Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The treatment of patients with hematologic malignancies has been revolutionized over the past decades as new therapeutic targets continue to be identified through cellular and molecular studies of these conditions. These investigations have spawned the discovery, clinical evaluation, and US Food and Drug Administration (FDA) approval of new mechanistic-based therapeutic agents. A surprising number of these agents have progressed rapidly from the discovery phase to validation, animal modeling, and successful clinical testing. The results have led to an explosion in the therapeutic armamentarium and an increase in the spectrum of drugs, including small molecules, monoclonal antibodies, radiolabeled antibodies, drug immunoconjugates, immunotoxins, and complex delivery systems. This chapter provides information on new and existing therapeutic agents available for the treatment of patients with hematologic malignancies. The chapter reviews the “classic” agents as well as the newly developed, target-based agents. Both cytotoxic and growth-inhibitory agents are covered; however, the use of therapeutic antibodies and antibody conjugates is reviewed within the chapters dealing with specific diseases.
Whereas hematologic malignancies are of clonal origin (i.e., they are derived from a single transformed cell), individual neoplastic cells from a patient's malignancy exhibit a great deal of phenotypic diversity and acquire secondary mutations that affect proliferation, drug sensitivity, and resistance. This diversity likely arises from the progeny of clonal populations and subsets of stem cells. In animal models, it has been shown that the clones themselves can give rise to progeny that can transmit the clonal malignancy after transplantation into secondary recipients, suggesting that stem cells are not required to transmit the malignant phenotype.
New evidence indicates that leukemia stem cells are more quiescent, have higher levels of protective proteins such as efflux pumps for drugs, and have higher levels of DNA repair proteins or antiapoptotic proteins than the more abundant cell making up the circulating population of cells. Tumor cell heterogeneity arises as a consequence of spontaneous mutational events, changes in gene promoter methylation, abnormal expression of transcription factors, lymphoid reactivity, and cytokine responsiveness. For example, a mutation or change in expression that renders a hematopoietic cell clone autonomous or growth factor–independent would be expected to render such cells less susceptible to adverse environmental conditions (e.g., growth factor withdrawal). Similarly, one would also predict that a genetic change facilitating cell cycle entry or disruption of cellular maturation would ultimately lead to overgrowth of affected clones. For obvious reasons, mutations that interfere with drug metabolism or the cell death pathway itself would provide a net survival advantage, particularly under the selection pressure of cytotoxic drug treatment.
Malignant myeloid and lymphoid cells have many reasons to have increased mutational rates. Genomic instability can arise from dysregulation of the cell cycle machinery because of a number of events, including perturbations of cyclins leading to MYC overexpression; AKT (protein kinase B) activation; disruption of replication sequences; loss of DNA repair enzymes such as mismatch repair (MMR) enzymes; loss of proper homologous recombination from a defect in the BRCA (BReast CAncer gene 2)–Fanconi pathways; and loss of ATM (ataxia telangiectasia mutated)/ATR (ataxia telangiectasia and Rad3 related) kinases, which can give rise to chromosomal recombination, loss, and microsatellite instability, and loss of checkpoint regulation. These events can give rise to intraclonal emergent point mutations, translocations, and intragenic losses that might not only result in malignant transformation, but also lead to disruption of genomic stability and selection in favor of proliferative and apoptosis-resistant subclones. Leukemic clonal evolution favors drug resistance.
Common mechanisms may be involved in events associated with malignant transformation and the development of mutations that result in tumor heterogeneity. For example, the cell cycle checkpoint and tumor suppressor gene, TP53 , is induced during DNA damage, leading to G 1 arrest and, if the damage is too severe to repair, cell death by apoptosis occurs. The presumed goal of this process is to eliminate cells that develop deleterious mutations as a result of damage to the genome. Loss of TP53 may not only increase cellular survival by inhibiting the cell death process, but may also promote the transmission of mutations that would otherwise be deleted. In this manner, a defect of the cell death pathway can have multiple consequences, including (1) selection of cells exhibiting a growth advantage over their normal counterparts, (2) development of drug resistance, and (3) promotion of mutations that result in either (1) or (2), as well as neoplastic cell heterogeneity. Age-dependent changes in these processes may explain the more favorable behavior of leukemias and lymphomas in response to chemotherapy in young patients than older patients.
A model of the relationship between tumor growth rate, the occurrence of spontaneous mutations, and the development of drug resistance was first described by Goldie and Coldman and is referred to as the Goldie and Coldman hypothesis. In this model, the size of a tumor depends on a complex interaction between tumor growth rate and cell loss, the latter stemming from the status of the cell death process, exhaustion of available nutrients, and outstripping of the blood supply. As tumors increase in size, the cell death rate tends to increase. The heterogeneous nature of additional mutations makes it likely that multiple mechanisms of resistance will develop as well. From an operational standpoint, this model has clear implications for the rational design of therapeutic strategies and provides a basis for early and intensive combination drug therapy. The successful implementation of this strategy is exemplified by the administration of dose-intensive multidrug regimens (i.e., the BEACOPP [bleomycin, etoposide, Adriamycin, cyclophosphamide, vincristine (Oncovin) procarbazine, and prednisone] regimen in Hodgkin lymphoma, CODOX-M-IVAC [cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate alternating with ifosfamide, etoposide and high-dose cytarabine] in Burkitt lymphoma [non-Hodgkin lymphoma (NHL)]) and combinations of cytotoxic agents with monoclonal antibodies, such as CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine [Oncovin], and prednisone)–rituximab, which are potentially curative when given early in the course of the disease. Other examples include combined use of multitargeted agents such as lenalidomide and bortezomib for myeloma, fludarabine, cyclophosphamide and rituximab for chronic lymphocytic leukemia (CLL), or maneuvers to overcome resistance of pretreated disease outside of cell-based therapies. However, as predicted by the model, administration of these or other intensive regimens in patients with relapsed or late-stage disease generally fails because of a generalized resistance of tumor cells to all classes of chemotherapeutic agents.
The quest for anticancer agents for hematologic malignancies began with the nitrogen mustard class of compounds developed from the chemical warfare agent sulfur mustard gas used in World War I. Since that time, the National Cancer Institute (NCI) and the pharmaceutical industry have developed complex approaches to drug development, screening, and evaluation. Initial screening consisted of toxicity assessment against murine tumor cell lines. Currently, screening is directed toward numerous cell targets, including receptor and downstream signaling kinases; inducers of cell death pathways, including those at the cell surface; mitochondrial enzymes; nuclear DNA and DNA replication, processing and repair proteins, including base excision repair (BER), poly ADP ribose polymerase (PARP), checkpoint kinase 1 (CHK1), topoisomerases, and telomerase; and inhibitors of histone deacetylase (HDAC) and histone methylation, cell cycle proteins, proteasomes, and the mitosis and spindle machinery. Therapeutic agents targeting microenvironment, angiogenesis, and immune checkpoints round out the spectrum of therapeutic approaches. Whereas killing cells had been the backbone of chemotherapeutic approaches to human malignancies, the field of antineoplastic agent development, which in the first decade of the 21st century was at a tipping point, has now embraced novel targeted and highly effective compounds that block the function of specific kinases with surprising efficacy, even at late stages of disease. Ultimately a balanced approach is likely, with much more precision in drug selection, use of mechanism-based combinations, and attention toward and anticipation of tumor heterogeneity, complex subclonality, and emergence of predictable resistance patterns that dictate proactive drug selection and utilization.
Examples include Bruton tyrosine kinase (Btk) and phosphatidylinositol 3-kinase (PI3K) inhibitors in lymphoid malignancies as well as Janus kinase (JAK) inhibitors for treatment of myelofibrosis and polycythemia vera. A number of differentiating agents (including methyltransferase inhibitors altering DNA methylation and gene expression), kinase inhibitors, and immunomodulatory and cytostatic agents complete the antineoplastic armamentarium. The field of drug development also has to contend with much more complex assessments of toxicities due to both on- and off-target effects, as well as normal tissue effects. In the sections that follow these toxicities will be noted because they restrict the utility of many drugs and toxicities need to be monitored when using new drug combinations.
Identification of critical targets for therapy in cancer cells and the microenvironment starts with the recognition of a known or novel target that appears critical for cancer development, dormancy, growth, or metastasis. The NCI Division of Cancer Treatment has a well molecularly characterized cell bank of malignancies that are available for new drug screening. A more contemporary cell line resource is the Cancer Cell Line Encyclopedia (CCLE), developed to assist in drug analysis. It is a compilation of gene expression, chromosomal copy number, DNA sequence, and RNA data from 947 human cancer cell lines collected across databases and repositories. Many cell lines have drug sensitivity profiles from more than 20 anticancer drugs. Both academia and pharma utilize high-throughput gene knockdown (shRNA and siRNA) library approaches, drug screening using large compound libraries, and in silico screening based on protein and drug structures for docking analysis. Repurposed drugs that are FDA approved or for which there are extensive data provide a rich resource for lead compound identification. Medicinal chemistry can then be applied to improve potency and specificity, solubility, kinetic profile, and tissue penetration and residence. For instance, to screen for a kinase inhibitor, cells overexpressing an activated kinase may be used. After an initial in vitro screen, human tumor cell activity is evaluated using a series of athymic mouse xenograft studies targeting tumors from tissues that show promise in in vitro assays. More informative data is generated from banks of patient-derived tumor cell lines, often using Rho-associated kinase (ROCK) inhibition, and using patient-specific tumor xenografts for drug screening. These in vivo models consist of primary malignancies grown in immunodeficient mice, typically NSG (NOD [non-obese diabnetic] scid gamma) mice. Some genetically engineer mice to express needed human cytokines that promote leukemic cell growth, or reconstitute mice with a human immune system to evaluate immunomodulatory drugs and cell-based immune responses. Another model system mimics the human disease by establishing mice with specific chromosomal translocations and oncogene mutations found in human cancers. Drug efficacy endpoints are complex, since target effects may produce cell death, senescence, apoptosis, differentiation, polyploidy, inhibition of metastasis, and loss of cancer stem cell populations. Drugs with promising efficacy and novel mechanisms of action then go on to formulation and toxicology testing, and are ultimately developed for phase I clinical testing through academic centers, industry, or the NCI Cancer Therapy Evaluation Program. Effective drug screening includes evidence of target effect specificity and potency, consideration of effects across tumor types, recognition of predictors of sensitivity and resistance that depend on the mechanism of action and known pathways of resistance, characterization of genetic changes associated with resistance and acquired resistance, and pharmacokinetic preclinical analysis. In all instances, animal studies often fail to provide accurate prediction of therapeutic efficacy, pharmacokinetics, emergence of resistance, or even toxicities.
Variants of patient-derived and specific tumor xenograft (PDX) models, which allow for direct passage of human tumors in mice, include orthotopic models that assess the tissue microenvironment for tumor growth and drug efficacy, metastatic models that allow for removal of primary tumor and metastasis to the brain, lungs, and bone, and direct implantation and metastasis, for instance into colon, spleen, or marrow sites.
