Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pharmacological management of PDA in the very preterm neonate remains a controversial topic, as the risks and benefits of the pharmacotherapeutic options remain unclear.
NSAIDs (indomethacin and ibuprofen) and acetaminophen are the most common and effective pharmacological agents used for PDA closure.
No major differences in the efficacy between the three agents have been established in comparative effectiveness trials. However, choice of therapy may be guided by their respective adverse effect profiles.
Wide variations in the timing, dosage, and type of pharmacological agent have been reported internationally.
Future research should attempt to tease out the interaction between patient characteristics; the timing of treatment; choice, route, and dosage of the medication; and degree of hemodynamic significance of the PDA.
This chapter reviews the epidemiology and current state of pharmacological management (prophylactic and therapeutic) of the patent ductus arteriosus (PDA) in the very preterm neonate. Until now, the optimal management of PDA has been controversial in the scientific community, with no clear consensus or generally accepted guidelines for management. Most of the dispute stems from several sources: the natural history of PDA, the hemodynamic significance of PDA, especially concerning its impact on end-organ perfusion and its potential long-term consequences on neurodevelopment, variable efficacy of the available treatments, unpredictable side effects of pharmacological and surgical therapy, and the lack of information on patient-important clinical outcomes and long-term neurodevelopmental outcomes associated with treatment. The wide variations in PDA management reported worldwide reflect our poor understanding of these uncertainties. While efforts are underway to expand our current understanding of the condition, clinicians should continue to weigh the risks and benefits of different treatment options when deciding the correct clinical course of action.
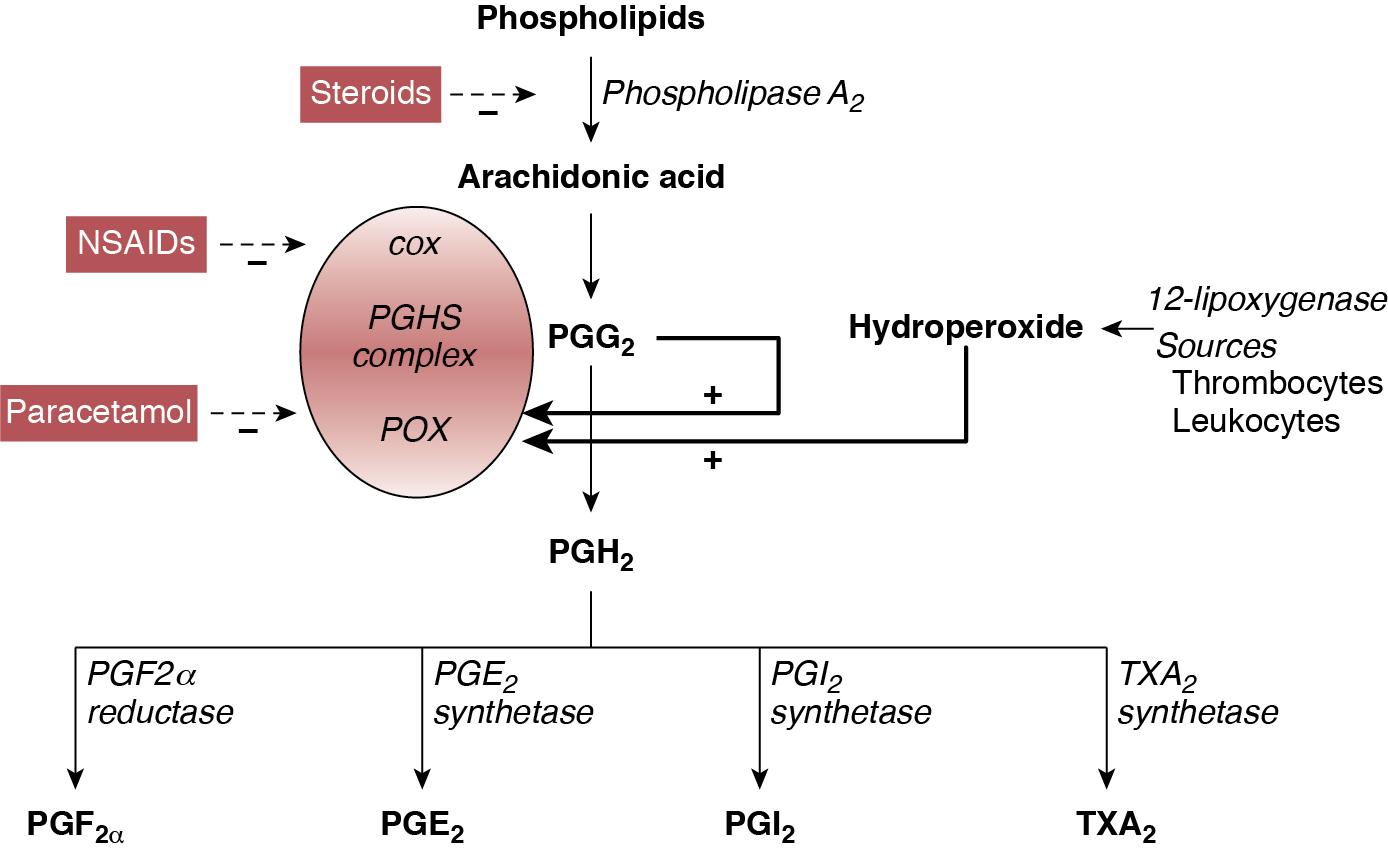
Apart from pharmacological therapy, conservative management and mechanical closure (surgical and percutaneous transcatheter route) of PDA are employed in the management of PDA. Conservative management approaches may range from watchful waiting to non-pharmacological shunt modulation strategies such as increasing the positive end-expiratory pressure. In contrast, pharmaceutical agents for PDA therapy are specifically used to stimulate ductal closure. Pharmacological agents stimulate PDA closure via inhibition of prostaglandin production, which play a significant role in maintaining ductal patency in utero and during the first 1–2 postnatal weeks. These agents are specifically designed to target either cyclooxygenase (COX) or peroxidase (POX), the second and third enzymes in the process