Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Multimodal information is often required for diagnostic or research purposes as each imaging technique provides complementary data, for example about anatomy, physiology, or metabolism. Positron emission tomography (PET) measures the distribution and concentration of certain molecules in the body, magnetic resonance imaging (MRI) reflects proton density and tissue relaxation times, and computerized tomography (CT) maps the electron densities of tissues.
Imaging hardware has constantly evolved to adapt to ever-changing needs. Technological advances have led to faster acquisition, higher spatial resolution, and better image contrast. In parallel, efforts have been made to develop hybrid devices that acquire complementary information from two combined different imaging modalities in the same imaging session, to address their technical limitations, maximize patient convenience, and minimize spatial distortions between the two datasets.
Initially, the need for accurately aligning molecular and anatomical images led to the development of combined PET/CT scanners ( ). While hybrid PET/CT scanners have rapidly become well established clinically ( ), the development and adoption of hybrid PET/MRI has been considerably slower due to technological and methodological challenges ( ; ). Indeed, the possibility of combining PET and MRI was first mentioned in 1991 ( ), simultaneous data acquisition was first demonstrated in vivo in 1997 ( ), in humans a decade later ( ), and clinical systems became commercially available in 2010.
In this chapter, the state-of-the-art integrated PET/MRI scanners will first be described. Next, the traditional methods for addressing the most important methodological challenge, attenuation correction, will be presented. Additionally, opportunities enabled by the simultaneous acquisition such as MR-assisted PET motion correction and image enhancement will be discussed. Finally, the deep learning approaches, recently introduced to further enhance the traditional methods, will be presented.
Several designs have been considered for integrating PET and MRI ( ; ). The first MR-compatible PET systems used photomultiplier tubes (PMTs) coupled to scintillator crystals to convert the annihilation photons into an electrical signal. However, as PMTs are very sensitive to magnetic fields, they had to reside outside the magnet and long optical fibers were used to couple them to the scintillator crystals placed inside the bore. A prototype small animal scanner using this concept was used to simultaneously acquire, for the first time, PET images and 31 P N spectra ( ). Although several improvements were subsequently proposed, the PMT-based approaches had limited performance and were exclusively used for proof-of-concept imaging studies in small animals ( ). In fact, the first PMT-based human whole-body PET/MRI scanner, the Philips TF Ingenuity (Philips Healthcare, Amsterdam, Netherlands), allowed only sequential imaging. For this purpose, separate minimally-modified PET and MRI scanners were placed in the same room and shared a revolving bed but maintained enough distance to minimize electromagnetic interference. Data acquired from each modality independently were fused using software. The first two Philips TF Ingenuity systems were installed at Mount Sinai Medical Center in New York, USA, and at Geneva University Hospital in Switzerland, in 2010 ( ).
The development of solid-state semiconductor-based photo sensors, called avalanche photodiodes (APDs), that preserve the light sensitivity of PMTs and are insensitive to magnetic fields ( ), allowed PET detectors to be positioned in the bore of the MRI scanner. This enabled simultaneous data acquisition using a practical approach that would eventually be scaled up to humans.
Several APD-based PET/MRI inserts for high-field small animal MRI systems were first developed ( ; ; ). In parallel with these advancements in the preclinical arena, and with the end goal of developing a fully integrated PET/MRI scanner, Siemens Healthineers (Erlangen, Germany) introduced a 3T MR-compatible PET insert for human brain imaging, called BrainPET ( ). The BrainPET prototype had an internal diameter of 36 cm and an axial field-of-view of ∼19 cm which allowed the imaging of the whole human brain in one bed position. Two MRI head radiofrequency coils (an outer birdcage transmit/receive coil and an inner 8-channel receive array) designed to minimally attenuate 511 keV photons were positioned inside the BrainPET. Only four BrainPET prototypes were installed around the world between 2007 and 2010 at University of Tübingen (Tübingen, Germany), Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital (Charlestown, MA. USA), Forschungszentrum Jülich (Jülich, Germany) and Emory University (Atlanta, GA, USA).