New anticancer agents are assessed through a series of clinical trials termed phase I , phase II , and phase III ( Table 58.1 ). The purpose of phase I clinical trials is to establish the safe and optimal biochemically active dose of the compound in question with acceptable toxicity that can be used in disease-targeted phase II testing. During phase I development, pharmacokinetics and pharmacodynamic measures are studied in detail so that appreciable information can be forthcoming from the very first set of patients targeted for treatment and to allow confirmation of these observations in larger phase II disease-focused trials. Dose schedule and route of administration are key considerations in early phase I development. Numerous considerations have guided dose-escalation strategies that accompany phase I trial development. The starting dose is typically 10% of the lethal dose in animals adjusted for species dose equivalency. In classic phase I development, a modified Fibonacci dose schedule is used. Groups of three patients are treated at each of the following doses until the maximum tolerated dose is observed: 1N (N, the starting dose), 2N, 5N, 7N, 9N, 12N, and 16N. Typically, the maximum tolerated dose is defined as the maximum dose not causing irreversible toxicity of any type and causing less than grade 4 toxicity in any organ. Newer agents often have off-target toxicities, and these complicate drug evaluation because the dose-dependent toxicities are replaced by rashes, cardiac effects from prolonged QTc intervals, activation or inhibition of other pathways, and unusual side effect profiles such as pleural and pericardial polyserositis. Typically, if one dose-limiting toxicity is observed, the patient cohort is expanded to six patients, and if two patients develop dose-limiting toxicity, typically defined as grade 4 toxicity except as previously noted, then further entry at this dose is not pursued, and the next lower dose level is used to establish the maximum tolerated dose with a total of six patients accrued at that dose level.
| Phase | Patient number | Objective | Patient population | Dosing | Comments |
|---|---|---|---|---|---|
| 0 |
|
|
Eligible subjects may have different diseases |
|
Phase 0 trials in oncology are infrequent |
| I | 20 to 200 |
|
Eligible subjects from one of several cancers vs. studies restricted to a group of disease or a single disease |
|
Classic and adaptive designs available |
| II | 50 to >100 |
|
Eligible subjects restricted to one or few disease targets |
|
|
| III | 100 to >1000 |
|
Eligible subjects restricted to one or few disease targets |
|
Most frequently are randomized, may be placebo controlled and blinded |
| IV | 100 to >1000 |
|
Eligible subjects restricted to one or few disease targets | Most often dosing at approved range | Usually observational No randomization |
Alternative strategies of drug escalation have included the use of toxicity grades to enhance dose escalation in early drug development, allowing that if no toxicity is observed, fewer patients might be accrued to each dose. Using the modified Fibonacci scheme, one patient is entered at each dose level until grade 2 toxicity is observed, at which point cohorts of three patients are entered at each level. Early in drug development, level skipping may take place if no toxicity is observed. The overall impact of this is to reduce the number of patients treated at suboptimal doses of therapy and to increase the number of patients evaluated at biologically active doses. Other dose escalation strategies include Bayesian optimized interval design studies, which permit variable cohort size and incorporate methods to dose escalation of two drugs in combination.
More importantly, biomarker-driven studies can be used to optimize inhibition of the target rather than treating to toxicity. This can then be extended in phase II to determine whether the drug effects on its biochemical target correspond to efficacy and tolerance.
Phase II drug development uses the dose established in phase I to evaluate therapeutic efficacy. Early phase II studies are conducted typically in a two-stage design, whereby a certain number of responses need to be observed in the first stage to proceed to full trial accrual. Modifications include seamless phase Ib/II designs in specific diseases with dose escalations and efficacy assessments in a disease-specific manner. Phase II combination therapies optimize therapeutic efficacy, often with dose escalation of one or both agents. For instance, topotecan was found in phase I testing to have efficacy against refractory leukemias, leading to combinations of topotecan with ara-C. When a new agent is combined with an established agent, the new agent undergoes dose escalation. In the past, strategies for combination chemotherapeutic agents have included the use of non–cross-resistant agents with nonoverlapping toxicities. Mechanism-based therapeutic combinations have been more successful. In rare diseases, and now with genomic-driven studies, randomized phase II trials in a specific disease comparing standard to novel therapy can be used as evidence for drug approval, a “registration” trial.
Most phase III trials randomize patients between an established standard therapy and a new therapy that has appeared promising in the phase II setting. These studies are usually multi-institutional, and many involve large national (e.g., National Clinical Trials Network) and international cooperative groups. The endpoint of these studies is disease response, time to progression (TTP), survival, and patient tolerance. Phase III trials are often registration trials designed with the FDA and intended to guide drug approval for a disease indication. All trials must have rigorous objective data collection, safety and quality review, and reporting. All clinical trials should be registered at clinicaltrials.gov with public reporting of results.
Malignant hematopoietic cells proliferate more and differentiate less than their normal counterparts. The cell cycle consists of a series of stages through which normal and neoplastic cells proceed during the course of cellular replication (shown schematically in Fig. 58.1 ). The cell cycle is divided into G 1 (pre-DNA synthetic phase), S phase (in which DNA replication takes place), G 2 (post-DNA synthetic phase), and mitosis (M), during which chromosomal division and segregation occur. In addition, nonproliferating, resting cells reside in G 0 , a phase that may theoretically last for an indefinite period. Such cells remain in G 0 until they are induced to cycle (at G 1 ) by specific triggers (e.g., hematopoietic growth factors). The growth fraction of a tumor represents the percentage of cycling cells relative to the total cell population. The generation time represents the time required for a cell to proceed through a single cell cycle (generally 24 to 36 hours for hematopoietic tissues). Surprisingly, in the case of acute myeloid leukemia (AML), the generation time of leukemic blasts is not shorter than that of normal hematopoietic progenitors and may be longer. The proliferative advantage of malignant hematopoietic cells (and of many nonhematopoietic tumors) stems, at least in part, from the fact that a higher percentage of cells are in cycle at any one point in time (i.e., the growth fraction is higher). The doubling time represents the period required for a tumor to double in mass and is, in general, inversely related to the tumor’s growth fraction. Tumor-doubling times range from longer than 120 days in the case of some solid tumors (e.g., lung and colon) to less than 2 weeks (in some leukemias and lymphomas). Tumors with high growth fractions and short doubling times tend to be more sensitive to chemotherapy than slowly growing neoplasms with low growth fractions and long doubling times.
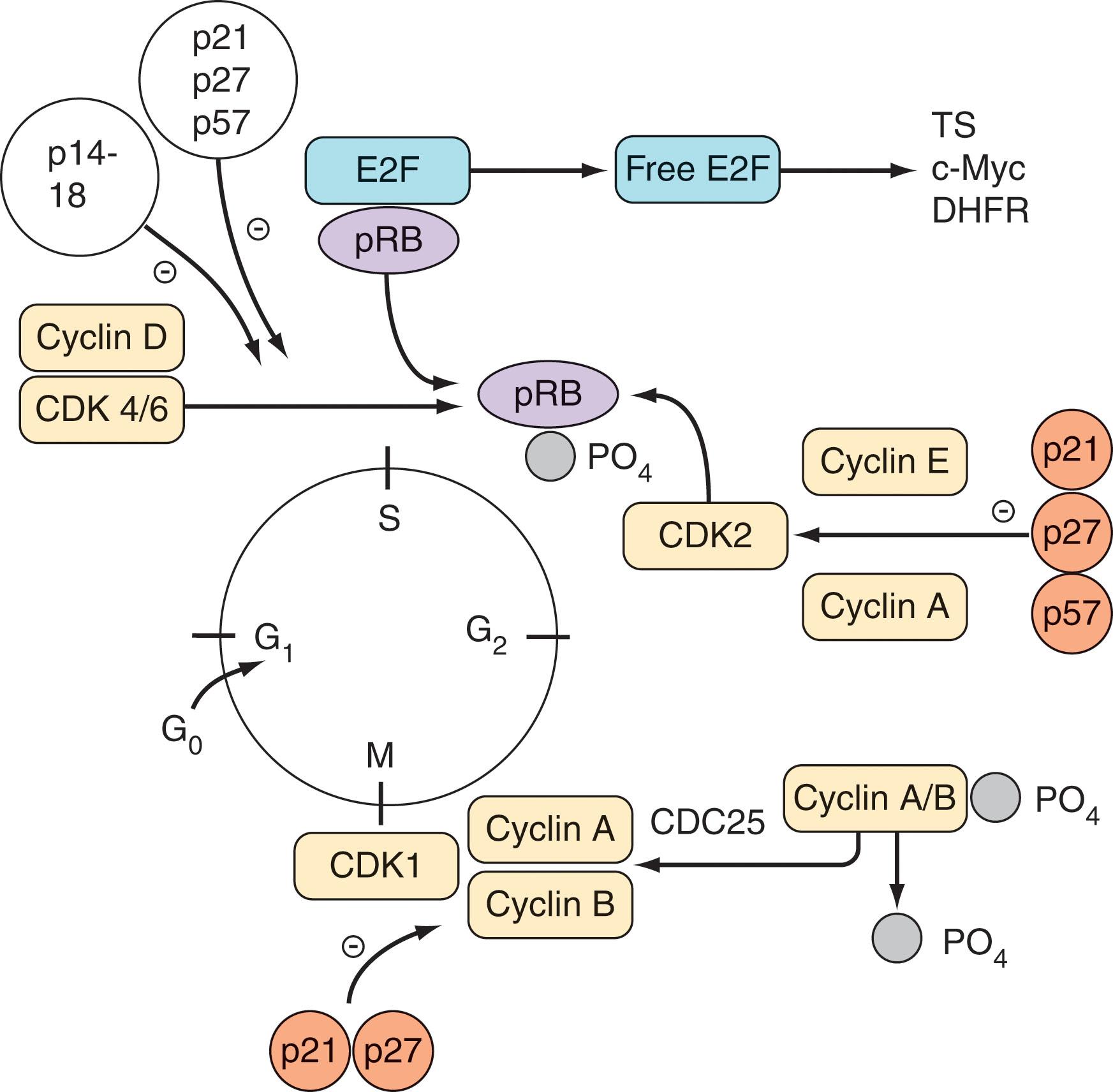
Cell cycle progression is governed by a complex network of proteins consisting of cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors. Progression through S phase is regulated primarily by CDK2 in association with cyclins A and E; progression through G 2 M is regulated by CDK1 (p34cdc2) and cyclins A and B; and progression through G 1 involves CDKs 4 to 6 in conjunction with cyclin D. CDK inhibitors fall into two major categories: the low-molecular-weight inhibitors (pINK14, -15, -16, -17, and -18), which primarily inhibit cyclin D (and to some extent, CDK2) complexes, and the higher molecular-weight inhibitors, p21, p27, and p57, which are more universal in their actions and inhibit most or all CDKs. Signals for the progression of cells through G 1 S are essential for maintenance of the neoplastic phenotype. In the commonly accepted model of G 1 S progression, inactivation of the retinoblastoma protein (pRb) is required. In quiescent cells, pRb is in an active dephosphorylated state and bound to the transcription factor E2F. Phosphorylation of pRb by CDK4, CDK6, and CDK2 leads to release of E2F, which is then free to activate diverse genes essential for S-phase progression, such as MYC (also known as c-Myc), TS (thymidylate synthetase), and DHFR (dihydrofolate reductase). Conversely, induction of CDK inhibitors (e.g., by transforming growth factor-β [TGF-β] or differentiation-inducing agents) results in inactivation of CDK4, CDK6, and CDK2, dephosphorylation of pRb, inactivation of E2F, and inhibition of the progression through S phase. Aberrant expression of cyclins and CDK inhibitors is commonly encountered in hematopoietic malignancies.
In addition to growth control, cell cycle proteins are intimately involved in the regulation of programmed cell death (apoptosis) and checkpoint control mechanisms. Consequently, cell cycle regulatory proteins can exert a major influence on the response of neoplastic cells to cytotoxic agents. For example, when cells undergo DNA damage, they may arrest in G 2 M or G 1 , during which repair occurs, or if the damage is too severe, the cells undergo apoptosis. In particular, the tumor suppressor gene TP53 and its downstream inducible target p21 have been implicated in the G 1 arrest process after genotoxic insult. Dysregulation of various cell cycle regulatory proteins can have a major impact on the sensitivity of neoplastic cells to chemotherapeutic agents. Loss of the TP53 gene renders cells resistant to diverse chemotherapeutic agents, presumably by preventing cells from undergoing repair in G 1 and thereby inhibiting the cell death processes and allowing DNA damage to accumulate, culminating in cellular transformation. Conversely, transfection of P53-negative cells with wild-type P53 restores responsiveness to most drugs. Dysregulation of the CDK inhibitors p21 (a downstream target of P53) and p27 increases the sensitivity of neoplastic cells to various cytotoxic agents, possibly by uncoupling S-phase progression and mitosis. After DNA damage, checkpoints block the cell cycle, but loss of the CDK inhibitors p21 or p27 prevents cells from arresting in G 1 and cells die during G 2 M. Mutations in the E2F protein have been shown to lengthen S phase and increase the sensitivity of malignant cells to S-phase–specific agents. Furthermore, cells lacking functional pRb have been shown to be significantly less sensitive to the actions of antimetabolites, including methotrexate.