of prostaglandin synthesis, respectively. These agents include the COX inhibitors indomethacin and ibuprofen and the POX inhibitor acetaminophen (paracetamol) ( Figure 17.1 ). Adding to the complexity of pharmacological management is the question of when to treat, which may include prophylactic (treat all without assessing), early asymptomatic (detect and treat early before PDA becomes significant during first week after birth), and symptomatic (treat when PDA becomes clinically and hemodynamically significant) treatment.
Variations in the management of PDA in very preterm and very-low-birth-weight infants have been well reported in North America, Europe, Australia, and Asia. These differences have been described for all aspects of treatment, including if, when, and how to treat PDA. Surveys conducted over the past 20 years regarding the practitioner’s approach to treatment of PDA have yielded consistently variable results. In North America a survey of 100 Canadian neonatologists in 1998 revealed a wide variation in practices regarding management of PDA both within and between centers. Fluid restriction and indomethacin were used for treatment by 89% of neonatologists surveyed, while surgery was reserved for patients unresponsive to pharmacological agents or had contraindications. Use of echocardiography for diagnosis of PDA also varied among clinicians. Almost a decade later, 56 fellowship program directors in the United States were surveyed regarding the management of PDA. A quarter of respondents were using prophylactic indomethacin for prevention of interventricular hemorrhage (IVH) and 9% used indomethacin to treat asymptomatic PDA. In cases of persistent PDA three-quarters of respondents indicated use of more than one course of indomethacin, with nearly half reporting usage of two courses and half reporting three courses, if needed. Most respondents were keen on administering indomethacin below 2 weeks of age and used echocardiography criteria to determine PDA treatment.
Hoellering and Cooke also surveyed neonatologists from Australia and New Zealand in 2007 for management of PDA in neonates of 28 weeks’ gestation or less or birth weights less than 1000 g. Expectant (or conservative) management of PDA was favored by 35% of clinicians, while 32% used echocardiographic-targeted prophylaxis, 16% used pre-symptomatic treatment, and 17% used a prophylactic approach; however, nearly half of participating units reported using more than one approach, often depending upon the preference of the individual practitioner. Interestingly, 86% of physicians used long courses of indomethacin and nearly one-quarter of respondents indicated that their approach was not influenced by published literature. This raises important questions regarding the level of effect that individual units or practitioners have on the outcomes of neonates with PDA.
