Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter addresses:
Penicillin Allergy/Anaphylaxis
Antibiotic-Associated Colitis
Drug-Seeking Behavior
Acute Acetaminophen Toxicity
Opioid Side Effects
Oral Drug-Induced Osteonecrosis of the Jaws
Intravenous Drug-Induced Osteonecrosis of the Jaws
The use of pharmacotherapy is an important primary or adjunctive modality of treatment in the management of the surgical patient. Antibiotics, anesthetics, and analgesics are the most commonly used medications in oral and maxillofacial surgery. Despite appropriate use of medications, side effects and complications are observed. In this section we present seven cases associated with the use of medications encountered in surgery, along with discussion of related topics. Two cases of drug-induced osteonecrosis of the jaws (DINOJ) are presented—one involving oral and the second involving intravenous bisphosphonates. These two cases are separated since they represent two clinically distinct situations. Cases directly related to anesthesia and anticoagulation therapy are presented separately in the anesthesia and medicine sections.
The undesired effects of medications may result from a single factor or a combination of factors related to the drug: (1) Immunologically mediated reactions ranging from mild cutaneous manifestations to life-threatening anaphylaxis reactions; (2) unwanted systemic or local (side) effects (e.g., antibiotic-associated colitis or bisphosphonate-induced osteonecrosis of the jaws); (3)inappropriate dosing (e.g., acetaminophen overdose); (4) abuse and addictive potential leading to physiologic or social complications (e.g., opioid abuse or addiction); (5) compromised or deficient metabolism and elimination capabilities of the patient leading to toxicities (e.g., renal or hepatic failure); (6) failure of the drug to treat the intended condition, leading to exacerbation of the disease process (e.g., antibiotic resistance); and (7) patient noncompliance compromising treatment (e.g., concomitant alcohol ingestion with opioid or benzodiazepine use).
As the future unfolds, the pharmacological management of oral and maxillofacial surgery patients will become more complex. The greater array of medications on the market will require identification of new unwanted sequelae and interactions of drugs. Integration of technology will allow easier access to information to help ameliorate this emerging phenomenon.
A 21-year-old woman admitted for treatment of an open mandibular body fracture complains of the sudden appearance of a rash and shortness of breath after receiving her intravenous antibiotics (anaphylaxis is more common with parenteral administration of medications).
The patient was admitted that day with a diagnosis of an open right mandibular body fracture secondary to assault. The patient did not report any known drug allergies (most frequently, patients do not have a previous history), and the admitting surgeon ordered intravenous penicillin G, morphine sulfate, and nothing-by-mouth (NPO) status in preparation for surgical treatment in the operating room. Upon arrival of the patient on the hospital ward, the nursing staff administered the first dose of intravenous aqueous penicillin G. Approximately 5 to 10 minutes later, the patient developed multiple circumscribed, erythematous, and raised pruritic wheals on her skin (symptoms generally develop within 5 to 60 minutes after exposure; earlier onset is seen with parenteral introduction of the allergen). The patient also reported feeling short of breath and the onset of wheezing (secondary to bronchospasm), nausea, and cramping abdominal pain.
The patient reports no known drug allergies. She has no history of food, environmental, or seasonal allergies. She has no family history of drug allergies. ( Multiple drug allergy syndrome is a term that may be applied to individuals who have experienced allergic reactions to two or more non-cross-reacting medications. People who are allergic to another drug are likely at increased risk of reacting to penicillin. The reasons are not clear, but genetics may play a role. Genetics may also play a role in the expression of penicillin allergy between family members; however, currently the studies are limited.)
Anaphylaxis is a serious allergic reaction that is rapid in onset and may cause death. Allergic anaphylaxis involves the production of symptoms via an immunologic mechanism. Nonallergic anaphylaxis (previously known as an anaphylactoid reaction) produces a very similar clinical syndrome but is not immune mediated (direct activation of mast cell). Treatment for the two conditions is similar.
General. The patient is a well-developed, well-nourished woman in moderate distress who is sitting up and leaning forward in bed.
Vital signs. Her blood pressure is 98/60 mm Hg (hypotension), heart rate 128 bpm (tachycardia), respirations 28 per minute (tachypnea), temperature 36.7°C, and Sa o 2 100% on 2 L per nasal cannula.
Neurologic. The patient's Glasgow Coma Scale score is 15; she is alert and oriented × 3 (place, time, and person).
Maxillofacial. Examination is consistent with a mandibular body fracture.
Cardiovascular. She is tachycardic at 128 bpm. The heart rate and rhythm are regular, with no murmurs, gallops, or rubs. Cardiovascular symptoms and signs occur in up to 45% of anaphylactic episodes; they include hypotonia (collapse), syncope, dizziness, tachycardia, and hypotension.