In vivo the growth of tumors is limited by various factors such as vascular supply, nutritional requirements, and possibly physical restraints. Consequently, the rate of tumor growth declines as the number of cells increases. To the extent that tumor-doubling times are inversely correlated with drug responsiveness, large, late-stage tumors are less susceptible to cytotoxic drugs than early-stage tumors, with higher growth fractions. Most chemotherapeutic drugs kill by first-order kinetics. The implication of this phenomenon is that it requires the same drug dose to reduce the number of tumor cells from 104 to 101 cells as it does to reduce the tumor burden from 1010 to 107 cells.
Hematopoietic malignancy-initiating or stem cells, such as leukemia stem cells, appear to explain resistance and treatment failure. These cells express high levels of hematopoietic stem cell proteins and markers and are resistant to cell cycle–specific agents because of an increase in quiescent cell populations. They overlap with normal hematopoietic stem cells (HSCs) and there appears to be a set of HSCs that contain preleukemic-promoting mutations that both predispose and may be sufficient for conversion to leukemic stem cells. The proteins expressed by these cells have become targets of therapy, including alterations in DNA damage, quiescent cell cycle factors such as the thrombopoietin receptor, MPL, stem cell proliferation signals such as NOTCH and WNT protein families, and niche occupancy proteins such as KIT.
Cytotoxic agents may be divided into several categories with respect to their effects on the cell cycle or the cell cycle specificity of their actions, or both.
Noncycle-active drugs kill both cycling and noncycling cells in all phases of the cell cycle. Examples include steroids and antitumor antibiotics (except bleomycin).
Cycle-active, nonphase-specific drugs are more active against cycling cells and can kill cells in each phase of the cell cycle. However, such drugs may preferentially kill cells in a particular phase of the cell cycle. Examples include alkylating agents, cisplatin, and 5-fluorouracil (5-FU).
Cycle-active, phase-specific drugs primarily kill cells in a specific phase of the cell cycle. Examples include most antimetabolites, which are active against cells engaged in DNA synthesis (S-phase cells), and microtubule-active drugs (e.g., vinca alkaloids, taxanes), which kill cells in G 2 M.
An example of a cytokinetically rational approach to chemotherapy involves the combination of a noncycle-active agent (e.g., daunorubicin) with a cycle- and a phase-specific agent (e.g., ara-C, fludarabine, decitabine, gemcitabine, clofarabine, and nelarabine). From a theoretical standpoint, administration of a noncycle-active agent may reduce tumor mass, leading in turn to an increase in the growth fraction caused by recruitment of cells into cycle. Such cells would then be more susceptible to a cycle- and phase-specific agent, particularly one administered over a prolonged interval. In the case of hematopoietic malignancies, attempts have been made to recruit neoplastic cells into the more susceptible S phase of the cell cycle through the use of hematopoietic growth factors. The success of such a strategy has been limited because of several factors, including the inability of growth factors to increase the S-phase fraction significantly, the lack of selectivity of this strategy, and the theoretical possibility that growth factors may protect neoplastic cells from apoptosis.
Unfortunately, cytokinetic differences between normal and neoplastic tissues have been difficult to exploit. Both normal hematopoietic stem cells and hematologic malignant stem cells have a low proportion of cells in G 1 . However, prolonged dosage schedules can provoke these malignant cells into cell cycle and may explain their efficacy. Consequently, rapidly dividing normal tissues such as gastrointestinal epithelium and normal hematopoietic progenitors tend to be very sensitive to most chemotherapeutic agents. As a result, mucositis and myelosuppression represent frequent dose-limiting toxicities for many cytotoxic drugs.
Newer agents that target cell cycle proteins are often first utilized in patients with hematologic malignancies. As noted in the section on CDK and CHK1 inhibitors, these agents can have potent cytotoxic effects on dividing cells.
Traditional agents are classified by their site of action, and as such all have a targeted mechanism, albeit not that of newer kinase targeted agents. They are divided into alkylating agents, antimicrotubule agents, antimetabolites, topoisomerase I or II inhibitors, platinum analogs, and miscellaneous agents. The pharmacology and cellular mechanisms of action of these agents are schematically presented in Fig. 58.2 .
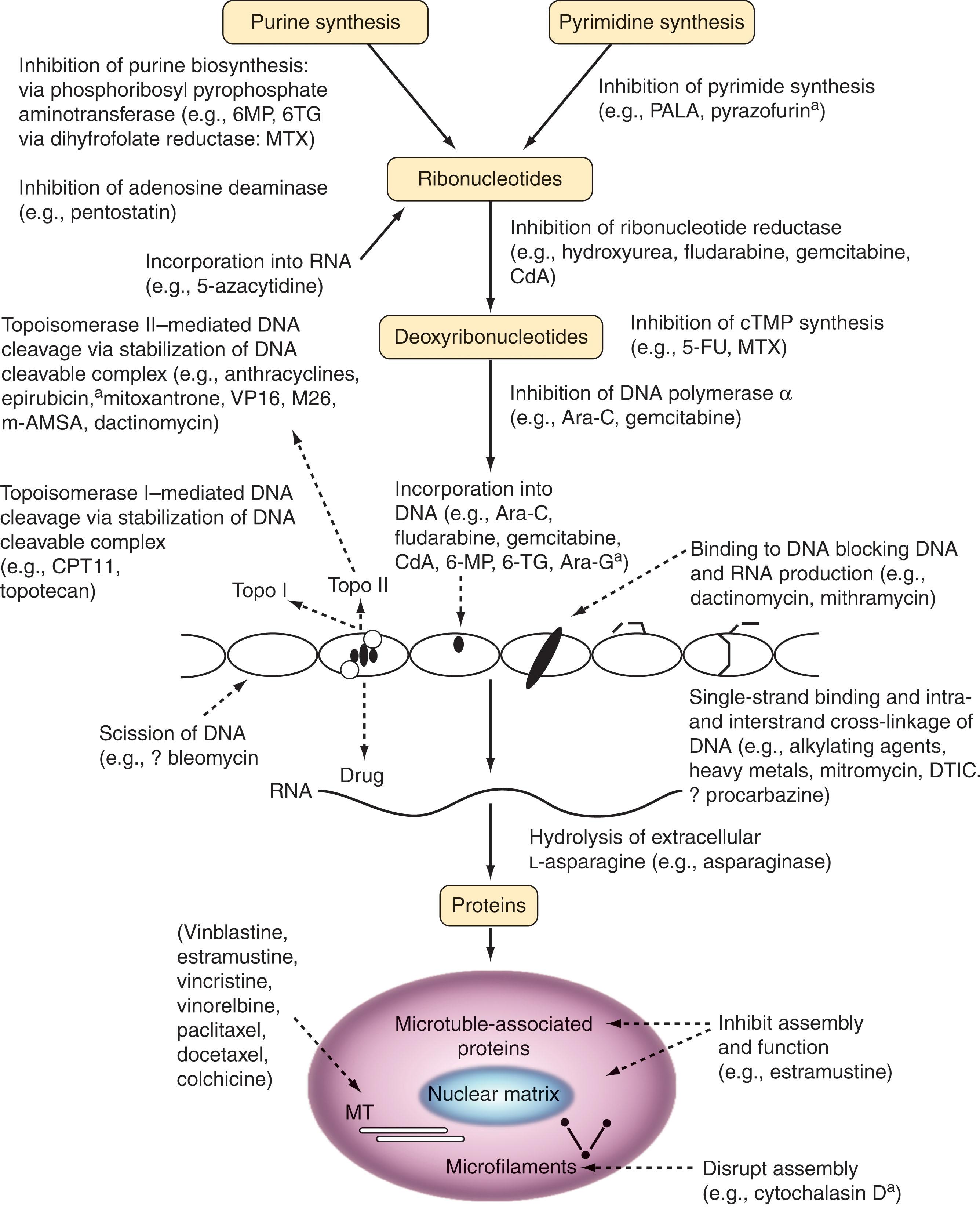
Drug treatment for cancer began with the use of the mustard class of alkylating agents, initially mechlorethamine (nitrogen mustard), which entered into clinical use in the mid-1940s. Alkylating agents are used in many regimens but are rapidly being supplanted by newer classes of agents.
All alkylating agents undergo molecular rearrangements to form covalent bonds to DNA bases. Some form monoadducts, while others form cross-links either with intrastrand or interstrand bases, or with adjacent proteins. All alkylating agents are cytotoxic to tumor cells through interruption of DNA replication, induction of DNA damage repair and stress response, or through checkpoints in the cell cycle that result in apoptosis, senescence, or necrosis. All alkylating agents induce DNA strand breaks directly or through the damage response. Alkylating agents can also cause DNA mutations that can induce a cytotoxic response or can alter proteins, leading to immune responses to novel protein sequences.
Efficient removal of the DNA adducts reduces the lesion burden, while loss of DNA damage recognition can invoke damage tolerance, as in the case of loss of MMR and temozolomide tolerance. Detoxifying enzymes (glucuronidating enzymes and metabolizing enzymes residing in the liver) can serve as acceptor molecules for alkylation, alter the agent prior to DNA attack, or metabolize the parent compound. Loss of TP53 results in loss of cell cycle checkpoint induction of DNA repair signals. Increased AKT signaling promotes cell proliferation to compensate for the DNA damage response-associated toxicity.
The nitrogen mustard class includes mechlorethamine, cyclophosphamide, 4-hydroperoxy cyclophosphamide, ifosfamide, chlorambucil, and melphalan. These drugs all share a common bischloroethyl group attached to nitrogen and a substituted “R” group that provides drug specificity ( Fig. 58.3 ). All nitrogen mustards react with DNA in an SN2 reaction, a bimolecular nucleophilic displacement reaction also called a second-order reaction. Although numerous sites are targeted for alkylation, nucleophiles in DNA, including nitrogen (N), oxygen (O), and phosphate (P), attract the chloroethyl moiety attached to the R–N backbone, and the chlorine is displaced by the nucleophilic atom to form an aziridinium moiety. The remaining chloroethyl group is then attracted to a second nucleophilic atom, forming a second aziridinium intermediate, leading to a second alkylation, forming a cross-link. Both intrastrand and interstrand cross-links are formed. The N7 guanine position is the most critical for cytotoxic cross-link formation. Clinical use of mechlorethamine is limited because the MOPP regimen (mechlorethamine, vincristine [Oncovin], procarbazine, prednisone) has been replaced by ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, dacarbazine [DTIC]) in Hodgkin lymphoma (see Chapter 78, Chapter 80 ). Local use of mechlorethamine occurs in a dermatologic suspension for the treatment of cutaneous T-cell lymphomas (CTCLs; see Chapter 89 ).