In a survey of 24 European Societies of Neonatology and Perinatology, Guimaraes and colleagues reported data on 45 responses from 19 countries. Most neonatal units used intravenous indomethacin (71%), followed by intravenous ibuprofen (36%) and oral ibuprofen (29%); some units reported use of multiple agents. Approximately half of the centers used a second course and one-quarter of them used a third course of pharmacotherapy in the event of persistent ductus. Nearly all (96%) units treated hemodynamically significant PDA (hsPDA), but a quarter also treated non-hsPDA. Only one neonatal unit preferred surgical ligation as the first-line therapy. In France nearly three-quarters of the 49 neonatal units surveyed between 2007 and 2008 reported the use of both clinical and echocardiography criteria to decide on treatment for PDA, whereas the remaining relied on echocardiography criteria alone. Most units also used echocardiography to diagnose PDA, but the criteria used to describe hsPDA differed. All units used ibuprofen to treat PDA, with most units using a standard course (see Section 3.2 on Ibuprofen below). Between one-half and two-thirds of centers indicated a tendency to use a second course when either the first course failed or if the duct reopened after successful closure. In the event of contraindications to medical treatment or ductal malformation, 39% of units considered surgery as the primary treatment.
Irmesi et al. recently collated information from published randomized trials of PDA management around the world. They identified that treatment with indomethacin and ibuprofen was more prevalent in the United States and Canada, whereas ibuprofen was the most common agent used in Europe. Worldwide variations were further exposed in a recent international survey of investigators from 335 neonatal units in 11 high-income countries. The results indicated that Japan, Sweden, Finland, and the Tuscany region of Italy routinely perform echocardiogram screening for PDA. Treatment rates of pre-symptomatic PDA based on routine echocardiography results alone, regardless of a patient’s clinical status, was in the range of 6–85% (6% of units in Canada; 7% in Illinois;19% in Israel; 50% in Sweden; 40% in Spain; 27% in Switzerland; 50% in Australia and New Zealand; 40% in Finland; 75% in Tuscany, Italy; and 85% in Japan) among those who conduct echocardiography screening.
Apart from the survey data described above, reports of actual practices in the management of PDA have recently been published. A recent cohort study from the iNEO (International Network for Evaluating Outcomes of Neonates) collaboration that included 39,096 infants born between 24 and 28 weeks’ gestation from across 6 countries (139 NICUs) demonstrated a wide variation in PDA treatment practices. While the overall PDA treatment rate was 45% in this cohort (13–77% by NICU), the observed to expected PDA treatment ratio ranged from 0.30 to 2.14. It was further noted that the relationship between the observed to expected PDA treatment ratio and primary composite outcome of death and severe neurological injury followed a U-shaped curve, suggesting that both low and high PDA treatment rates were associated with worse clinical outcomes. In another study Hagadorn and colleagues examined trends in the management of PDA in 19 US children’s hospitals between 2005 and 2014 and linked the data to neonatal outcomes. Approximately three-quarters of infants with PDA were treated with pharmacological management or surgery, with wide variation noted among hospitals. There was a steady decline in the number of neonates treated over the years, with the odds of treatment decreasing by 11% in each year of the study period. The trend of reducing treatment was temporally associated with a decline in mortality; however, bronchopulmonary dysplasia (BPD), periventricular leukomalacia, retinopathy of prematurity (ROP), and acute renal failure increased. In a population-based cohort study Edstedt Bonamy et al. evaluated regional variations and their relationship with outcomes in PDA management across 19 regions in 11 European countries between 2011 and 2012. The proportion of neonates ≤31 weeks’ gestation who received PDA treatment varied from 10% to 39% between units, and it was independent of perinatal characteristics of patients. Variations in PDA treatment rate were not associated with neonatal outcomes.