Pulmonary. The patient has bilateral wheezing. Respiratory symptoms and signs occur in up to 70% of anaphylactic episodes; they include nasal congestion and discharge, a change in voice quality, a sensation of throat closure or choking, stridor, shortness of breath, wheezing, and cough.
Abdominal. The abdomen is soft, tender to palpation (secondary to spasm of intestinal smooth muscles), and nondistended with no rebound tenderness and normal bowel sound. Gastrointestinal symptoms and signs occur in up to 46% of anaphylactic episodes; they include nausea, vomiting, diarrhea, and crampy abdominal pain.
Skin. The patient has urticaria (commonly known as “hives,” urticaria consists of circumscribed areas of raised erythema and edema of the superficial dermis). Skin symptoms and signs occur in up to 80% to 90% of anaphylactic episodes; they include generalized hives; itching or flushing; swollen lips, tongue, and uvula; periorbital edema; and conjunctival swelling.
The clinical presentation of anaphylaxis is variable and may include any combination of common signs and symptoms. Anaphylaxis is under-recognized and undertreated; the goal is early recognition and treatment with epinephrine. Diagnostic criteria were published by an expert panel in 2006 with the intention of helping clinicians recognize anaphylaxis.
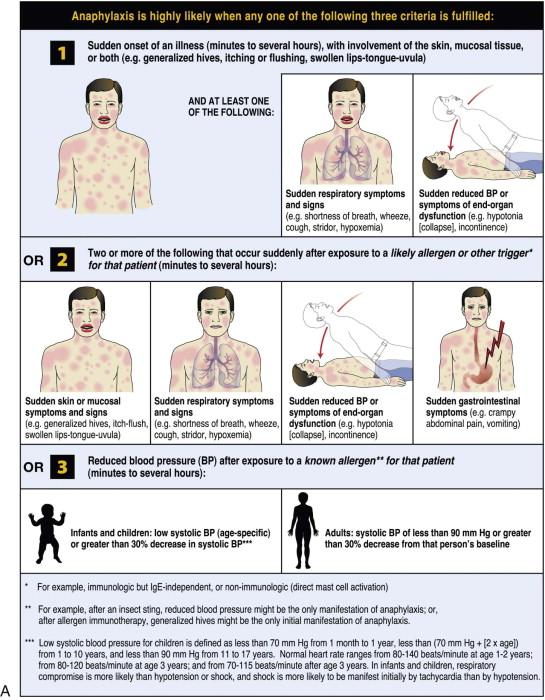
The World Allergy Organization (WAO) has developed a poster that presents the key clinical criteria for both the diagnosis and initial treatment of anaphylaxis ( Figure 2-1 ). These criteria reflect the different clinical presentations; anaphylaxis is highly likely when any one of the criteria is met. It was acknowledged that no single set of criteria can provide 100% sensitivity and specificity, but it is believed that the WAO's proposed criteria are likely to capture more than 95% of cases of anaphylaxis. The majority of anaphylactic reactions include skin symptoms, which are noted in more than 80% of cases. Thus at least 80% of anaphylactic reactions should be identified by criterion 1, even when the allergic status of the patient and the potential cause of the reaction might be unknown.
In the acute phase of anaphylaxis, no imaging studies are indicated (any unnecessary delay may compromise other lifesaving interventions).
During an acute anaphylactic episode, no laboratory tests are indicated. However, once the patient's condition has been stabilized (or if the diagnosis is in question), in addition to a complete blood cell count (CBC) and comprehensive metabolic panel (CMP), the following tests can be obtained:
Plasma histamine level. This is elevated within 5 to 10 minutes after the onset but remains elevated for only 30 to 60 minutes because of rapid metabolism (histamine is released secondary to IgE-mediated mast cell degranulation).
Urinary N-methyl histamine. A metabolite of histamine, N-methyl histamine remains elevated for several hours. A 24-hour urine sample for N-methyl histamine may be useful.
Serum tryptase. This peaks 60 to 90 minutes after the onset of anaphylaxis and remains elevated for up to 5 hours. Tryptase is a protease specific to mast cells. It is the only protein that is concentrated selectively in the secretory granules of human mast cells. Normal levels of either tryptase or histamine do not rule out the clinical diagnosis of anaphylaxis.
Immediate allergic anaphylactic reaction induced by intravenously administered penicillin G
Box 2-1 outlines the differential diagnosis of anaphylactic shock.