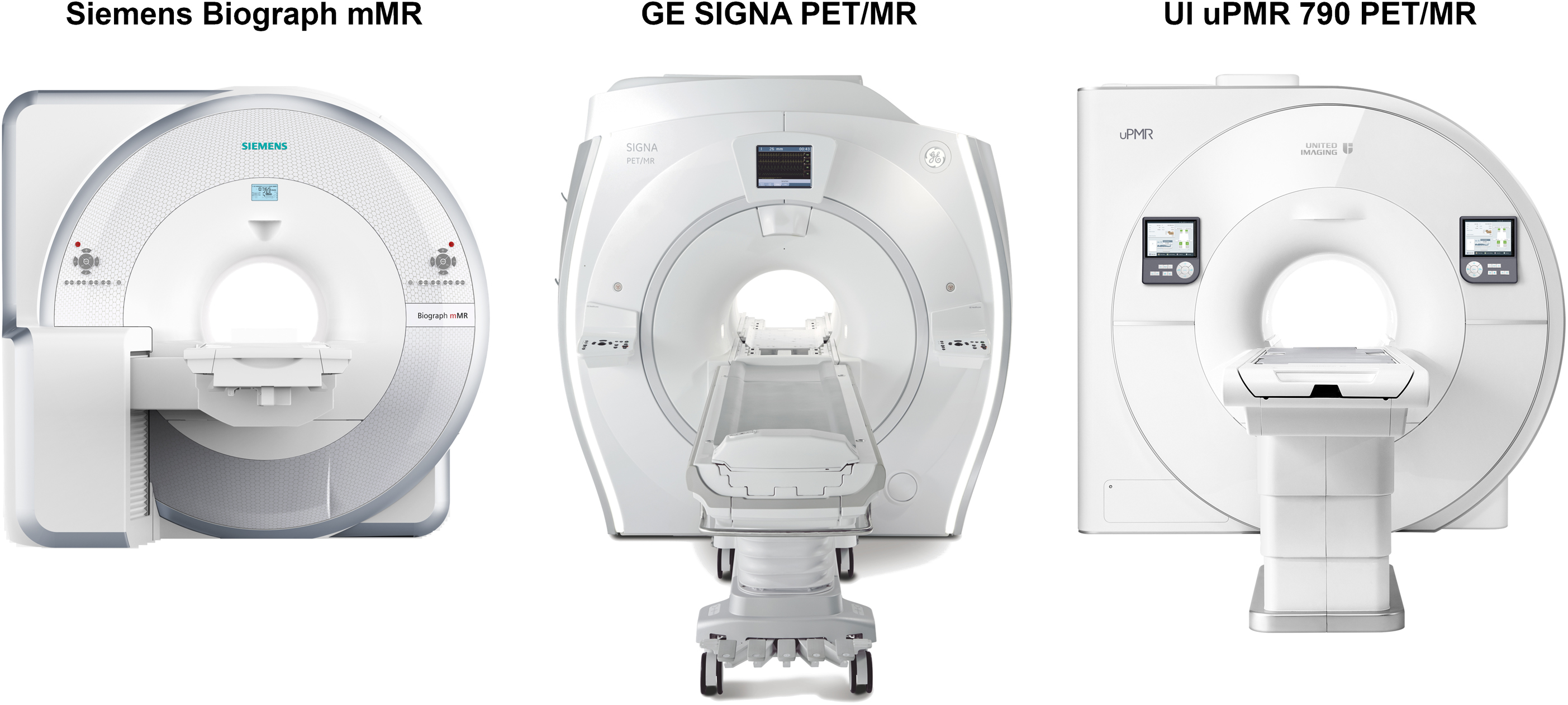
Siemens also introduced the first fully integrated whole-body PET/MRI system, called Biograph mMR. In this scanner, the APD-based PET detectors were positioned between the gradient and the radiofrequency coils and the data acquisition software was also fully integrated. The PET detector block consists of an 8 × 8 array of 4 × 4 × 20 mm 3 LSO crystals readout by an array of 3 × 3 APDs. These blocks are arranged in eight rings with 56 detectors each, providing a transverse field-of-view of 59.4 cm and an axial field-of-view of 25.8 cm. A water-based cooling system was also integrated to ensure optimal PET performance even while running the most demanding MRI sequences. The MRI system is based on the 70 cm diameter bore Siemens Magnetom 3T Verio MRI scanner. In terms of performance, the PET component of the mMR was similar to that of the PET/CT scanners commercially available at that time (e.g., Siemens mCT). Its average spatial resolution at 10 mm radius from the center of the field-of-view is 4.3 mm full width at half maximum (FWHM), peak noise-equivalent count rate is 184 kcps at 23.1 kBq/mL, scatter fraction is 37.9%, while its sensitivity is 15 cps/kBq at the center of the field-of-view ( ). The Siemens Biograph mMR system received the CE mark and FDA approval in 2011. The first system was installed at the Technical University Munich (Munich, Germany) in 2010 and the second at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital (Charlestown, MA, USA) in 2011.