In Canada conservative management of PDA in neonates between 23 and 32 weeks’ gestation increased from 14 to 38% between 2006 and 2012, while using pharmacotherapy alone and surgical treatment alone decreased from 58 to 49% and 7.1 to 2.5%, respectively, and both pharmacotherapy and surgical ligation dropped from 21 to 10% (all P < 0.01) during the same time period. With an increase in conservative management, there was a reduction in the composite outcome of mortality or major morbidity between 2009 and 2012 compared to 2006 and 2008; however, there remains the possibility of confounding by indication. Slaughter et al. attempted to adjust for residual confounding parameters by incorporating clinician preference-based variation in practice as an instrument in their analyses. They reported that though an infant’s chance of receiving pharmacotherapy increased by 0.84% for each 1% increase in the hospital’s annual pharmacotherapy rate for treatment of PDA, there was no association between pharmacotherapy and mortality and mortality or BPD in neonates of ≤28 weeks’ gestation. This finding suggests that conservative management of PDA may be a rational approach for a subset of preterm infants with PDA. However, more work needs to be done to identify the right patient population who would benefit from treatment of the PDA ( Chapter 19 ).
In a prospective cohort from France Rozé et al. evaluated the role of early screening (before day 3 of postnatal life) in neonates <29 weeks’ gestation. The authors determined that screened infants were more likely to be treated for PDA than unexposed infants (55% vs. 43%; odds ratio [OR] 1.62, 95% confidence interval [CI] 1.32–2.00). Screened neonates were at lower odds of mortality (OR 0.73, 95% CI 0.54–0.98) and pulmonary hemorrhage (OR 0.60, 95% CI 0.38–0.95). However, when instrumental variable analyses using unit preference for early screening were conducted, there was no statistically significant association between early screening and mortality (OR 0.62, 95% CI 0.37–1.04), suggesting that questions about screening, prophylaxis, and treatment could only best be answered in well-designed randomized trials. The variations reported above in both survey designs and in studies comparing the evolution of approaches and their relationship with outcomes indicate that the management of PDA is widely variable within neonatal units and at regional, national, and international levels.
Pharmacological interventions for PDA can be divided into two groups: agents used for symptomatic treatment (i.e. management of pulmonary edema and heart failure) and agents that induce PDA closure. Based on symptoms associated with pulmonary hyperperfusion and heart failure, diuretics such as furosemide have been used to reduce overall fluid overload. Use of furosemide in the context of PDA management remains controversial. Animal studies suggest furosemide may stimulate renal production of prostaglandin E2, thereby contributing to ductal patency. Further, three randomized controlled trials (RCTs) exploring the role of furosemide in indomethacin-treated infants for symptomatic PDA have failed to demonstrate any benefit. In addition, loop diuretics, such as furosemide, are associated with various side effects, including electrolyte imbalance, nephrocalcinosis, and hearing impairment. However, a recent large observational study of 43,576 infants demonstrated that exposure to furosemide for any indication was associated with reduced odds of PDA treatment (adjusted odds ratio = 0.72, 95% CI 0.65–0.79). Therefore controversy remains on the role of furosemide in the management of symptomatic PDA. While the Canadian Pediatric Society suggests considering the use of furosemide for conservative management of symptomatic PDA, further research is required to strongly recommend its use.