Other forms of shock —Hemorrhagic/hypovolemic, cariogenic, septic
Flush syndromes —Carcinoid, postmenopausal hot flashes, red man syndrome (vancomycin), oral hypoglycemic agents with alcohol, medullary carcinoma of the thyroid, idiopathic
Excess endogenous production of histamine —Systemic mastocytosis, basophilic leukemia, hydatid cyst
Respiratory distress —Asthma/chronic pulmonary obstructive disease exacerbation, foreign body aspiration, vocal cord dysfunction, pulmonary embolism
Nonorganic diseases —Panic attacks, globus hystericus, Munchausen's stridor
Other conditions —Ingestion of sulfites or monosodium glutamate, hereditary angioedema, neurologic condition (stroke or seizure), drug overdose
The initial management of anaphylaxis is to perform a focused examination; discontinue the suspected medication; call for help; administer intramuscular injection of epinephrine; place the patient, if possible, in a Trendelenburg position (to maximize perfusion of vital organs); administer supplemental oxygen; establish a stable airway (with intubation if necessary); obtain venous access (preferably with two large-bore [16-gauge] peripheral intravenous catheters) for volume resuscitation; and continuously monitor the vital signs and level of consciousness.
Immediately upon diagnosis, 0.3 to 0.5 ml of 1 : 1,000 epinephrine (0.3 to 0.5 mg in adults and 0.01 mg/kg in children) should be injected intramuscularly (IM) into the anterolateral thigh (injection at this site has been shown to be more effective than subcutaneous or upper arm [deltoid] injection). The site can be massaged to facilitate absorption. This dose may be repeated every 10 to 15 minutes, up to a total of three doses. The therapeutic effects of epinephrine include:
α 1 adrenergic agonist: Increased vasoconstriction, increased peripheral vascular resistance, and decreased mucosal edema (in the upper airway)
β 1 adrenergic agonist: Increased inotropy and chronotropy
β 2 adrenergic agonist: Increased bronchodilation and decreased release of mediators from mast cells and basophils
Patients with severe upper airway edema, bronchospasm, or significant hypotension or who do not respond to IM injection (may not be perfusing muscle tissue) and fluid resuscitation should receive 0.5 to 1 ml of 1 : 10,000 epinephrine intravenously at 5- to 10-minute intervals. Alternatively, a continuous infusion of 1 to 10 µg/min of epinephrine (titrated to effect) may be administered (this is preferred over bolus dosing of epinephrine, because bolus dosing is associated with more adverse effects). Patients receiving intravenous epinephrine require continuous cardiac monitoring because of the potential for arrhythmias and ischemia, which occur most commonly with this route of administration. If intravenous access cannot be established, the epinephrine can be administered via an endotracheal tube (3 to 5 ml of 1 : 10,000 epinephrine).
It has been recommended that the epinephrine be administered early, because this can prevent progression to severe symptoms. Delayed administration has been implicated in contributing to fatalities.
Nebulized albuterol (β 2 agonist) for respiratory symptoms may be administered, and intravenous aminophylline (bronchodilator) can be considered, although its effectiveness for anaphylaxis is questionable. These are adjunctive treatments to epinephrine. Large volumes of fluids may be required to treat hypotension caused by increased vascular permeability and vasodilatation. Any patients with evidence of intravascular volume depletion (e.g., hypotension, low urine output, low or no response to injected epinephrine) should receive volume replacement. Normal saline is preferred initially. Additional pressors, such as dopamine (5 to 20 µg/kg/min), norepinephrine (0.5 to 30 µg/kg/min), or phenylephrine (30 to 180 µg/kg/min), may be required.
Antihistamines also are considered adjunctive to epinephrine. The purpose for using antihistamines is to relive itch and hives. A combination of H 1 and H 2 blockers may be superior to either agent alone. Thus, diphenhydramine (H 1 -receptor blocker) 25 to 50 mg intravenously/intramuscularly every 4 to 6 hours can be used with cimetidine (H 2 -receptor antagonist) 300 mg intravenously every 8 to 12 hours. Alternatively, ranitidine (H 2 -receptor antagonist) 1 mg/kg intravenously can be used.
Most authorities also advocate the administration of corticosteroids (methylprednisolone 1 to 2 mg/kg/day); their benefit is not realized for 6 to 12 hours after administration, but they may be helpful in the prevention of biphasic reactions. They can be stopped after 72 hours, because all biphasic reactions reported to date have occurred within 72 hours.
Patients currently taking β-blockers pose a challenge, because these drugs may limit the effectiveness of epinephrine. These patients may develop resistant hypotension, bradycardia, and a prolonged course. Atropine (anticholinergic) may be given for bradycardia. Some clinicians recommend administering glucagon. Glucagon exerts a positive inotropic and chronotropic effect on the heart independent of catecholamines. A 1-mg intravenous bolus followed by 1 to 5 mg every hour may improve hypotension in 1 to 5 minutes, with maximal benefit at 5 to 15 minutes. All patients with anaphylaxis should be monitored for the possibility of recurrent symptoms after initial resolution.