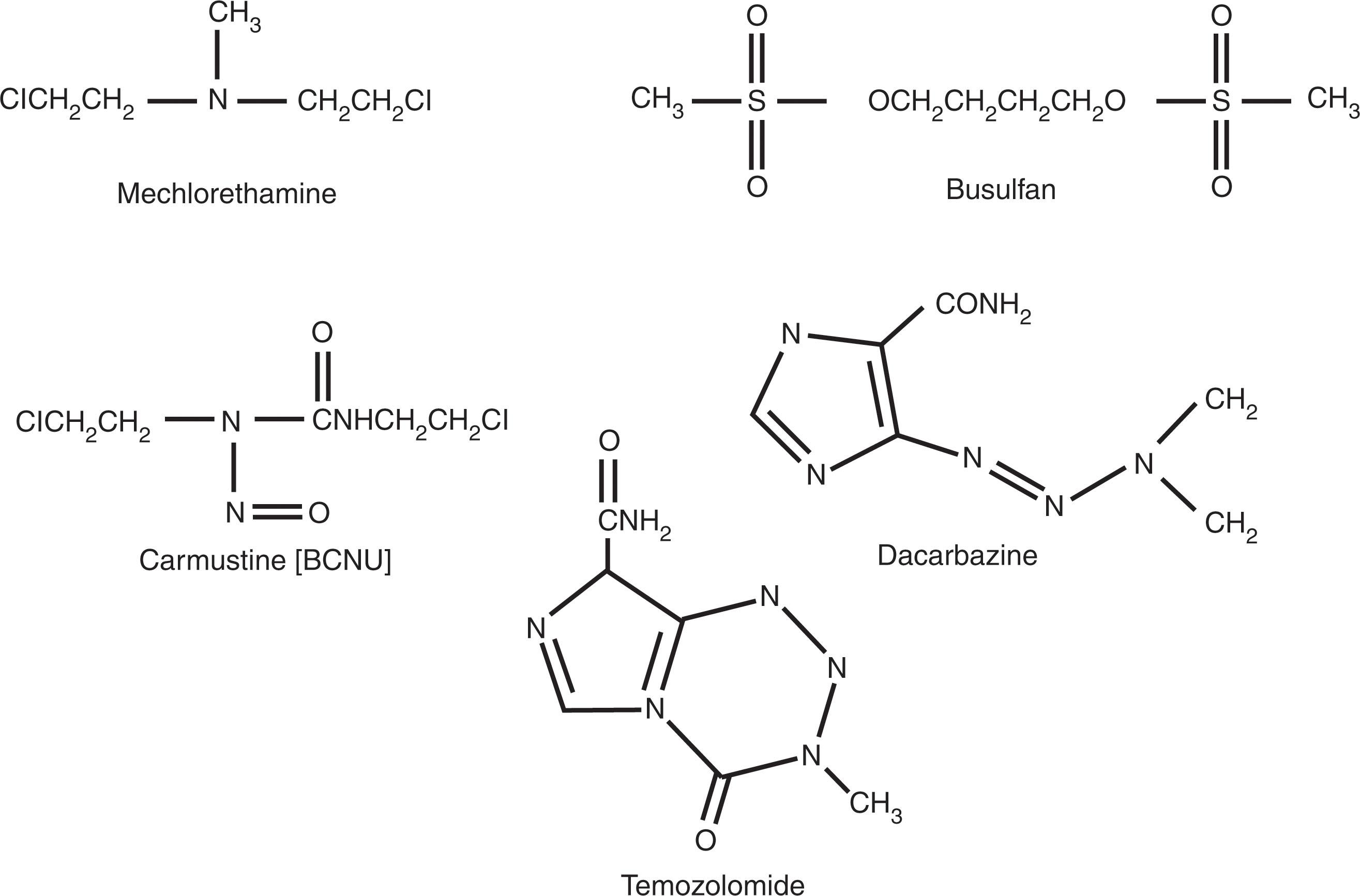
Cyclophosphamide is a nitrogen mustard with a ringed structure off the end-chloroethyl backbone that decreases spontaneous decomposition (see Fig. 58.3 ). Enzymatic activation is required through multifunction P450 enzymes in the liver. Phenobarbital and corticosteroids may alter activation. The bioavailability of cyclophosphamide orally and intravenously is quite similar, although most use of the drug is by bolus IV injection. Cyclophosphamide is detoxified through oxidation to 4-keto-cyclophosphamide and carboxyphosphamide by aldehyde dehydrogenase. Cyclophosphamide is used in doses as small as 50 to 100 mg/day orally and in bolus doses of 400 to 700 mg/m 2 for solid tumors and 750 mg/m 2 in combination with doxorubicin and vincristine and prednisone as part of the CHOP regimen for NHLs, or alone or with bortezomib for patients with myeloma. It is also used at doses of up to 60 mg/kg/day for 4 days in autologous and allogeneic bone marrow (BM) transplantation protocols. Cyclophosphamide is used in numerous treatment protocols for NHLs and high-dose therapy regimens designed to eradicate tumor and BM in patients with lymphomas and leukemias and those undergoing BM transplantation. It is commonly used with granulocyte colony-stimulating factor (G-CSF) for mobilizing hematopoietic stem cells to be collected before autologous transplantation.
Cyclophosphamide is metabolically activated by cytochrome P450 mixed function oxidases in the liver to 4-hydroxycyclophosphamide. 4-Hydroxycyclophosphamide is further converted to aldophosphamide and then to phosphoramide mustard, the alkylated species, and acrolein. High levels of aldehyde dehydrogenase detoxify cyclophosphamide in hematopoietic stem cells and, thus, high doses are not marrow ablative. Acrolein is a highly reactive aldehyde and the cause of hemorrhagic cystitis. Mercaptoethane sulfonate (Mesna) is used to provide prophylaxis against hemorrhagic cystitis caused by cyclophosphamide and ifosfamide, and is now standard for doses of cyclophosphamide and ifosfamide above 1000 mg/m 2 . Mesna is given in divided doses every 4 hours or as a continuous infusion for 18 to 24 hours in a dose equivalent to either cyclophosphamide or ifosfamide. Other than hemorrhagic cystitis, BM suppression is dose limiting and can be rescued by reinfusion of autologous or allogeneic hematopoietic progenitor cells. Other toxicities include alopecia and cardiac toxicity, which is unusual and most often seen after high-dose therapy.
4-Hydroperoxycyclophosphamide, a chemically stable form of the reactive intermediate of cyclophosphamide, 4-hydroxycyclophosphamide, is more toxic to committed hematopoietic progenitors such as colony-forming unit–granulocyte/macrophages (CFU-GM), burst-forming unit–erythroids (BFU-E), and colony-forming unit–erythroids (CFU-E).
Melphalan, or phenylalanine mustard, has an amino acid side chain that alters its cellular uptake and stabilizes its structure, allowing PO administration (see Fig. 58.3 ). It is available in both PO and IV forms, and has a similar effect on DNA cross-linking as cyclophosphamide and the other nitrogen mustards. Melphalan uptake by cells is by means of a neutral amino acid transporter. Its rate of cross-link formation is much slower than that of mechlorethamine, presumably because of delayed metabolism. Oral melphalan is used predominantly for the standard treatment of multiple myeloma (MM) and IV in high-dose regimens in preparation for stem cell transplantation (see Chapter 91 ) for MM patients.
Chlorambucil has been used for more than 40 years for the treatment of CLL. Chlorambucil is the phenylbutyric acid derivative of nitrogen mustard and is very stable, entering the cell by diffusion rather than by a specific uptake mechanism. It is typically administered orally on a daily basis or intermittently. It appears to have greater bioavailability than melphalan and a more consistent half-life of approximately 2 hours.
Busulfan is an alkylsulfonate unique among alkylating agents because of two sulfur groups and lack of a chloroethyl moiety (see Fig. 58.3 ). Busulfan, similar to the nitrogen mustards, reacts predominantly at the N 7 position of guanine and produces an N 7 –N 7 biguanyl DNA cross-link, although the precise nature of this cross-link appears different than that of the nitrogen mustards. The pharmacokinetics of busulfan is important for its use in high-dose therapy for ablation of the BM in patients undergoing autologous transplantation for acute leukemia or allogeneic stem cell transplantation (see Chapter 104, Chapter 105, Chapter 106, Chapter 107 ). Because the incidence of veno-occlusive disease is lower in patients receiving high-dose busulfan with predosing pharmacokinetics performed, this is now recommended in high-dose regimens, the target being an area under the curve (AUC) of 1125 μmol/L × min (range 900 to 1350) with every 6-hour dosing. Busulfan is a potent stem cell toxin, killing both early and late hematopoietic progenitor cells and damaging the BM stroma. Other toxicities of busulfan include nausea and vomiting, and pulmonary interstitial and intra-alveolar edema leading to fibrosis. The pulmonary fibrosis is distinct from the interstitial pneumonitis, which accompanies allogeneic stem cell transplantation and is not related to cytomegalovirus (CMV) or other viral infections.
Four chloroethyl nitrosoureas and one methyl nitrosourea are in clinical use. These agents are different from the nitrogen mustards in that they alkylate through an SN 1 reaction, forming a highly reactive intermediate in the presence of N, O, and P nucleophiles in DNA. The commonly used clinical agent is (2-chloroethyl)- N -nitrosourea (BCNU). N -([4-amino-2-methyl-5-pyrimidinyl] methyl)- N -(2-chloroethyl)- N -nitrosourea (ACNU) is commonly used in Japan. A third agent, N -(2-chloroethyl)- N -cyclohexyl- N -nitrosourea (CCNU), is used predominantly as an PO nitrosourea in children with brain tumors. All of these compounds have high hydrophobicity, actively penetrating the blood–brain barrier.
The DNA alkylation sites include N 7 and O 6 of guanine. Chloroethylation at the O 6 position of guanine appears critical to cytotoxicity. DNA cross-linking by chloroethyl nitrosoureas include at 1-(3-cytosinyl), 2-(1-guanyl) ethane, and 1-2-bis(7-guanyl) ethane. The former is responsible for much of the cytotoxicity observed with the chloroethyl nitrosoureas. It is formed after alkylation at the O 6 position of guanine. This adduct undergoes intramolecular rearrangement to a circular intermediate, N 1 . O 6 ethanoguanine is formed, which can then rearrange by attack at the opposite hydrogen-bonded base N 3 of cystine, forming the interstrand cross-link. This is a unique DNA cross-link and is poorly recognized by DNA repair processes, leading to marked cytotoxic potency of this cross-link.
The pharmacokinetics of the chloroethyl nitrosoureas reveal a very short half-life. BCNU is predominantly used for high-dose treatment of recurrent lymphomas. Regimens including BCNU induce sustained complete remission (CR) rates of approximately 40% to 60%. Doses of between 300 and 600 mg/m 2 have been safely administered. The chloroethyl nitrosoureas cause profound and cumulative BM suppression at conventional doses of 120 to 150 mg/m 2 , limiting treatment to three to five cycles at 6-week intervals.
Complications of high-dose BCNU therapy include pulmonary toxicity and renal toxicity at doses higher than 600 mg/m 2 . Pulmonary toxicity, as evidenced by a decrease in the DL CO (diffusing capacity of the lung for carbon monoxide), occurs in up to 40% of patients. It can be managed with high-dose oral steroids during the inflammatory phase. Interstitial nephritis with glomerulosclerosis, interstitial fibrosis, and dropout of tubules has been reported with BCNU or CCNU.
Four methylating alkylating agents are in clinical use. These include procarbazine, DTIC, streptozotocin, and temozolomide. Procarbazine and DTIC are triazines. Streptozotocin is a monofunctional methyl nitrosourea derivative with an attached sugar moiety, and temozolomide is an imidazotetrazine. All react with DNA by undergoing SN 1 reactions forming a methyldiazonium ion, resulting in methylation of N 7 guanine (67%), O 6 guanine (9%), O 4 and O 2 thymine (2%), and N 3 adenine (3%). None form DNA cross-links. However, all induce high levels of DNA methylation, and their recognition and repair results in both single- and double-strand breaks. N 7 methylguanine is removed through the BER system. Recognition by the N -methylpurine glycosylase results in removal of the adducted base with formation of an abasic site that is recognized by the apurinic (AP) endonuclease, which then cleaves the backbone at the AP site. Subsequently, the free 5′ sugar is released by DNA lyase, with repair initiated by β-polymerase and DNA ligase. BER effectively removes N 7 methylguanine and N 3 methyladenine, and restores DNA to normal. Inhibition of BER by the investigational agent methoxyamine (TRC102) blocks this pathway and increases toxicity.