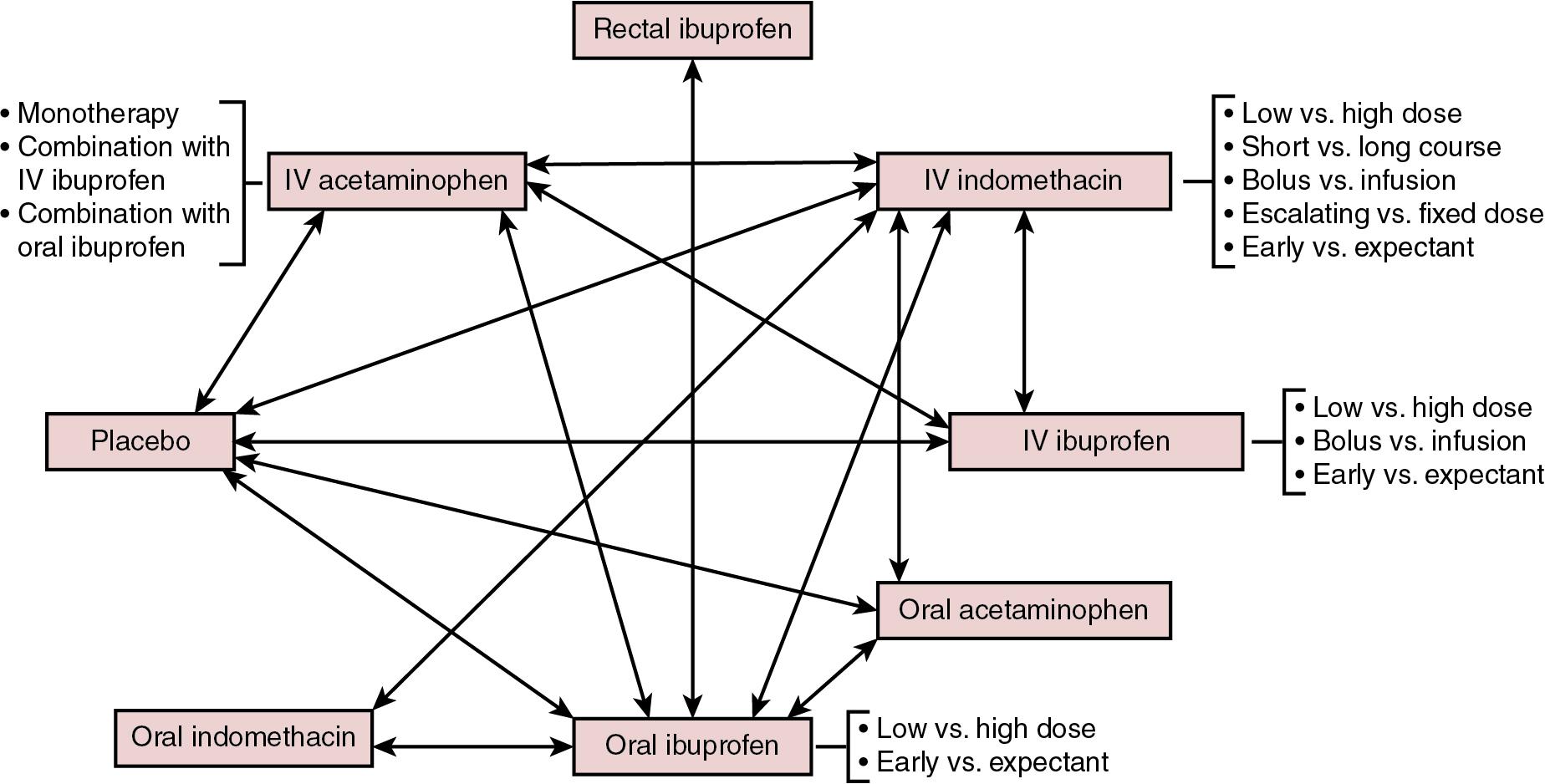
As previously mentioned, the three main pharmacological agents used to induce PDA closure are indomethacin, ibuprofen, and acetaminophen (paracetamol), each of which is described below. More than 80 RCTs have been conducted over the past 40 years exploring different dosages, duration, and routes of administration of these drugs. The complexity of management of PDA is summarized in Figure 17.2 , where strategies that have been tested in randomized trials are delineated.
Indomethacin is a potent and non-selective inhibitor of the COX enzyme and promotes PDA closure by inhibiting the synthesis of prostaglandins, including prostaglandin E2. The half-life of indomethacin is 4–5 hours longer on average in preterm neonates <32 weeks’ gestation compared to those >32 weeks’ gestation (17.2 ± 0.8 vs. 12.5 ± 0.5 hours) and thus prolonged accumulation can occur in very preterm neonates.