Although APDs allowed fully integrated PET/MRI scanners for human imaging to be developed, their performance is inferior to that of PMTs, particularly in terms of timing resolution. Therefore, the Biograph mMR does not have time-of-flight (TOF) capability, which refers to the ability to measure the difference in the arrival times of the two photons very accurately (<1 ns) to estimate the position of the positron-electron annihilation along the line-of-response. Including the TOF information in the image reconstruction reduces the uncertainty in the localization of the site of annihilation from the whole line-of-response to a distribution around the true site with a Gaussian probability determined by the FWHM timing resolution. TOF imaging requires specialized reconstruction algorithms but offers improved signal-to-noise ratio in the resulting images.
Around the time the Biograph mMR was developed, a new type of photon detectors, called Geiger-mode APD (G-APD, also commonly known as silicon photomultipliers, SiPMs) were successfully tested for PET applications ( ; ). They have performance characteristics comparable to PMTs, including excellent timing performance and are not affected by magnetic fields. Similar to the early developments of PMT- and APD-based systems, several SiPM-based prototypes were initially developed for small animal imaging ( ). Eventually, General Electric Healthcare (Waukesha, WI, USA) introduced the Signa TOF PET/MRI, the first TOF whole-body integrated PET/MRI scanner. The PET detector gantry has transverse and axial fields-of-view of 60 and 25 cm, respectively. It was designed to fit between the body RF coil and the MRI gradient set of the GE Discovery 750w 3T MRI scanner and consists of five rings of 112 detector blocks (LYSO coupled to arrays of SiPMs). The PET component of the Signa TOF PET/MRI has radial, tangential, and axial spatial resolutions of 4.4, 4.1, and 5.3 mm FWHM, respectively, a peak noise-equivalent count rate of 218 kcps at 17.8 kBq/mL, scatter fraction is 43.6%, a sensitivity of 23.3 cps/kBq at the center of the field-of-view, while offering a timing resolution of <400 ps ( ). The GE Signa TOF PET/MRI system also received 510 K clearance and CE mark in 2014, with the first installations at Stanford University (Palo Alto, CA, USA), University of California at San Francisco (San Francisco, CA, USA), and University of Zurich (Zurich, Switzerland) in 2014.