O 6 methylguanine mispairs with thymine during DNA synthesis, resulting in a lesion recognized by the MMR system. Mispair recognition proteins are MSH6, MSH3, and MSH2. Recognition of the mispair recruits additional proteins to the complex, including MLH1 and PMS1/PMS2. These proteins initiate exonuclease cleavage of a long patch in the newly synthesized strand of DNA. This is then repaired by polymerases-δ and -ε. If unrepaired, a thymine is repeatedly inserted opposite the O 6 methylguanine, resulting in multiple single-strand breaks. Cells expressing high levels of the DNA repair protein for O 6 methylguanine, O 6 methylguanine-DNA methyltransferase (MGMT), are approximately 10-fold more resistant to methylating agents than MGMT-negative cells. Cells lacking MMR are very resistant to methylating agents. Acquisition of MMR defects is associated with acquired resistance to methylating agents and cisplatin, which is also recognized by this protein complex.
Procarbazine was synthesized as a monoamine oxidase inhibitor and has been used since the 1950s for the treatment of Hodgkin lymphoma and NHL, as well as a component of combination therapies for gliomas. DTIC is metabolically activated by cytochrome P450 microsomal oxidoreductases, ultimately leading to formation of the methyldiazonium ion and DNA methylation. DTIC is used in combination with ABVD for treating Hodgkin lymphoma (see Chapter 79, Chapter 80 ) and is also used for patients with metastatic malignant melanoma in combination with BCNU, cisplatin, and tamoxifen. Activation of DTIC requires hydroxylation of one terminal methyl group caused by demethylation forming 5-[3-methyl-triazen-1-yl]-imidazole-4-carboxamide (MTIC), with spontaneous decomposition to the methyldiazonium ion, which alkylates the DNA, as noted earlier. Maximum tolerated doses of DTIC are approximately 1000 mg/m 2 , with myelosuppression and gastrointestinal toxicity (including severe watery diarrhea) being the most common side effects.
Temozolomide represents an imidazotetrazinone. It differs from DTIC in that it is chemically degraded to the monomethyl triazine, MTIC, at neutral pH and does not require P450 enzymatic demethylation. Compared with DTIC, temozolomide has much more consistent pharmacokinetic parameters, including peak serum concentrations, volume of distribution and clearance, and conversion to MTIC. Clinical studies documented considerable activity in acute leukemias. The dose-limiting toxicity was thrombocytopenia and, less frequently, neutropenia, with maximum tolerated doses of 1000 mg/m 2 given over 5 days on a daily or twice-daily regimen. Nausea and vomiting were the other common side effects, easily controlled with antiemetics.
Bendamustine is comprised of a 2-chloroethylamine nitrogen mustard alkylating group, a benzimidazole ring, and a butyric acid side chain. Its mechanism of action is unknown but appears to be different than other alkylating agents, causing DNA damage that is repaired predominantly by the base-excision repair system and has activity against lymphoid cell lines resistant to alkylating agents. Myelosuppression with leukopenia and thrombocytopenia is the most frequent side effect, with mild nonhematologic side effects including nausea, fatigue, constipation, and diarrhea. Phase III studies comparing standard treatment with bendamustine and rituximab in front-line treatment of CLL and indolent lymphomas (see Chapter 76 ) have established this combination as a first-line treatment. Bendamustine is also active against MM, and combinations with steroids and bortezomib or the immunomodulatory agents thalidomide and lenalidomide have been reported to result in high response rates in patients with relapsed or refractory disease.
Alkylating agents induce dose-limiting myelosuppression and cause sublethal DNA damage to hematopoietic progenitors, causing mutational events that lead to malignant transformation to preleukemic and leukemic states. A concern exists about the use of hematopoietic growth factors after exposure to alkylating agents (see Chapter 59 ). There is evidence of increased cytotoxicity to hematopoietic progenitors during simultaneous exposure to these agents and growth factors. Treatment-related AML (t-AML) accounts for approximately 15% of all adult AML. Approximately 50% of t-AML patients have a preleukemic phase compared with only 10% of patients with de novo AML. CRs are achieved in 15% to 30% of patients with t-AML and a mean remission duration of 2 months. Chromosomal abnormalities and gene mutations characteristic of t-AML establish this as a distinct disease requiring novel therapeutic approaches. Loss or deletion of all or part of the long arm [q] of chromosomes 5 or 7 is common, as are trisomy 8 and deletions of the short arm of chromosomes 12, 17, and 21.
Historically, patients with Hodgkin lymphoma treated with mechlorethamine and procarbazine in the MOPP regimen or with CCNU were at the highest risk if exposed to radiation as well as an alkylating agent combination. Patients with polycythemia vera treated with chlorambucil were at much higher risk than patients treated with phlebotomy alone, which can contribute to a shift in treatment strategy. Patients with myeloma and ovarian cancer have developed t-AML, especially after prolonged exposure to alkylating agents. Patients treated with alkylating agents for benign diseases such as nephritis, lupus, psoriasis, rheumatoid arthritis, and Wegener granulomatosis also have an increased risk of t-AML. The mean latency between exposure and t-AML from alkylating agents is 4 to 5 years, in contrast to t-AML from etoposide, which has a latency period as short as 1 year. The cumulative risk of developing t-AML is between 10% and 17% at 4 to 6 years for myeloma patients treated with melphalan and between 2% and 10% at 7 to 10 years in patients with Hodgkin lymphoma. Alkylating agent-associated t-AML has also been recognized in patients with breast and colon cancer.
The antimicrotubule drugs include the vinca alkaloids (e.g., vincristine, vinblastine, vinorelbine and vindesine), taxanes (e.g., paclitaxel and docetaxel), and epothilones (ixabepilone).
The vinca alkaloids are naturally occurring (vincristine and vinblastine) or semisynthetic (vinorelbine) nitrogenous bases derived from the pink periwinkle plant, Catharanthus roseus . Paclitaxel was originally isolated from the bark of the Pacific yew, Taxus brevifolia . Paclitaxel can also be isolated from other members of the Taxus genus and from a fungal endophyte that grows on the Pacific yew. Docetaxel is derived semisynthetically from 10-deacetyl-baccatin III, which is obtained from the needles of the European yew, Taxus baccata .
The vinca alkaloids bind to the protein tubulin at a site distinct from that of the taxanes and, at low concentrations, inhibit microtubule dynamics. At higher concentrations, these vinca alkaloids disrupt microtubules and mitotic spindle, resulting in cell cycle mitotic arrest and apoptosis of cells (see Fig. 58.3 ). In contrast, after binding to α-tubulin, taxanes kinetically stabilize microtubule dynamics at their plus ends and shift the equilibrium toward tubulin polymerization into microtubule bundles. This also causes mitotic arrest and apoptosis of cells. The mitotic arrest caused by antimicrotubule drugs is associated with phosphorylation of the B-cell lymphoma (BCL2) protein and increased intracellular levels of the BCL2-associated X protein (Bax), which promote apoptosis.
As natural products, overexpression of the efflux pump multidrug resistance gene-1 (MDR-1) and the ABCB-1 transporter mediate resistance. Intracellular resistance to mitotic spindle and microtubule formation is mediated by numerous pathways and proliferative signals including MYC, nuclear factor kappa-B (NFκB), and AKT.
Antimicrotubule agents, particularly the vinca alkaloids, are used in the management of lymphomas and leukemias, and continue to be used in the mainstay of clinical chemotherapeutic regimens. Because of their mechanism of action, they are best used in multiagent combinations, in which potentiation of efficacy with other classes of agents, such as antimetabolites and DNA-damaging agents, provide better therapeutic responses and well-tolerated treatments.
Within this class are two important agents used for hematologic malignancies, hydroxyurea and methotrexate. They both serve to disrupt nucleotide synthesis, and slow or stop DNA and RNA synthesis.
Hydroxyurea inhibits ribonucleotide diphosphate reductase, blocking de novo synthesis of purines and pyrimidines. S-phase arrest is commonly observed. Given this exquisite cell cycle specificity, resting and quiescent cells are rarely affected and there is virtually no hematopoietic stem cell toxicity. As a consequence of disruption of nucleotide synthesis and direct binding to telomere binding factors, telomere synthesis and function are compromised in leukemic cells. In cells arrested in S phase, apoptosis and senescence are observed.
Hydroxyurea is used in the treatment of myeloproliferative neoplasms including essential thrombocythemia, polycythemia vera, and myelofibrosis (see Chapter 69, Chapter 70, Chapter 71, Chapter 72 ). It may also be used for acute cytoreduction prior to induction therapy of AML and for management of chronic myelomonocytic leukemia. It is commonly used for extended periods of time to induce fetal hemoglobin and reduce sickle crisis in patients with sickle cell anemia (see Chapter 42, Chapter 43 ).
Folic acid analogs block the formation of thymidine by inhibition of thymidylate synthase, resulting in accumulation of uridine-5’-monophosphate (UMP), leading to high levels of uridine-5’-triphosphate (UTP) which is incorporated into DNA and causes purine nucleotide pool imbalance, slowing DNA synthesis. These agents bind thymidylate synthase and DHFR and disrupt cell cycle progression and cell division. Methotrexate polyglutamation results in higher affinity binding to DHFR and improved inhibition. Aminopterin, the first antifolate, was used by Sidney Farber (reported in 1948) and became one the first chemotherapeutic agents to be administered with success to children with acute lymphocytic leukemia (ALL). Methotrexate is the agent currently used in hematologic diseases, while the family of agents includes pemetrexed and trimetrexate. Inhibition of folate-dependent methyl transfer enzymes disrupts purine synthesis pathways. Toxicity of methotrexate is reversed by N 5 -formyl FH 4 , or leucovorin, which serves as a direct folate coenzyme. Since it has no tumor selectivity, normal tissues in active cell cycle are affected, including mucosa, BM, and hair follicles, resulting in mucositis, pancytopenia, and alopecia. Prolonged use is associated with pulmonary and liver fibrosis. High-dose therapy and excessive periods of high blood levels result in interstitial nephritis, sometimes requiring dialysis.
The most common resistance is due to amplification of DHFR expression by upregulation of translation and gene amplification, including the establishment of minichromosomes with the DHFR gene. A second mechanism is decreased affinity of DHFR to methotrexate. A third mechanism is decreased thymidylate synthase, and the fourth mechanism of resistance is impaired methotrexate polyglutamate formation.
While use has diminished in recent years, methotrexate remains part of the regimen for maintenance in children and adults with ALL (see Chapter 66 ). It is also used to depress T-lymphocyte proliferation after allogeneic stem cell transplantation to prevent acute graft-versus-host disease (GVHD). High-dose methotrexate, with careful blood level monitoring to prevent acute renal toxicity and mucositis, and leucovorin rescue, is effective in the management of CNS leukemias, primary CNS lymphoma, and highly proliferative fraction (c-myc- positive and high KI67) intermediate- and high-grade lymphomas, including Burkitt.
The nucleoside analogs exhibit structural similarities to naturally occurring nucleosides and are incorporated into either DNA or RNA with lethal consequences. Alternatively, they block key enzymes in de novo purine or pyrimidine biosynthesis. There are two broad categories:
Pyrimidine analogs (e.g., ara-C, 5-azacytidine, gemcitabine)
Purine analogs (e.g., 6-thioguanine [6-TG], 6-mercaptopurine, fludarabine, chlorodeoxyadenosine, deoxycoformycin, clofarabine, nelarabine)
These categories are not mutually exclusive; for example, some nucleoside analogs (e.g., ara-C and 6-TG) also inhibit enzymes involved in DNA or deoxyribonucleotide biosynthesis. These agents are predominantly cycle-active agents and in most cases are phase- specific, being primarily active against cells in S phase. Because the growth fraction of hematologic malignancies tends to be higher than that of nonhematologic malignancies, nucleoside analogs are particularly useful in the former disorders. In contrast to alkylating agents, nucleoside analogs have limited carcinogenic and leukemogenic potential. The fluorinated pyrimidines (e.g., 5-FU) are generally not used in treating hematologic disorders and are not discussed further.