In the current patient, penicillin was immediately discontinued and 0.3 mg of 1 : 1,000 epinephrine was injected intramuscularly into the right anterolateral thigh. Synchronously a code was called; the patient was placed in a Trendelenburg position; and supplemental oxygen at 10 LPM was administered via a nonrebreather mask. Two 16-gauge peripheral intravenous lines were started at each antecubital fossa, and a bolus of normal saline was given. The patient also received intravenous medications (i.e., 50 mg diphenhydramine, 300 mg cimetidine, 100 mg methylprednisone) and nebulized albuterol. The vital signs were continuously monitored. An additional 0.3 mg of 1 : 1,000 epinephrine was given intramuscularly after 12 minutes. The patient remained stable, and marked improvement was noted. She was subsequently transferred to the intensive care unit (ICU) for observation.
After an uneventful overnight stay in the ICU, the patient was taken to the operating room the next day, where she underwent open reduction with internal fixation (ORIF) of the mandibular fracture. The anesthesia team was informed of her hospital course (in case the patient experienced a biphasic recurrence, with the signs and symptoms of anaphylaxis occurring during anesthesia). All early symptoms of anaphylaxis usually observed in the awake patient (e.g., malaise, pruritus, dizziness, and dyspnea) are absent in the anesthetized patient. The most commonly reported initial features are pulselessness, difficulty in ventilating, desaturation, and decreased end-tidal CO 2 . Also, cutaneous signs may be difficult to notice in a completely draped patient.
Upon discharge, the patient was thoroughly informed of her allergy. She was provided with a medical alert bracelet, and follow-up was arranged with allergy care specialists.
Complications of anaphylaxis range from full recovery to anoxic brain injury and death despite adequate response and treatment. The factors that determine the course of anaphylaxis are not understood. At the onset of an episode, it is not possible to predict how severe it will become, how rapidly it will progress, or whether it will resolve spontaneously (as a result of endogenous production of compensatory mediators, such as epinephrine) or become biphasic or protracted. The rapidity of onset of symptoms makes this uncommon condition difficult to treat. Early recognition and treatment are essential. It is estimated that anaphylaxis causes approximately 1,400 to 1,500 fatalities per year in the United States. Between 5% and 20% of patients experience biphasic anaphylaxis, with a recurrence of symptoms after apparent initial resolution (typically 1 to 10 hours after initial resolution). Some cases of recurrence have been reported up to 72 hours later. Protracted anaphylaxis also has been reported, with persistence of symptoms for hours, days, or even weeks despite therapy.
Anaphylaxis is known to be difficult to recognize clinically for several reasons, including the broad differential that needs to be considered. Concurrent use of central nervous system (CNS)–active medications, such as sedatives, hypnotics, antidepressants, and first-generation sedating H 1 antihistamines, can interfere with recognition of anaphylaxis triggers and symptoms and with the ability to describe symptoms. In patients with concomitant medical conditions, such as asthma, chronic obstructive pulmonary disease, or congestive heart failure, symptoms and signs of these diseases can also cause confusion in the differential diagnosis of anaphylaxis. Death most commonly results from intractable bronchospasm, asphyxiation from upper airway edema, or cardiovascular collapse.
Penicillin allergy is reported by up to 10% of people in the United States. It has also been recognized that 80% to 90% of patients who report a penicillin allergy are not truly allergic to the drug. Of significance is that many people are falsely labeled as being penicillin allergic.
Most clinicians simply accept a diagnosis of penicillin allergy without obtaining a detailed history of the reaction. In their review, Salkind and colleagues stress the importance of a thorough history when faced with a penicillin-allergic patient ( Box 2-2 ). However, it has been shown that patients with a vague history have also been found to have an IgE-mediated allergy. The time elapsed since the last reaction is important, because penicillin-specific IgE antibodies decrease with time (approximately 80% of patients with IgE-mediated penicillin allergy have lost sensitivity after 10 years). Nonetheless, it is prudent to refer any patient with a history of IgE-mediated penicillin allergy for testing. Penicillin is the most common cause of drug-induced anaphylaxis. It causes an estimated 40% to 50% of all anaphylactic deaths in the United States.
What was the patient's age at the time of the reaction?
Does the patient recall the reaction? If not, who informed him or her of it?
How long after beginning penicillin did the reaction start?
What were the characteristics of the reaction?
What was the route of administration?
Why was the patient taking penicillin?
What other medications was the patient taking? Why and when were they prescribed?
What happened when the penicillin was discontinued?
Had the patient taken antibiotics similar to penicillin (e.g., amoxicillin, ampicillin, cephalosporins) before or after the reaction? If yes, what was the result?