Around 2017, United Imaging Healthcare (Shanghai, China) announced their combined uPMR 790 HD TOF PET/MRI system that integrates SiPM-based detectors with TOF capabilities into a 3T MR scanner. The PET detector gantry has transverse and axial fields-of-view of 60 and 32 cm, respectively. The PET detector system was designed to be installed between the gradient coil and radiofrequency body coil of the uMR 780 and the complete PET ring consists of 20 modules, each of them having 5 × 14 blocks with 14 blocks along the axial direction; individual blocks contain four SiPM detector channels coupled with a 7 × 8 array of 15.5 × 2.76 × 2.76 mm 3 LYSO crystals. The PET component of the uPMR 790 HD TOF PET/MRI has radial, tangential, and axial spatial resolutions of 2.72, 2.86, and 2.81 mm FWHM, respectively, a peak noise-equivalent count rate of 129.2 kcps at 14.7 kBq/mL, a scatter fraction of 37.9%, a sensitivity of 15.9 cps/kBq at the center of the field-of-view, and a timing resolution of 474 ps ( ). The uPMR 790 HD TOF PET/MRI scanner received FDA clearance in 2019.
To summarize, Siemens, General Electric, and United Imaging are the three equipment manufacturers that currently offer fully integrated whole-body PET/MRI systems capable of acquiring data simultaneously ( Fig. 1.1 ). Table 1.1 summarizes the main technical and performance characteristics of these systems.
| Biograph mMR | Signa TOF PET/MR | uPMR 790 HD TOF PET/MR | |
|---|---|---|---|
| Vendor | Siemens healthineers | General electric healthcare | United imaging healthcare |
| Original MR scanner | Magnetom 3T Verio | Discovery MR750w 3T | uMR 780 3T |
| FDA clearance | 2011 | 2014 | 2019 |
| Spatial resolution (radial/tangential/axial @ FWHM) | 4.3/4.3/4.3 mm | 4.4/4.4/5.3 mm | 2.72/2.86/2.81 mm |
| Peak noise-equivalent count rate (NECR) | 184 kcps | 218 kcps | 129.2 kcps |
| Scatter fraction | 37.9% | 43.6% | 37.9% |
| Sensitivity | 15.0 cps/kBq | 23.3 cps/kBq | 15.9 cps/kBq |
| TOF capability | No | Yes | Yes |
| Timing resolution | 2.93 ns | <400 ps | 474 ps |
Annihilation photons have a high probability of interacting with the subject before reaching the PET detectors. Consequently, the number of detected photons is reduced or “attenuated” in a particular line-of-response. This leads to underestimation of the radiopharmaceutical concentration in structures located closer to the center of the body and to image artifacts. Relatedly, the annihilation photons can undergo Compton scattering but still reach the PET detectors. These scattered photons are assigned to different lines-of-response than the one passing through the point of positron annihilation, leading to loss of contrast ( ; ; ; ; ). Precise corrections for both physical phenomena are required to produce quantitative images that reflect the true distribution of the radiopharmaceutical and to reduce artifacts. The photon attenuation along each line-of-response can be estimated if the properties of the object are known ( ). However, this is challenging in integrated PET/MRI systems because no direct relation exists between tissue linear attenuation coefficients and MRI signal intensities ( ).
Most of the early developments focused on solving this issue in the head and can be broadly classified in segmentation and atlas-based methods ( ; ). The former approaches were used to perform tissue estimation and classification into homogeneous classes ( ; ). The latter were used to generate detailed pseudo-CT maps (i.e., synthetic maps comparable to those obtained from CT). A variety of approaches have been proposed for this purpose such as image registration and pattern recognition techniques to match MRI and CT data ( ), atlas-based methods relying on nonrigid registration of a parametric atlas ( ), as well as enhanced methods based on nonrigid registration of a nonparametric multi-atlas followed by a label fusion step depending on patch similarity measures ( ; ; ).