Intravenous indomethacin has been used in various dosing regimens. Initial animal studies demonstrated that intravenous indomethacin in doses of 0.2–0.4 mg/kg substantially reduced PDA diameter in fetal lambs. Most RCTs conducted in preterm neonates have used three doses of 0.1–0.2 mg/kg/dose every 12–24 hours apart; however, many modifications of this strategy have been carried out. In one study dose escalation of indomethacin starting from 0.2 mg/kg and increasing to 1 mg/kg in non-responders resulted in a 98.5% PDA closure rate. It should be noted that higher doses are typically associated with increased risk of side effects. In another study a high-dose (0.2–0.5 mg/kg/dose) and low-dose (0.1 mg/kg/dose) regimen of indomethacin were compared in cases of persistent PDA following conventional treatment with the three conventional doses. Although the authors reported no difference in PDA closure rates (55% vs. 48%, respectively), the infants exposed to the higher dosage displayed increased rates of renal compromise and moderate to severe retinopathy.
Though the most common route of administration of indomethacin is intravenous, it has been used orally, rectally, and intra-arterially. Six studies of oral use (ranging between 9 and 74 neonates) have reported PDA closure rates of 66–67%. Intra-arterial use in 26 neonates was successful in 76% of cases, whereas a 66% closure rate was observed in a small group of neonates treated either orally ( n = 1) or rectally ( n = 5). Both of these routes have not been widely used due to concerns of damage to mucosal layers from local direct effects of indomethacin, as well as the effects on prostaglandin synthesis inhibition affecting mucosal integrity of the gastrointestinal tract, especially the ileum.
The usual duration for one course of indomethacin treatment is 48–72 hours. For some neonates, ductal closure can be a lengthy remodeling process and may need prolonged treatment. Five randomized trials compared PDA closure rates in neonates treated with a prolonged course of indomethacin versus routine treatment using a three-dose course and reported no difference in PDA closure rates but identified that an increased risk of necrotizing enterocolitis (NEC) was associated with longer indomethacin exposure (relative risk [RR] 1.87, 95% CI 1.07–3.27). Some practitioners advocate for an echocardiogram to be performed after the last dose (third dose of a routine course) and to continue treatment until the duct closes. However, based on concerns of adverse effects, a prolonged course is not recommended by most.
Typically, indomethacin is administered as a slow infusion to avoid rapidly rising concentrations characteristic of bolus infusions. The potential impact of indomethacin concentration on cerebral, renal, and splanchnic blood flow has led to recommendations for infusion to be administered over a 20- to 30-minute period. Studies have reported reduced blood flow, and similar or higher closure rates (81% vs. 43%; P = 0.03), with bolus infusion compared with continuous infusion. However, as suggested by a previous systematic review, the evidence may be too limited to draw a conclusion regarding the superiority of either approach. Pharmacokinetic data from a small series of neonates suggest that in neonates who had lower plasma levels, faster clearance, and shorter half-life, the drug was less effective. In addition, there was a 20-fold variation in the plasma levels 24 hours after indomethacin administration among neonates.
Indomethacin is a potent medication for PDA closure, with historically proven rates of ductal closure. Closure rates following an initial course vary from 48 to 98.5% depending on dose, duration, and method of administration. , , However, it is important to keep in mind that the majority of the placebo-controlled RCTs on indomethacin efficacy were conducted in the 1980s and 1990s and included more mature preterm infants with high spontaneous PDA closure rates. Therefore these numbers stated above may not be reflective of the PDA closure efficacy of indomethacin in extremely-low-gestational-age infants. Many times a repeat course is provided when either a PDA fails to close following the first course or reopens after initial closure. The reported success rates with a second course are approximately 40–50%. Very rarely is a third course of indomethacin attempted, as exposure to more than two courses has been associated with periventricular leukomalacia. It is unclear what pathological mechanisms play a role in this association but the indomethacin-induced prolonged decrease in cerebral blood flow might contribute to this phenomenon. The efficacy of indomethacin declines with decreasing gestational age and increasing postnatal age. Data regarding efficacy of indomethacin beyond 2 months of age suggest its ineffectiveness at this age. Similarly, it is unclear whether indomethacin is as useful in the treatment of periviable neonates of 22 and 23 weeks’ gestation as the efficacy decreases with decreasing gestational age.