Allergic drug reactions are one type of adverse drug reaction (ADR). An ADR has been defined by the World Health Organization as any noxious, unintended, and undesired effect of a drug that occurs at doses used for prevention, diagnosis, or treatment. ADRs can be categorized into two types: type A reactions, which account for 85% to 90% of all ADRs and can affect any individual (e.g., diarrhea in response to antibiotics), and type B reactions, which are hypersensitivity reactions that occur in susceptible patients. Although it has been difficult to determine the frequency of drug-induced allergic reactions specifically, it is known that they account for only a small proportion of ADRs, approximately 6% to 10%.
An allergic drug reaction can be classified as immediate (reaction occurs within 1 hour of administration and is usually IgE mediated) or delayed (reaction occurs after 1 hour, at times days or weeks after treatment, and is not IgE mediated). Anaphylaxis is an example of an immediate reaction. Late reactions can range from a rash that develops during treatment with amoxicillin to life-threatening conditions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN). In rare cases, certain β-lactams can cause interstitial nephritis, hepatitis, or a vasculitis with or without signs of serum sickness.
Allergic reactions can also be classified by the immune mechanism involved, as described by Gell and Coombs. In this classification, type I represents an IgE-mediated response, whereas types II, III, and IV are non–IgE dependent. Type II, type III, and type IV reactions are classified as delayed reactions because they generally occur more than 1 hour after drug administration.
The clinical syndrome of anaphylaxis results from activation and release of mediators from mast cells and basophils (e.g., histamine). The cross-linking of mast cell–bound IgE with antigens causes the release of these mediators, with manifestations that include increases in vascular permeability (causing edema), vasodilatation (causing hypotension), respiratory smooth muscle contraction (causing bronchospasm), stimulation of the autonomic nervous system (causing tachycardia), mucus secretion, platelet aggregation, and recruitment of inflammatory cells.
Middle-aged and elderly patients are at increased risk of severe or fatal anaphylaxis because of known or subclinical cardiovascular diseases and the medications used to treat them. In the healthy human heart, mast cells are present around the coronary arteries and the intramural vessels, between the myocardial fibers, and in the arterial intima. In patients with ischemic heart disease, the number and density of cardiac mast cells is increased in these areas, and mast cells also are present in the atherosclerotic plaques. During anaphylaxis, the mediators released from cardiac mast cells contribute to vasoconstriction and coronary artery spasm.
Foods are the most common trigger for anaphylaxis in children, teens, and young adults (food triggers differ according to local dietary habits). Insect stings and medications (e.g., penicillin, radiocontrast media) are relatively common triggers in middle-aged and elderly adults. Natural rubber latex (NRL) may trigger anaphylaxis in health care settings, where it is found in equipment such as airway masks, endotracheal tubes, blood pressure cuffs, and stethoscope tubing and also in supplies such as disposable gloves, catheters, adhesive tape, tourniquets, and vials with NRL closures. Prompt recognition and treatment are critical in anaphylaxis.
For patients whose history appears to indicate an IgE-mediated response, skin testing to confirm allergy is useful if there is a compelling reason to use penicillin. Penicillin skin testing is performed by three classic methods: prick, intradermal, and patch. Skin testing itself carries a risk of fatal anaphylaxis, and the facility must be prepared to respond should a reaction occur. Studies have shown that among patients who test positive on a penicillin skin test, approximately 2% will react to a cephalosporin.
Patients who are allergic to penicillin and in whom the administration of a penicillin antibiotic is very desirable or even essential can be managed by desensitizing the patient to penicillin. Desensitization is accomplished by administering increasing doses of penicillin over a period of 3 to 5 hours. The mechanism whereby clinical tolerance is achieved is not entirely clear. The most likely hypothesis is that desensitization works by making mast cells unresponsive to the specific antigen. The desensitization procedure should be undertaken in an ICU setting, where continual monitoring is available. Also, the clinician must be at the bedside or readily available.
It has been shown that desensitization is an acceptable, safe approach to therapy in patients who are penicillin allergic but require β-lactams for treatment. Oral desensitization is safer than parenteral desensitization. There are no specific contraindications to desensitization. However, patients who are unable to withstand the consequences of an acute allergic reaction and its management are poor candidates.
A 65-year-old man, status post incision and drainage (I & D) of a severe facial infection, who was previously admitted to the hospital for treatment of an odontogenic infection, complains of the new onset of severe “watery diarrhea.”
The patient was admitted 6 days earlier and was taken to the operating room on that day, where I & D of the right submandibular/medial masticator and submental spaces, with extraction of a grossly carious right mandibular first molar, was performed. He remained intubated for 3 days and was maintained on intravenous clindamycin (900 mg every 8 hours). A previously placed nasogastric tube was also removed. The patient continued to do well and was transferred to the ward from the ICU on the fourth postoperative day, with continuation of the intravenous clindamycin therapy.
On hospital day 6, the patient reported lower abdominal pain and cramping of over 12 hours' duration. He also reported experiencing nausea, malaise, fever, and chills. He had several episodes of profuse, watery diarrhea, which were documented by the nursing staff. There was no evidence of blood in his stool, but he has had minimal oral intake since the symptoms began.