Ara-C has been used in the treatment of acute leukemias—particularly acute nonlymphocytic leukemias, aggressive lymphomas including Burkitt, and CNS lymphomas for over 30 years. It is incorporated into DNA during replication and inhibits polymerase-α, is cell cycle specific, and penetrates the blood–brain barrier. In rapidly replicating cells, it stalls polymerase function, leading to replication fork collapse and strand breaks at the replication fork, signaling apoptosis and differentiation.
Although ara-C remains the backbone of therapy for aggressive hematologic malignancies, resistance has been observed that is both specific to the drug and nonspecific. Specific resistance could be due to nucleotide transport down regulation, uncommon in dividing cells. Inside the cell, ara-C is phosphorylated by deoxycytidine kinase to the active 5′-triphosphate derivative ara-cytidine triphosphate (CTP), whereas catabolism of ara-C by cytidine deaminase (CDD) to the nontoxic metabolite arabinoside uridine is a major pathway of detoxification that can be overcome by high-dose therapy. Low penetration into the cerebrospinal fluid is overcome by bolus administration of very high doses: 1 to 2 g/m 2 over 1 hour. Other resistance mechanisms appear nonspecific and result in leukemia tolerance to cell cycle arrest and induction of apoptosis, tolerance to DNA strand breaks, and rapid cell division after therapy.
Standard induction therapy for AML includes a 5- to 7-day continuous infusion of 100 to 200 mg/m 2 or high-dose ara-C at doses of 1 to 3 g/m 2 over 1 hour every 12 hours. Many modifications to these schedules have improved tolerance without sacrificing efficacy. For CNS leukemia, a depo form of ara-C has been developed with improved tolerance and sustained cerebrospinal fluid levels.
Subcutaneous (SC) administration has provided responses in older individuals with AML or myelodysplastic syndromes (MDS) who do not respond well to more intensive induction therapy.
These agents were initially developed as antimetabolites but are more effective at low doses that inhibit the function of histone demethylation and are described below.
Developed as an agent for the treatment of pancreatic cancer, gemcitabine has gained therapeutic attention for use in patients with Hodgkin lymphoma and NHL, particularly in the relapsed state.
Like other nucleotide analogs, gemcitabine is a prodrug that is phosphorylated by deoxycytidine kinase to gemcitabine diphosphate (FdCDP) and gemcitabine triphosphate (dFdCTP), which, when incorporated into DNA, stalls the polymerase-α, which adds one more deoxynucleotide to the elongating strand. The polymerase replication complex then falls off the DNA, causing replication fork collapse and chain termination. In addition, FdCDP is a potent inhibitor of ribonucleoside reductase, causing depletion of deoxyribonucleotide pools and further encouraging dFdCTP incorporation while disrupting DNA synthesis.
Specific resistance is caused by upregulation of deoxycytidine deaminase, which metabolizes gemcitabine to 2,2′-difluorodeoxyuridine. Nonspecific resistance emerges due to upregulation of membrane transporters, although their role in clinical resistance is less clear.
Numerous studies have identified the utility of adding gemcitabine to cisplatin and other agents for the treatment of both relapsed Hodgkin lymphoma and NHL, and CTCLs. The combination of oxaliplatinum or cisplatin and gemcitabine is effective and well tolerated, and the majority of patients remain eligible for autologous stem cell collection and transplantation.
Fludarabine was introduced 25 years ago as an agent with potent activity against lymphoid malignancies and remains an active agent in CLL and other low-grade lymphomas. It has since emerged as an agent in combination that is effective in leukemias, and in induction therapy for nonmyeloablative conditioning prior to allogeneic stem cell transplantation. It is recognized for its immunosuppressive function and inducing tolerance to allografts transplantation. Fludarabine phosphate is the 2-fluoro, 5′-monophosphate derivative of vidarabine (9′-β- d -arabinofuranosyladenine [ara-A]) and is converted to the di- and triphosphate by intracellular kinases, as are gemcitabine and ara-C. It has greater potency because it confers resistance to deamination by adenosine deaminase (ADA) and improved solubility. It is incorporated into DNA as a nucleotide and causes chain termination. It is a potent inhibitor of cytosolic 5′-nucleotidase II. It is also a substrate for uracil glycosylase, causing abasic sites as the first stem in BER and has recently been used in combination with TRC102, which binds to these sites and prevents their repair. Like other nucleoside analogs, it causes replication fork collapse, double-strand breaks, and induction of P53, leading to apoptotic signaling. Its efficacy against normal lymphoid T and B cells appears linked to both cytotoxicity against proliferating cells and resting cells; the latter effect is mediated by interference with the normal activity of the nucleotide excision repair pathway.
Fludarabine is a complex agent. Resistance is multifactorial including active transport, decreased cytosolic 5′-nucleotidase II and deoxycytokine kinase, and Ku80 binding to telomerase. Other nonspecific mechanisms include mutations in p53 or loss due to chromosome deletion, which is important in many cases of CLL, and other proliferation-associated genes such as NOTCH1 , SF3B1 , and BIRC3 . Low-level miR-34a is also associated with fludarabine resistance.
Fludarabine, at a standard dose of 25 mg/m 2 for 5 days and rituximab with or without cyclophosphamide were the mainstay of primary treatment for CLL (see Chapter 76 ). Fludarabine has also been used in refractory leukemias, marginal cell and other low-grade lymphomas. Recognition of the appearance of lymphopenia after treatment led to studies establishing fludarabine as part of the nonmyelosuppressive preparative regimens for allogeneic transplantation. This is most often at a dose of 30 to 35 mg/m 2 for 5 days and used in combination with radiation, melphalan, or cyclophosphamide. In addition to lymphopenia, multiple cycles are associated with prolonged myelosuppression and a chronic peripheral neuropathy.
Clofarabine (2-chloro-2′-arabino-flouro-2′-deoxyadenosine) is a purine analog with activity in patients with relapsed acute leukemia. Its activation requires cellular uptake and conversion to the triphosphate nucleotide. It then decreases ribonucleotide reductase; alters nucleotide precursors; inhibits and reduces the function of antiapoptotic proteins such as Bcl-X(L), Mcl-1, and Bax with dephosphorylation of akt; and inhibits DNA synthesis.
It appears to be more active against B-cell than T-cell, especially in AML and MDS. It is well tolerated. Recent studies show its significant efficacy in ara-C–refractory AML. Reversible liver toxicity and myelosuppression can be dose-limiting (see Chapter 60, Chapter 61, Chapter 62 ).
Nelarabine (9-β- d -arabinofuranosylguanine) is another FDA-approved purine analog for the treatment of refractory T-cell leukemias and lymphomas (see Chapter 66, Chapter 67, Chapter 68 ).
As a nucleotide analog, it preferentially accumulates in T cells and is incorporated into DNA, causing chain termination and inhibiting DNA synthesis. The FDA approved this drug after analyzing the results of two phase II clinical trials, one in pediatric T-cell ALL and the other in adults with T-cell lymphoblastic lymphoma. In both cases, patients had relapsed after at least two induction regimens. Because CRs were seen in 13% of the 39% of pediatric patients and in 18% of the 28 adult patients, the FDA granted approval. Neurologic toxicity is dose limiting. Good response rates, including CRs, have been seen in patients with refractory T-cell leukemias.
Reduced ara-G incorporation into DNA is likely due to altered nucleotide transport, increased nucleotidase activity, reduced nucleoside kinase activity, and nonspecific resistance mechanisms as described earlier for this class of agent.
Inhibitors of DNA topoisomerases I and II include such drugs as doxorubicin, daunorubicin, mitoxantrone, etoposide, and topotecan. Before describing the specific inhibitors, a brief review of the drug targets (topoisomerase enzymes) will be presented ( Table 58.2 ).
| Topoisomerase I | Topoisomerase IIα and Topoisomerase IIβ | ||
|---|---|---|---|
| Size of monomer (kDa) | 100 | 170 | 180 |
| mRNA (kb) | 4.2 | 6.2 | 6.5 |
| Chromosome | 20q12–13.2 | 17q21–22 | 3p24 |
| DNA cleavage | Single-strand breaks | Double-strand breaks | Double strand |
| Covalent intermediate | 3′PO 4 -Tyr 723 | 5′PO 4 -Tyr 804 | 5′PO 4 -Tyr 821 |
| ATP requirement | No | Yes | Yes |
| Nuclear location | Nucleoli, diffuse | Nuclear matrix and scaffold, nucleoli | NucleoliNuclear matrix |
| Cell cycle dependence | None | Yes, maximum in G 2 /M | None |
| Nuclear localization signal | NH 2 -end | COOH-end | COOH-end |
| Phosphorylation | By CK II and PKC (increases activity) | By CK II, PKC, p34 odc2 , MAPK | Increases mass to 190 kDa |
| Role | In replication, transcription, and recombination | In replication, transcription, chromosome condensation/segregation, and recombination | rRNA transcription |
| Inhibitors | Camptothecins | (see Table 58.3 ) |
Topoisomerase I is a ubiquitous enzyme whose function in vivo is to relieve the torsional strain in DNA, specifically to remove positive supercoils generated in front of the replication fork and to relieve negative supercoils occurring downstream of RNA polymerase during transcription. Topoisomerase I is catalytically active as a 100-kDa monomer and is concentrated in nucleoli, although smaller amounts are found in a diffuse nuclear distribution. The gene for this enzyme is located on human chromosome 20q12–13.2. Topoisomerase I does not require adenosine triphosphatase (ATP) for catalytic activity. It binds double-strand DNA over 15 to 25 bp (with a preference for supercoiled or bent DNA) followed by cleavage of one DNA strand and forming a transient covalent phosphotyrosyl bond at the 3′-end of DNA. DNA torsional strain is then relieved by a “controlled rotation” mechanism (see Fig. 58.1 ), subsequent to which the cleaved DNA is religated. The three-dimensional crystal structure of human topoisomerase I, both in covalent and noncovalent complexes with DNA, has defined the structural elements of the enzyme that contacts DNA. The association between topoisomerase I and the 3′-end of cleaved DNA has been termed the cleavable complex , which is stabilized by topoisomerase I inhibitors.
Two isoforms of human topoisomerase II (α and β) exist. They act as homodimers to cleave double-stranded DNA and require ATP for full activity. Their role in vivo is to relieve torsional strain in DNA, and their cellular distribution is determined by nuclear localization signals contained in the C-terminal domain. These isoforms are distinct in that they have different-size monomers (see Table 58.2 ), their genes are located on separate chromosomes, their nuclear distribution is different, and only the α-isoform shows cell cycle variations in amount and activity (with maximal activity being in G 2 /M). The mechanism of action of topoisomerase II involves several steps ( Fig. 58.4 ): DNA recognition and binding (curved and supercoiled DNA, as well as DNA crossovers, are preferred), the sequential cleavage of the two strands of DNA with covalent attachment of a monomer to each 5′-end of the cleaved DNA, passage of another DNA duplex through the break site (e.g., to relieve DNA torsional strain or decatenate daughter chromosomes at the end of replication), religation of the cleaved DNA, and ATP hydrolysis-dependent enzyme turnover. The binding of ATP by topoisomerase II is required for the strand passage reaction. Again, the association between topoisomerase II monomers and the 5′-end of the cleaved DNA has been termed the cleavable complex , the stabilization of which generally correlates with the cytotoxic activity of specific topoisomerase II inhibitors.