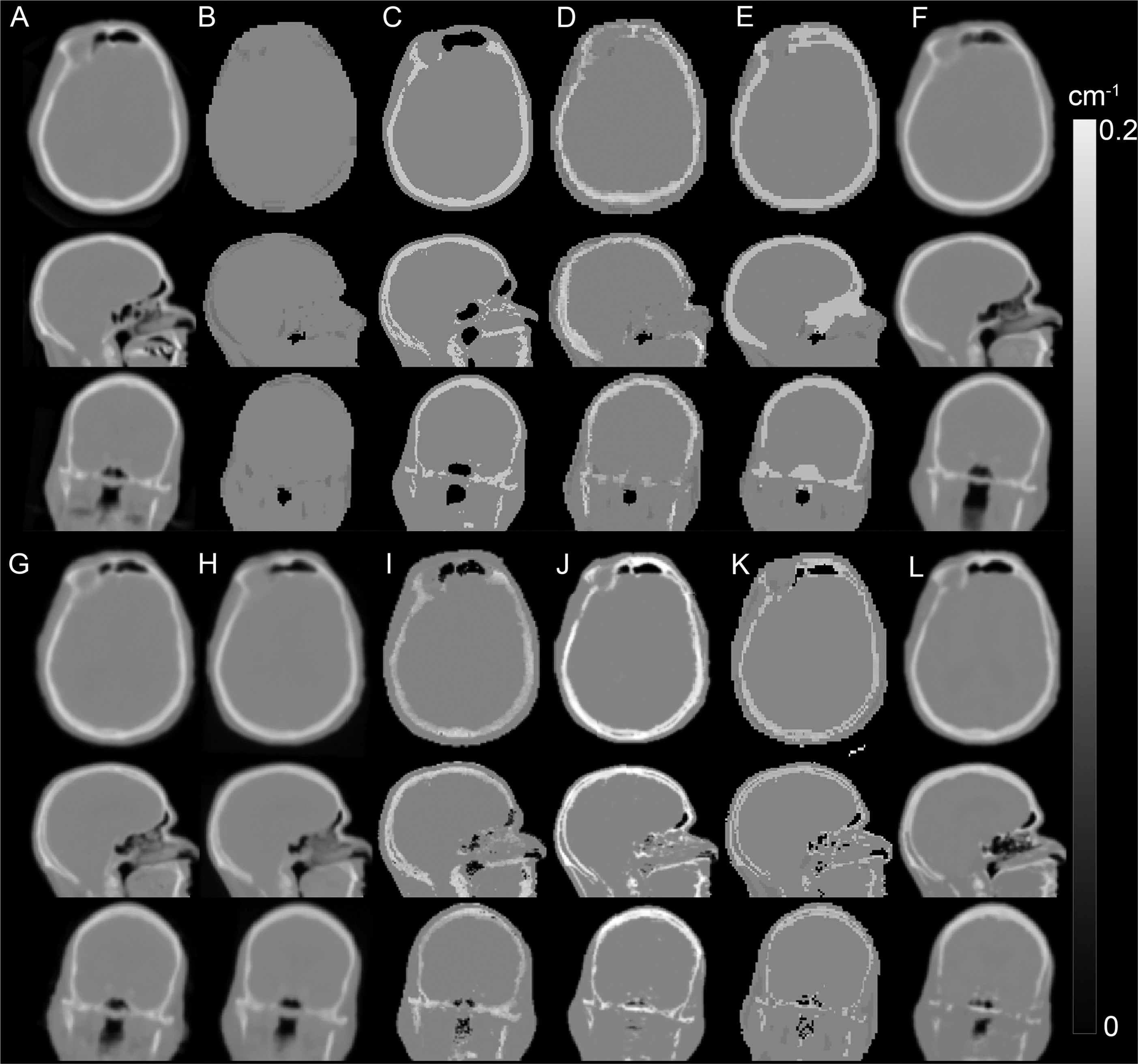
Eleven head attenuation map generation methods were evaluated in a multi-center setting using the data obtained from 337 brain PET/MRI and same day low dose CT studies. Globally, all the methods tested were on average within 5% of the CT-based reference, although there were differences in terms of robustness and presence of outliers. Four of the methods showed regional average errors in PET estimates within ±3% compared to those obtained using the reference method. The conclusion of the authors was that “the challenge of improving the accuracy of MR-[attenuation correction] in adult brains with normal anatomy has been solved to a quantitatively acceptable degree, which is smaller than the quantification reproducibility in PET imaging” ( Fig. 1.2 ) ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here