Allergies. The patient has a penicillin allergy (history of rash).
Current medications. He is receiving clindamycin 900 mg intravenously every 8 hours and morphine sulfate 2 mg every 4 hours as needed for pain.
General. The patient is an obese man in mild distress who is resting in bed.
Vital signs. His blood pressure is 110/68 mm Hg, heart rate 118 bpm (tachycardia secondary to elevated temperature and gastrointestinal fluid losses), respirations 18 per minute, and temperature 38.8°C (fever secondary to release of inflammatory mediators in the gastrointestinal tract).
Maxillofacial. With decreasing facial edema, drains in the submandibular and submental spaces are nonproductive; removal is pending.
Abdominal. The abdomen is soft, nontender, and nondistended, with hyperactive bowel sounds in all four quadrants. There is no guarding or rebound tenderness (these would be indicative of peritonitis).
Plain radiographic imaging studies of the abdomen (e.g., kidney-ureter-bladder [KUB]) can be used to assist in the diagnosis of Clostridium difficile –associated diarrhea. Plain radiographs may reveal a dilated colon suggestive of ileus. (Patients with severe disease may develop colonic ileus or toxic dilatation with abdominal pain and distension with minimal or no diarrhea). A diffusely thickened or edematous colonic mucosa is often better visualized on an abdominal computed tomography (CT) scan. Thickening can sometimes be seen on abdominal plain films.
Colonoscopy or sigmoidoscopy is a more invasive diagnostic modality that is reserved for cases in which rapid diagnosis is necessary or stool samples cannot be obtained secondary to ileus. The finding of pseudomembranes is pathognomonic for C. difficile colitis. Because of the increased risk for intestinal perforation, endoscopy should be used sparingly in patients with suspected C. difficile –associated diarrhea.
Because this patient is relatively stable, abdominal imaging and/or endoscopy is not indicated.
A basic metabolic panel (BMP) demonstrated an elevated sodium level (148 mEq/L) and elevated BUN and creatinine (secondary to dehydration). Serial CBCs demonstrated elevation of the white blood cell (WBC) count, from 12,000 to 20,000 cells/µl, with bandemia.
The patient's stool guaiac test was negative for blood. Enzyme-linked immunosorbent assay (ELISA) for C. difficile toxin was positive.
The gold standard test for diagnosis of C. difficile –mediated disease is a cytotoxin assay. Although this test is highly sensitive and specific, it is difficult to perform, and the results are not available for 24 to 48 hours. In addition, the testing facility must be equipped with tissue culture capabilities. ELISA can be used to detect C. difficile toxin (A and/or B) in stool. This test has a sensitivity of 63% to 99% and a specificity of 93% to 100%. ELISA can be quickly performed (2 to 6 hours) and is the laboratory test most frequently used to diagnose C. difficile infection.
The average range for peripheral WBCs in patients with C. difficile –associated diarrhea is 12,000 and 20,000 cells/µl, but occasionally the count is higher. An important indicator of impending fulminant colitis is a sudden rise in peripheral WBCs to 30,000 to 50,000 cells/µl. Because progression to shock can occur even in patients who have had benign symptoms for weeks, early warning signs, such as the leukocytosis, can be invaluable.
Resolving odontogenic infection now complicated by C. difficile– associated diarrhea.
In otherwise healthy adults, the first step is to discontinue the precipitating antibiotic and to administer fluids and electrolytes to maintain hydration. For many patients, antibiotic-associated diarrhea is a mild and self-limited illness that responds to the discontinuation of antibiotics, supportive care, and fluid and electrolyte replacement. Specific pharmacotherapy for C. difficile –associated diarrhea should be initiated once the diagnosis of C. difficile has been confirmed or in highly suggestive cases of severely ill patients ( Box 2-3 ). The use of opiates and antidiarrheal medications has previously been discouraged; however, some studies have shown that evidence supporting this hypothesis is lacking. Additionally, antimotility agents may be beneficial in providing symptomatic relief and reducing environmental contamination with infectious stool.
Discontinue antibiotics.
Initiate supportive therapy. Prophylactic antibiotic therapy should not be given routinely. Once the diagnosis of C. difficile diarrhea has been confirmed and specific therapy is indicated, metronidazole given orally is preferred. If the diagnosis is highly likely and the patient is seriously ill, metronidazole may be given empirically before the diagnosis is established.
Vancomycin given orally is reserved for the following conditions:
The patient has failed therapy with metronidazole.
The causative organism is resistant to metronidazole.
The patient is allergic to or cannot tolerate metronidazole or is being treated with ethanol-containing solutions.
The patient is pregnant.
The patient is a child under 10 years of age.