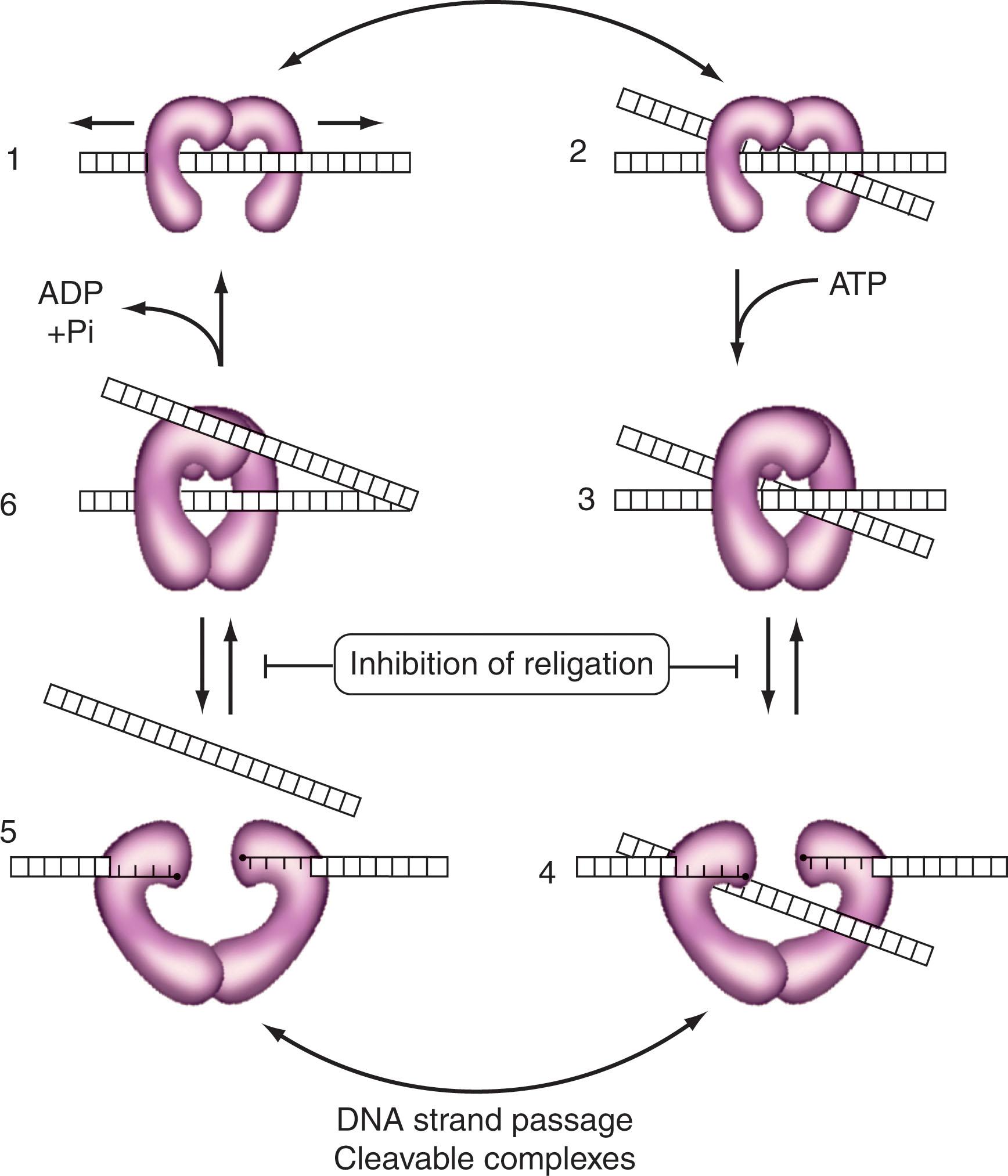
Because topoisomerase I and II inhibitors convert their respective enzymes into DNA-damaging agents, it is usually true that the more enzyme target a cell contains (provided it is in the nucleus), the more cytotoxic is the specific inhibitor. An exception to this generalization is CLL cells, which have abundant topoisomerase I but are not very sensitive to topoisomerase I inhibitors because topoisomerase I inhibitors are S-phase-specific and CLL cells have very few cells in S phase. Finally, in addition to topoisomerase I and II, a mammalian DNA topoisomerase III has been described and found to be essential for early embryogenesis in the mouse. In addition to the presumed lethality of a homozygous deletion of the topoisomerase II gene, topoisomerases I and III appear to be essential for cell growth and division in mammals. The specific role of topoisomerase III in humans is unknown at present.
Camptothecin (CPT-11 or irinotecan) is a plant alkaloid first identified in 1966 from the tree Camptotheca acuminata . Early clinical studies with camptothecin were stopped primarily because of hemorrhagic cystitis resulting from conversion of the sodium salt form to the active lactone form owing to its acidic pH in the bladder. Renewed interest in camptothecin occurred in 1985 when topoisomerase I was identified as the target of this drug and as new more water-soluble analogs became available. At present, two topoisomerase I inhibitors have been approved by the FDA as second-line agents for the treatment of ovarian carcinoma and colorectal cancer; these are topotecan and irinotecan (CPT-11; Fig. 58.5 ). Topotecan has been shown to be active in the treatment of MDS and inactive in the treatment of CLL. Responses to topotecan have also been seen in refractory MM, refractory large-cell lymphoma, and refractory acute leukemia.
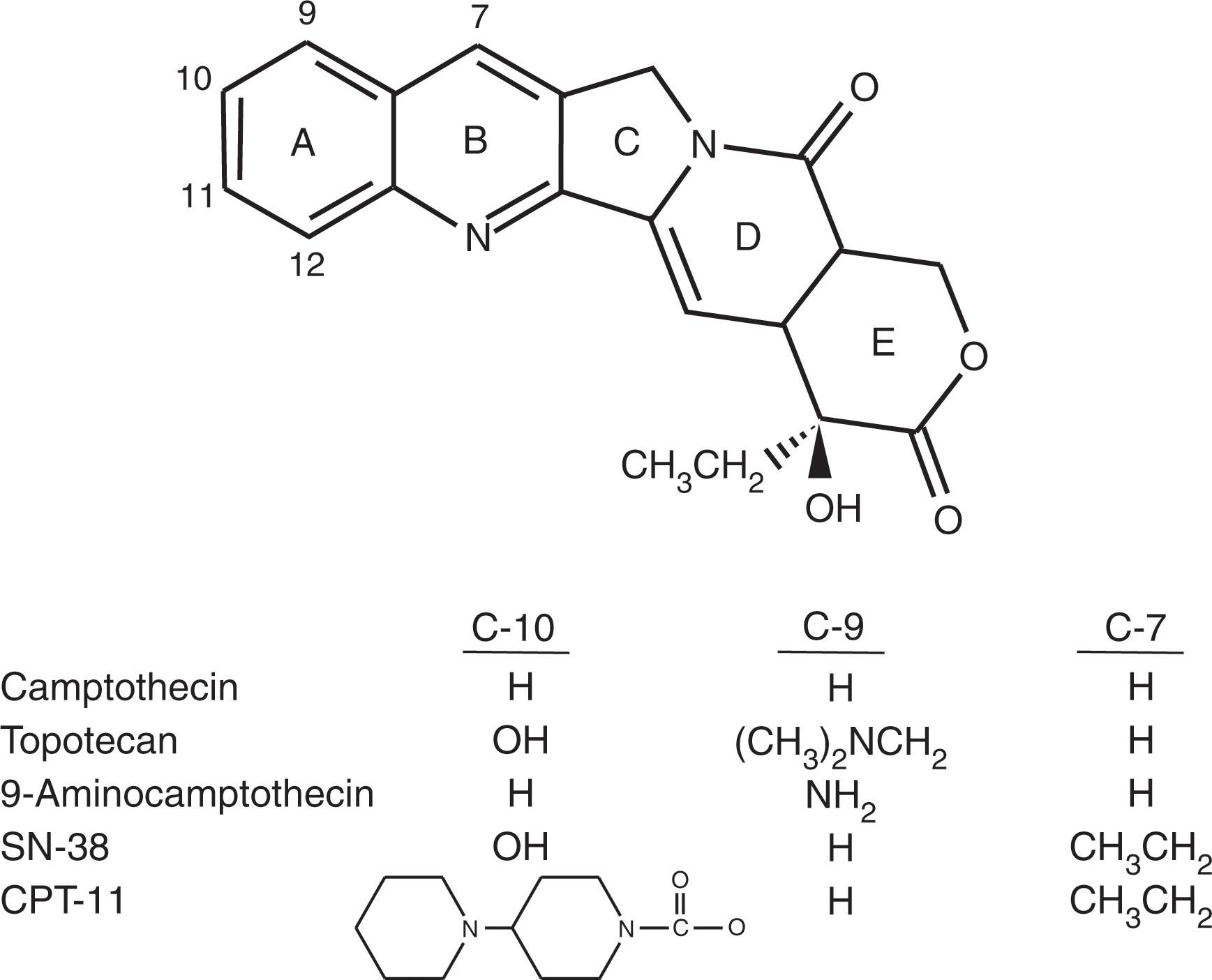
The lactone forms of topotecan and SN-38 (the active form of CPT-11 generated in vivo by the action of a carboxylesterase) are as much as 1000-fold more active inhibitors of DNA topoisomerase I than are their carboxylate forms. The lactone form predominates at an acidic pH. Topoisomerase I inhibitors stabilize the DNA-enzyme cleavable complex and thus inhibit DNA religation, but the production of DNA double-strand breaks results from a collision of the DNA replication fork with the ternary drug-enzyme-DNA complex, which is the lethal event (see Fig. 58.4 ). Topoisomerase I inhibitors are considered S-phase-specific agents because they require ongoing DNA synthesis to exert their cytotoxic effect.
Inhibitors of DNA topoisomerase II are commonly used for the treatment of hematologic malignancies. Three general types of topoisomerase II inhibitors exist ( Table 58.3 ). The first are the topoisomerase II poisons, typified by etoposide, which results in the stabilization of cleavable complexes. The second group are the catalytic inhibitors, represented by aclarubicin, merbarone, and the bis-2,6-dioxopiperazine derivatives (ICRF-193, ICRF-159, ICRF-187); these are drugs that, except for aclarubicin, do not bind DNA and do not stabilize cleavable complexes, but rather interfere with some aspect of topoisomerase II catalytic activity (e.g., ICRF-187 inhibits topoisomerase II ATPase activity). The final class includes drugs that can inhibit both DNA topoisomerases I and II, and are represented by intoplicine and saintopin.
| Drug | Topoisomerase II Inhibition Poison | DNA Suppressor | Free Radical Intercalation | Formation |
|---|---|---|---|---|
| Epipodophyllotoxins | +++ | − | − | + |
| VP-16 | ||||
| VM-26 | ||||
| Anthracyclines | ||||
| Doxorubicin | ||||
| Daunorubicin | ++ | ++ | ++ | + |
| Idarubicin | ||||
| Epirubicin | ||||
| Anthracenedione | ||||
| Mitoxantrone | ++ | ++++ | ++ | + |
| Acridine | ||||
| m -AMSA | +++ | + | + | − |
| Catalytic inhibitors | ||||
| Aclarubicin | − | +++ | + | − |
| Others (merbarone, fostriecin, bis-2,6-dioxopiperazines) | − | +++ | − | − |
DNA topoisomerase II poisons (see Fig. 58.5 for structures) are most likely cytotoxic because they trap DNA topoisomerase II complexes on nascent DNA in the nuclear matrix. The topoisomerase II poison-stabilized enzyme–DNA complex likely acts as a replication fork barrier and leads to the generation of irreversible DNA damage and cell death in proliferating cells. Whereas experiments in yeast show that although DNA synthesis is a major determinant for cell killing by topoisomerase I inhibitors, topoisomerase II poisons are also cytotoxic during other phases of the cell cycle.