Indomethacin is used for prophylactic, early asymptomatic, and symptomatic treatment. Prophylactic use is employed in the first 24 hours after birth, irrespective of the presence of a PDA. Since most (85%) PDAs in VLBW infants have been shown to close spontaneously before hospital discharge, this strategy predisposes many neonates to overtreatment. The underlying basis of prophylactic administration is to reduce the incidence and/or severity of peri/intraventricular hemorrhage (P/IVH) through modulation of the PDA shunt effect in addition to lowering cerebral perfusion via mechanisms independent of its effect on prostaglandin synthesis. A systematic review and meta-analysis of 19 studies identified a significant reduction in the incidence of symptomatic PDA (RR 0.44; 95% CI 0.38–0.50) and need for surgical ligation of a PDA (RR 0.51; 95% CI 0.37–0.71) with prophylactic use compared to placebo. Indomethacin use was also associated with reduced rates of any P/IVH (RR 0.88; 95% CI 0.80–0.98) and severe P/IVH (RR 0.66; 95% CI 0.53–0.82). However, there was no improvement in neurodevelopmental outcomes during early childhood despite a reduction in the severity of P/IVH. , This has created diverse opinions and practices regarding the use of prophylactic indomethacin in routine clinical settings. Certain subgroups, such as male sex, lack of antenatal corticosteroids, and extremely-low-gestational-age neonates, especially those born <27 weeks’ gestation, have been identified as potential candidates for prophylactic indomethacin. In addition, units with a higher underlying rate of P/IVH might use this approach as, in this situation; the benefits may outweigh the risks of prophylactic indomethacin administration.
Using indomethacin during the “early asymptomatic phase” significantly lowers the number of patients exposed to the drug compared to prophylactic measures described above, yet several patients who would have had a spontaneous closure will still be exposed. A systematic review of three RCTs reported a reduction in symptomatic PDA (RR 0.36, 95% CI 0.19–0.68) and duration of oxygen therapy following indomethacin use in the early asymptomatic phase; however, there was no difference in any other neonatal complications and no assessment of long-term neurodevelopmental outcomes. A recent RCT compared treatment with indomethacin and a placebo within the first 12 hours after delivery in infants who positively screened for a “large” PDA, irrespective of their effects on hemodynamic status. The trial was stopped prematurely due to unavailability of indomethacin. There was a significant reduction in pulmonary hemorrhage (2% vs. 21%), early P/IVH (4.5% vs. 12.5%), and need for later medical treatment of PDA (20% vs. 40%) with early asymptomatic treatment. However, there was no difference in the primary outcome of death or abnormal head ultrasound findings.
Another approach is to treat PDA when it becomes symptomatic or hemodynamically significant. This method prevents unnecessary exposure to indomethacin as far as the PDA is concerned. Early treatment is used to describe the administration of the medication within the first 5–7 postnatal days, while late treatment is considered to occur in the second week after birth. A meta-analysis of four trials conducted between 1980 and 1990 revealed a significant reduction in BPD (OR 0.39; 95% CI 0.21–0.76) and duration of mechanical ventilation in infants receiving early versus late symptomatic treatment. Furthermore, higher PDA closure rates by day 6 (73% vs. 44%; P < 0.001) and day 9 (91% vs. 78%; P <0.05) in early versus late treatment groups were shown in a randomized controlled trial. Infants treated early were more susceptible to side effects, such as lower urine output and higher creatinine levels, and experienced more severe adverse events. A recent systematic review of early treatment versus expectant management of the hemodynamically significant PDA showed that very early (defined as treatment initiated <3 days of age) or early treatment (defined as treatment initiated <7 days) with indomethacin was not associated with improvement in any clinically meaningful outcomes such as death, BPD, NEC, or need for PDA ligation, but it was associated with increased exposure to NSAIDs. Of note, a before-after observational study also showed that delayed initiation of treatment is feasible and may reduce exposure to pharmacologic agents; however, this approach may result in an increase in the combined outcome of death or BPD.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here