The patient is critically ill because of C. difficile –associated diarrhea or colitis.
There is evidence suggesting that the diarrhea is caused by Staphylococcus aureus .
For persisting symptoms, first-line therapy consists of metronidazole 500 mg orally three times daily for 10 to 14 days. Vancomycin is also an effective treatment, with a response rate greater than 95%. If the patient is pregnant or does not respond to or cannot tolerate metronidazole, then vancomycin should be initiated at 125 mg orally four times daily for 10 to 14 days. Response to therapy can be assessed by the resolution of fever, usually within the first 2 days. Diarrhea should resolve within 2 to 4 days; however, treatment is continued for 10 to 14 days. Therapeutic failure is not determined until treatment has been given for at least 5 days.
The best treatment is prevention. This includes the judicious use of antibiotics; hand washing between patient contacts (hand washing with soap and water may be more effective than the use of alcohol-based hand sanitizers, because C. difficile spores are resistant to killing by alcohol); rapid detection of C. difficile by immunoassays for toxins A and B; and isolation of patients who have C. difficile –associated diarrhea.
In the current case, the patient was placed on contact precautions. Current guidelines from the Centers for Disease Control and Prevention (CDC) indicate that patients should be placed under contact precautions and in isolation until the diarrhea has resolved. This patient was given a bolus of normal saline (NS) and started on maintenance fluids of ![]() at 110 ml/hr. Clindamycin was discontinued, and the patient was started on metronidazole 500 mg orally three times daily. His diarrhea resolved in 2 days, and he was subsequently discharged. He was given a nonopiate pain medication during his hospital stay and upon discharge. The patient was educated about his diagnosis, and it was recommended that he inform other practitioners of it before initiation of antibiotic therapy.
at 110 ml/hr. Clindamycin was discontinued, and the patient was started on metronidazole 500 mg orally three times daily. His diarrhea resolved in 2 days, and he was subsequently discharged. He was given a nonopiate pain medication during his hospital stay and upon discharge. The patient was educated about his diagnosis, and it was recommended that he inform other practitioners of it before initiation of antibiotic therapy.
Recurrence can develop and is usually due to the germination of persistent C. difficile spores in the colon after treatment or secondary to reinfection by the pathogen. Relapse is reported to occur in 15% to 20% of cases regardless of the initial treatment used. Some conditions identified as potential markers for relapse include previous relapses, chronic renal failure, marked leukocytes, and continued antibiotic use. In patients who have had more than one relapse, the recurrence rate can be as high as 65%; in such cases, avoidance of unnecessary antibiotics is strongly advised. Different agents, regimens, doses, and even unusual forms of therapy, such as fecal enemas, have been tried in these cases, with varying success.
Approximately 3% of patients develop severe C. difficile –associated diarrhea. The mortality rate in these patients ranges from 30% to 85%. Treatment of severe cases must be aggressive, with intravenous metronidazole and oral vancomycin used in combination. If ileus occurs, vancomycin can be administered by nasogastric tube with intermittent clamping, retention enemas, or both. If medical therapy fails or perforation or toxic megacolon develops, surgical intervention with colectomy and ileostomy is indicated but carries a high mortality rate.
Of concern is the fact that recent studies indicate the emergence of a new, more virulent strain of C. difficile that is associated with more severe disease (higher rates of toxic megacolon, leukemoid reaction, shock, need for colectomy, and death).This new strain is commonly designated as NAP1/BI/027 (the designation denotes the following: NAP1—a North American Pulse Field type 1 pattern on gel electrophoresis; BI—a BI pattern on restriction endonuclease analysis; 027—type 27 on ribotyping). Deletion of a gene in this new strain may be responsible for its greater pathogenicity. This deletion is thought to be responsible for production of 16 to 23 times more toxin A and B. The emergence of this virulent strain underscores the importance of judicious use of antibiotics (especially cephalosporins, clindamycin, and fluoroquinolones). Strict infection control measures, including contact isolation and enhanced environmental cleaning, are mandatory. Recent outbreaks of the more virulent strain in hospitals in the United States and Canada, which have been reported in the popular press, have increased public awareness of this disease process.
C. difficile is a gram-positive, spore-forming rod that is responsible for 20% to 30% of antibiotic-related cases of diarrhea. C. difficile infection results in more than 300,000 cases of diarrhea in the United States and is the most common cause of nosocomial diarrhea. The case mortality rate is approximately 1% to 2.5%.
Acquisition of C. difficile occurs primarily in the hospital setting or long-term care facilities, where the organism has been cultured from bed rails, floors, windowsills, and toilets, in addition to the hands of hospital workers who provide care for patients with C. difficile infection. The rate of C. difficile acquisition is estimated to be 13% to 20% in patients with hospital stays up to 2 weeks and 50% in those with hospital stays longer than 4 weeks.
