that are part of the growing cell wall structure. This inhibits the transglycosylation reaction and prevents incorporation.
Vancomycin and teicoplanin are used in intravenous form for the treatment of serious infections caused by Gram-positive cocci such as Staphylococcus aureus and coagulase-negative Staphylococcus. Vancomycin is the main treatment for patients with MRSA infections. It can also be given orally for the treatment of pseudomembranous colitis in the colon, usually caused by Clostridium difficile , which is rarely seen in children.
Like aminoglycosides, glycopeptides are nephro- and ototoxic and hence require TDM ( Box 36.2 ). Variations in protocol occur throughout different hospitals about how to monitor and adjust vancomycin dosing, and local policies should be followed. Teicoplanin is less toxic than vancomycin but still requires monitoring. Adverse drug reactions due to vancomycin include red man syndrome, characterized by flushing and erythematous skin usually of the upper body and face. This is caused by a non-specific mast cell degranulation and can be avoided with a slow infusion rate.
The following (A–H) is a list of drug metabolism pathways:
Acetylation
Glucuronidation
Hydration
Hydrolysis
Methylation
Oxidation
Sulfation
Reduction
Pick the mechanism from the list described above that best matches the descriptions below:
The major phase 1 pathway utilized by cytochrome P450 enzymes in the liver.
The major phase 2 pathway responsible for elimination of paracetamol in teenagers.
F. Oxidation
B. Glucuronidation
See below for discussion.
Pre-dose (trough) after 2nd–3rd dose:
<5 mg/L – increase frequency if able, if not, increase the dose by 10–20%
5–10 mg/L – increase dose by 10–20%.
10–15 mg/L – continue as in therapeutic range (sometimes need 15–20 mg/L in less sensitive MRSA strains – consult microbiology if cultures available)
15–20 mg/L – reduce dose by 10–20%
>20 mg/L – check trough before commencing and reduce frequency
Check electrolytes to identify acute kidney injury.
Most drugs need to be converted into more water-soluble compounds to become inactivated and excreted from the body. This can take place in a variety of sites (e.g. gastrointestinal tract, skin, plasma, kidney, lungs), but most are metabolized in the liver via hepatic enzymes.
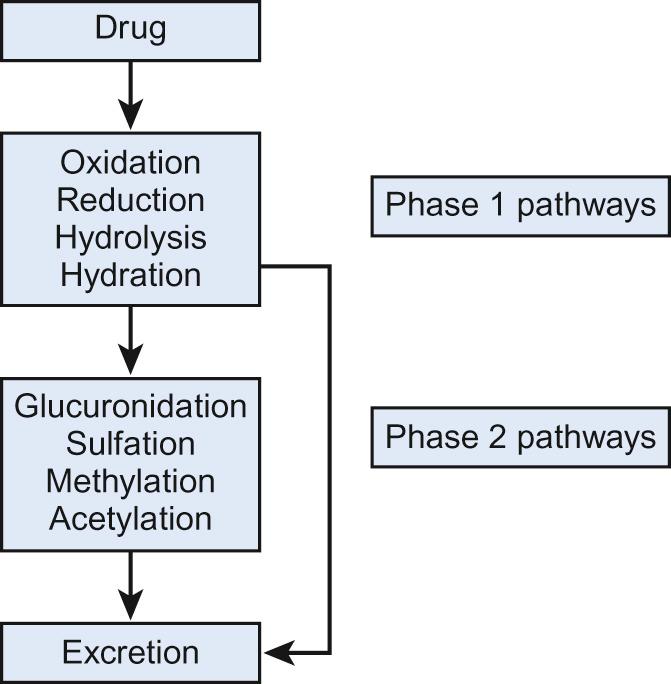
Two phases of drug metabolism are traditionally distinguished – phase 1 and phase 2. Phase 1 involves altering the structure of the drug, for example oxidation and hydrolysis. The major pathway is oxidation, which involves the cytochrome P450-dependent (CYP) enzymes that are present mainly in the liver. Phase 2 reactions conjugate the drug to another molecule (for example, glucuronidation of paracetamol in older children). Drugs may undergo metabolism and subsequent elimination by a combination of phase 1 and phase 2 pathways ( Fig. 36.2 ). This deliberate simplification of drug metabolism does not imply that only the parent compound itself is active and that metabolites are not. Some drugs are inactive and need to be metabolized to exert their effect; for example, carbamazepine, and enalapril. Other drugs may have metabolites with the same therapeutic effect as the parent compound, such as morphine and its metabolite morphine-6-glucuronide. Finally, some metabolites may be responsible for adverse drug reactions. Paracetamol is metabolized to the reactive metabolite N -acetyl- p -benzoquinone imine (NAPQI), which is associated with acute liver injury after overdoses (see below).
The most abundant and best studied phase 1 system is the cytochrome P450 (CYP450) family which consists of at least 56 genes that code for functional enzymes. More than half of all metabolized drugs are subject to CYP450-mediated metabolism . The most common subfamilies are CYP1A, CYP2A–2E and CYP3A/CYP3A4. They are responsible for the metabolism of many drugs, such as midazolam, ciclosporin, fentanyl and nifedipine ( Table 36.1 ). CYP1A2 accounts for 13% of total enzyme activity in the liver. Caffeine and theophylline are metabolized via the CYP1A2 pathway (see Table 36.1 ).
| Pathway | Drug |
|---|---|
| Oxidation | |
| CYP3A4 | Carbamazepine Diazepam Erythromycin Fentanyl Midazolam Nifedipine Ondansetron Rifampicin |
| CYP1A2 | Caffeine Theophylline |
| CYP2C9 | Phenytoin Ibuprofen |
| CYP2D6 | Amitriptyline Codeine Selective serotonin reuptake inhibitors |
| Glucuronidation | Paracetamol Morphine |
| Sulfation | Paracetamol |
These are a different set of enzymes and less is known about them. Glucuronidation and sulfation are the two major phase 2 pathways. Examples include UDP-glucuronosyltransferases (UGT), sulfotransferases (SULT) and glutathione-S-transferases (GST). The majority of these encompass large numbers of different enzymes which are differently regulated and metabolize different drugs.
The majority of drug-metabolizing enzymes develop in a different pattern and rate. However, there are three distinct patterns that have emerged from research studies. Individual enzymes show maximum activity either prenatally, postnatally or throughout development. Within the prenatal pattern, enzyme activity is high in the fetal liver as well as just before and after birth and then it declines. An example of this is CYP3A7.
The second group has a postnatal pattern. Here, the levels of the enzyme are low at birth but increase to adult ranges over the next few weeks or months. This is seen in a large number of enzymes, including CYP1A2, CYPC2C, CYP2D6 and CYP3A4.
Finally, in the constant pattern, activity remains stable from early fetal life through adulthood; examples are CYP3A5 and SULT1A1.
Age-related changes in drug metabolism have a major impact on a drug's effect. The majority of these are observed in neonates and infants, when typically the largest changes in enzyme activities occur. An important example of this is chloramphenicol. Historically, chloramphenicol was used to treat neonatal infections using adult doses of 12.5–25 mg/kg four times a day. Neonates have immature levels of UGT2B7, which converts chloramphenicol to the excreted water-soluble chloramphenicol glucuronide. Large numbers of babies developed cardiovascular collapse and irregular respiration, and in some cases died; this became known as grey baby syndrome because of chloramphenicol toxicity as a result of this immature metabolism. Consequently, dosing in neonates has been reduced to 12.5 mg/kg twice daily for those under one month of age, and is rarely used in neonates.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here