Essentially all of the topoisomerase II poisons (see Tables 58.2 and 58.3 ) are substrates for the drug efflux pump P-glycoprotein (PGP), and many are substrates for multidrug-resistance protein (MRP) and lung resistance-related protein. In addition, several point mutations and gene deletions have been defined in the gene for topoisomerase II, resulting in the production of an enzyme with altered catalytic or cleavage activity. The third mechanism of resistance is a decrease in expression of the enzyme such that there is less target for the inhibitor to “convert” to a DNA-damaging agent. This can result from a proliferation-dependent or cell cycle–dependent decrease in topoisomerase II, from a specific attenuation of topoisomerase II, or from an intrinsic absence of topoisomerase II (identified in some acute and chronic leukemias). The fourth resistance mechanism involves alterations in the subcellular distribution of the enzyme. Truncation of the COOH-end of topoisomerase II has resulted in the cytoplasmic distribution of enzyme caused by a loss of nuclear localization signals, so that the enzyme cannot interact with DNA in the presence of an inhibitor and the cell is resistant. However, mutations in the gene for topoisomerase II do not appear to be common, because only a single patient with AML has been found to have a point mutation. By contrast, CLL cells are resistant to topoisomerase II inhibitors because they express very low levels of the protein.
During a study of the effects of electric current on growing bacteria, the antibacterial and, later, the antitumor activities of the platinum compounds were fortuitously discovered. The antitumor agent cisplatin, its cis -carboxylester analog, carboplatin, and the diaminocyclohexane-containing oxaliplatin, are heavy-metal platinum complexes. They are activated when one of their ligands (cisplatin; chloride and carboplatin; carboxylester) is displaced by water, leading to the formation of positively charged aquated platinum complexes, allowing platinum to stably bind DNA, RNA, proteins, or other critical biomacromolecules (see Fig. 58.2 ). With DNA, platinum complexes form covalent links to the N 7 position of guanine and adenine. The N 7 adducts at d(GpG) or d(ApG) result in intrastrand or interstrand DNA cross-links that bend the DNA helix and inhibit DNA synthesis. The cytotoxicity of platinum analogs correlates with the total platinum binding to DNA, as well as with the intrastrand or interstrand cross-links. This results in DNA damage, which triggers apoptosis of sensitive cells.
Cisplatin, carboplatin, and oxaliplatin are used in the treatment of refractory lymphomas in a variety of combinations, and as part of high-dose and intensification therapy for lymphomas as definitive treatment including prior to autologous stem cell transplantation.
Among the agents included in this category, only plicamycin, bleomycin, l -asparaginase, gallium nitrate, and glucocorticoids are of current interest to hematologists.
Since the year 2000, the therapeutic arsenal available for treatment of hematologic malignancies has expanded to include a group of drugs that have collectively been termed as “targeted agents.” Traditional chemotherapeutic drugs affect specific targets, many times identified after the cytotoxic and clinical activity of these agents have been demonstrated. Dobbelstein and Moll describe three “waves” or “epochs” in anticancer drug development. The first wave includes traditional chemotherapeutic agents, comprised mostly of drugs that affect DNA replication, repair and cell division; these drugs are nonspecific and can affect normal cells, but take advantage of the increased proliferation of cancer cells to exert their cytotoxic activity. Second-wave drugs target cellular signals, including those mediated by surface receptors and protein kinases. Monoclonal antibodies are included in this second wave. The targets of signaling inhibitors are diverse, and include the products of oncogenes, essential for the development and survival of neoplasms (“oncogene addition”). The prototype of this class of agents is imatinib, targeting the chimeric product of BCR-ABL in chronic myelogenous leukemia (CML).
Other cellular signaling proteins are not the product of oncogenes but have an essential role in intracellular processes of malignant cells. This state is known as “nononcogene addiction” and has expanded the number of targets that can be used for cancer treatment with signaling inhibitors. Examples of the latter agent class include inhibitors of mammalian target of rapamycin (mTOR) and BTK. The third wave of anticancer drugs target cellular mechanisms and effector systems distinct from those targeted by drugs of the first two waves, but that are still essential for the survival of cancer cells. The cellular processes targeted by drugs of the third wave are those that contain several types of constitutive stress experienced by cancer cells (separate from replicative stress), including proteotoxic stress leading to abnormal protein folding and increased protein degradation (targeted by heat-shock protein inhibitors and proteasome inhibitors), DNA damage (targeted by PARP inhibitors), DNA modifications (targeted by hypomethylating agents and HDACs), prosurvival balance in organelles governing cell survival (targeted by BCL2 inhibitors), increased need for protein transport (inhibited by nuclear transport inhibitors), as well as transcriptional, ribosomal, metabolic, and oxidative stress, targeted by several other drugs in development. Other agents not easily included in these three waves include immunomodulatory drugs (thalidomide, lenalidomide, and pomalidomide), as well as agents promoting cancer cell differentiation (all- trans retinoic acid).
Imatinib mesylate is a phenylaminopyrimidine developed as an inhibitor of the constitutively active tyrosine kinase BCR-ABL, expressed in the leukemic cells of most patients with CML. The recognition that the constitutively active BCR-ABL played a central role in the pathogenesis of CML led to the search for potential inhibitors. The introduction of the tyrosine kinase inhibitor (TKI) imatinib for treatment of CML is a major milestone in the development of targeted therapy for hematologic and neoplastic disorders. The role of the BCR-ABL kinase in the pathogenesis of CML and ALL and the use of BCR-ABL inhibitors in the treatment of these disorders is discussed in Chapter 67, Chapter 68, Chapter 69 .
| Agent | Target | Approved indication |
|---|---|---|
| Acalabrutinib | BTK | CLL, MCL |
| Alectinib | ALK, RET | Alk positive metastatic NSCLC |
| Asciminib | BCR-ABL | |
| Azacitidine | DNA methyltransferase | MDS, CML, and AML |
| Belinostat | Histone deacetylase | PTCL |
| Bortezomib | Proteasome | Multiple myeloma, MCL AL amyloidosis |
| Bosutinib | BCR-ABL, KIT, Src, HCK, Lyn, MAPK1-2 | CML |
| Carfilzomib | Proteasome | Multiple myeloma |
| Ceritinib | ALK | NSCLC |
| Copanlisib | PI3K-alpha and PI3K-delta | Follicular lymphoma |
| Crenolanib | FLT3, PDGFRα, PDGFRβ, c-KIT | |
| Crizotinib | ALK, HGFR | ALK-positive non-small–cell lung cancer |
| Dabrafenib | BRAF | Melanoma with BRAF V600E mutation |
| Decitabine | DNA methyltransferase | AML |
| Enasidenib | IDH2 | AML |
| Everolimus | mTOR | Renal cell carcinoma |
| Fedratinib | JAK2, FLT3, RET | Intermediate - 2 or high-risk myelofibrosis |
| Gilteritinib | FLT3 | AML, MDS |
| Glasdegib | Hedgehog pathway | AML |
| Ibrutinib | BTK | CLL, MCL, Waldenström's macroglobulinemia, Marginal zone lymphoma, GVHD |
| Idelalisib | PI3Kδ | Relapsed CLL, SLL, and follicular lymphoma |
| Ipatasertib | AKT | |
| Ivosidenib | IDH1 | AML, cholangiocarcinoma |
| Ixazomib | Proteasome | Multiple myeloma |
| Lenalidomide | IMiD | Multiple myeloma, MCL, 5q- syndrome, DLBCL (in combination with tafasitamab) |
| Midostaurin | FLT3, c-KIT | AML |
| Nilotinib | BCR-ABL, Kit, Lck, Ephrins, PDGFRβ, MAPK11, ZAK | CML |
| Omacetaxine | Ribosomal protein translation | CML with resistance or intolerance to tyrosine kinase inhibitors |
| Pacritinib | JAK2, FLT3 | |
| Panobinostat | Histone deacetylase | Multiple myeloma, myelofibrosis |
| Ponatinib | BCR-ABL, Kit, PDGFRα, VEGFR, Src, Lyn, Lck, FGFR1–4, FLT3, TEK | CML with T315I mutation |
| Quizartinib | FLT3, CRS1R, SCFR, PDGFR | |
| Romidepsin | Histone deacetylase | CTCL, PTCL |
| Ruxolitinib | JAK1, JAK2, | Intermediate or high-risk myelofibrosis, GVHD |
| Sonidegib | Hedgehog pathway | Basal cell carcinoma |
| Sorafenib | BRAF, VEGFR, FLT3, PDGFRβ, Kit, FGFR1, Ret | Hepatocellular carcinoma |
| Tazemetostat | EZH2 | Follicular lymphoma, epithelioid sarcoma |
| Temsirolimus | mTOR | Renal cell carcinoma |
| Thalidomide | IMiD | Multiple myeloma |
| Umbralisib | PI3K-delta and CK1-epsilon | |
| Vemurafenib | BRAF | Melanoma with BRAF V600E mutation |
| Venetoclax | BCL2 | CLL, AML |
| Vismodegib | Hedgehog pathway | Basal cell carcinoma |
| Vorinostat | Histone deacetylase | CTCL, PTCL |
| Zanubrutinib | BTK | MCL |
Imatinib mesylate is the founding agent in the class of TKIs. It causes direct inhibition of the BCR-ABL kinase but is not absolutely specific for this molecule, as it also inhibits other kinases, including KIT, platelet-derived growth factor (PDGF), stem cell factor (SCF), and TEL-ARG.
Radiographic crystallography studies show that imatinib mesylate competitively binds to the ATP-binding site of the ABL kinase and stabilizes it in an inactive conformation. The 50% inhibitory concentration (IC 50 ) of imatinib mesylate for BCR-ABL is in the submicromolar range, and is substantially lower than the supramicromolar levels achievable in the plasma of patients receiving the drug by the oral route.
Despite the success with imatinib mesylate in the treatment of chronic-phase CML and, to a lesser extent, accelerated or blast-phase CML, the preexistence or development of resistance represents a major therapeutic challenge that can occur at any stage of the disease. Primary or secondary resistance can develop through a variety of mechanisms. BCR-ABL–dependent mechanisms correspond to the emergence of mutations in ABL kinase domain, which can be located in the imatinib binding site, the P loop, the catalytic domain, or the activation loop. Many of these mutations are present at the time of diagnosis and resistant clones emerge after exposure to imatinib. Resistance mechanisms independent of BCR-ABL include development of multidrug resistance mechanisms (e.g., PGP related); decreased levels of human organic cation transporter; acquisition of additional genetic abnormalities (clonal evolution) including acquisition of an additional Philadelphia-positive chromosome (Ph+), trisomy 8 and isochromosome 17q, increased expression of the BCR-ABL protein, and Src kinase overexpression.
Imatinib also has activity in diseases dependent on other kinases, such as PDGF receptor PDGFRα, PDGFRβ, or KIT, and is active against myeloproliferative disorders associated with eosinophilia and FIP1L1/PDGFRα or PDGFRβ fusion genes, as well as against systemic mastocytosis without KIT D816V mutation (see Chapter 71, Chapter 72 ).
Common adverse events due to imatinib include fluid overload and edema, and development of heart failure, fatigue, rash, and myelosuppression. Gastrointestinal side effects are common, and nausea and vomiting are frequent reasons for poor compliance.
Dasatinib is a second-generation ABL kinase inhibitor approved for the treatment of CML. Dasatinib is an oral multikinase inhibitor, affecting BCR-ABL, KIT, PDGFR, and Src kinases. It binds the ATP binding site of ABL in an opposite direction to imatinib, can inhibit active and inactive forms of the kinase, and requires fewer points of contact. This reduced structural stringency in kinase inhibition allows for inhibition of all kinase mutations, with the exception of the T315I mutation.
Compared with imatinib, dasatinib is several hundred times more potent as an inhibitor of ABL. Initial studies demonstrated dasatinib-induced responses in patients who had developed resistance to imatinib treated in the chronic, accelerated, and blast phase of CML. There is a high response rate in patients with wild-type sequences of BCR-ABL and also in patients with mutations in the ABL protein conferring resistance to imatinib, including E255V and Y253H . The notable exception is T315 mutations.
As a multikinase inhibitor, dasatinib has a role in myeloid-lymphoid neoplasms with eosinophilia and recurrent genomic translocations. Dasatinib has a broad toxicity profile, with pulmonary edema, pleural effusions, and thrombocytopenia being the more frequent adverse effects. Recently, higher rates of pulmonary hypertension have been observed in patients receiving dasatinib.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here