The risk factors for the development of symptomatic C. difficile –associated diarrhea are summarized in Box 2-4 . The most important modifiable risk factor for the development of C. difficile infection is exposure to antimicrobial agents. Even very limited exposure, such as single-dose surgical antibiotic prophylaxis, increases a patient's risk of both C. difficile colonization and symptomatic disease. The initiating event for C. difficile colitis is disruption of colonic flora, with subsequent colonization. Depending on host factors, a carrier state or disease results. The disruption is usually caused by broad-spectrum antibiotics. Clindamycin and broad-spectrum penicillins and cephalosporins are most commonly implicated. C. difficile colitis can occur up to 8 weeks after discontinuation of antibiotics.
Admission to the ICU
Advanced age
Antibiotic therapy
Immunosuppressive therapy
Multiple and severe underlying diseases
Placement of a nasogastric tube
Prolonged hospital stay
Recent surgical procedure
Residing in a nursing home
Sharing a hospital room with a C. difficile –infected patient
Antacid use
C. difficile produces two toxins that are responsible for its pathogenesis, toxin A and B. Both toxins play a role in the pathogenesis of C. difficile –associated diarrhea. Toxin B is approximately 10 times more potent than toxin A. The toxins bind to intestinal receptors, leading to disruption of the cellular skeleton and intracellular junctions. Protein synthesis and cell division are inhibited. Inflammatory mediators attract neutrophils and monocytes, increasing capillary permeability, tissue necrosis, hemorrhage, and edema. As colitis worsens, focal ulcerations occur, and the accumulation of purulent and necrotic debris forms the typical pseudomembranes.
The diagnosis of C. difficile colitis requires a detailed history, including use of any antibiotics over the past 3 months. A detailed description of the type, frequency, and consistency of diarrhea is important. The enzyme immunoassay that detects toxins A and B is the laboratory test most commonly used to diagnose C. difficile –mediated disease.
Fidaxomicin is a macrocyclic antibiotic that is bactericidal against C. difficile. It was approved by the U.S. Food and Drug Administration (FDA) in 2011. Trials have shown that fidaxomicin 200 mg twice daily has clinical cure rates similar to those of vancomycin; also, the recurrence rates with fidaxomicin are lower with non-NAP1 strains, but not with the NAP1 strain. Fidaxomicin may be appropriate in patients with relapsing C- difficile. Parameters for its most appropriate use are still being developed.
Probiotics, a group of agents designed to resist colonization and restore normal flora, have been tried in antibiotic-associated diarrhea. The most promising probiotic agent is Saccharomyces boulardii, a live, nonpathogenic yeast. Some studies have shown that when S. boulardii was given prophylactically to patients receiving antibiotics, it was safe and beneficial in reducing the incidence of C. difficile colitis. Lactobacillus GG, another popular probiotic, has been shown to improve intestinal immunity by increasing IgG and IgA levels at the intestinal mucosal level. However, despite some positive findings, conclusive studies are still lacking to recommend the use of probiotics for routine prevention of antibiotic-associated diarrhea. Although usually considered harmless, both S. boulardii and Lactobacillus therapy are capable of inducing fungemia and bacteremia, respectively.
Other types of diarrhea should be considered and ruled out based on the history and physical examination. These include infectious enteritis or colitis, bacterial gastroenteritis, viral gastroenteritis, amebic dysentery, inflammatory bowel disease (e.g., Crohn's disease), ulcerative colitis, and ischemic colitis. Antibiotic intolerance manifested as diarrhea in which there is no evidence of colitis usually resolves upon antibiotic withdrawal.
A 40-year-old man presents to your office, stating, “My tooth is killing me, and I ran out of my pain meds.”
The patient complains of a 3-day history of exquisite tooth pain. When interviewed, the patient immediately emphasizes that he has tried everything and that only “Percocet” helps with the pain. He states that he is unable to tolerate all nonsteroidal antiinflammatory drugs (NSAIDs) because of gastric upset, and he also states that he has a “very high tolerance” for pain medications because of a history of chronic pain associated with a herniated lumbar disc. The patient is given a prescription for 20 oxycodone/acetaminophen (325/5 mg) tablets, to take as needed, and he is scheduled for surgery the next morning. On the morning of surgery, he calls to cancel his appointment for “financial reasons” and requests more pain medication to last him through the weekend. You call his referring dentist to obtain a more detailed history and find out that this patient has called several times in the past requesting opioid pain medications without compliance with the proposed treatment plans (restoration versus extraction).
The patient has a history of back pain caused by lumbar disc herniation, which resulted from a fall at work (a previous history of chronic pain and narcotic analgesic use would likely indicate tolerance). He also has a history of depression (this may be a consequence of chronic pain, but patients with depression and/or anxiety also are more likely to exhibit dependence or addiction).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here