Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the United States, it is estimated that annually six out of every 1 million people undergo surgery for peripheral nerve tumors. The neurosurgeon is often consulted to diagnose and manage tumors involving the peripheral nerves of the extremities. Intraneural tumors arise from components of the nerve sheath (Schwann cells, perineural cells, fibroblasts), neurons, or invading metastases and cause expansion of the nerve. Extraneural tumors arise from adjacent structures and can secondarily compress or infiltrate the nerve. The majority of tumors involving the peripheral nervous system are benign tumors of the nerve sheath (schwannoma or neurofibroma). Tumors can arise sporadically or be associated with genetic syndromes that produce multiple tumors such as neurofibromatosis or schwannomatosis.
This chapter focuses on the diagnosis and management of peripheral nerve tumors in the extremities. Special emphasis is placed on the management of schwannomas, neurofibromas, and malignant peripheral nerve sheath tumors (MPNSTs).
Patients with a peripheral nerve tumor usually present with a visible or palpable mass (may be symptomatic or asymptomatic), sensory alterations, pain, weakness, or autonomic dysfunction, or the tumor may be an incidental finding ( Table 190.1 ). Many peripheral nerve tumors are first noticed as asymptomatic lumps. There may be swelling or a history of swelling around the lump. Sensory alterations might consist of anesthesia, hypoesthesia, hyperesthesia, paresthesias, or dysesthesia in the distribution of the affected nerve. Stimulation of the skin with innocuous mechanical or thermal stimuli might elicit pain (allodynia) or patients might have a heightened response to painful stimuli (hyperalgesia). The area surrounding the tumor might spontaneously ache or be tender to palpation. Palpation of the mass might elicit paresthesias or dysesthias. Rarely, involvement by the tumor of a motor or mixed nerve might produce true weakness or, more commonly, subtle functional impairment with fine motor tasks. Autonomic dysfunction can manifest as alterations in the coloration, texture, or temperature of the skin or in the amount of sweat. On occasion, peripheral nerve tumors cause complex regional pain syndrome.
| Visible or palpable mass |
| Pain |
| Altered sensation (anesthesia, hypoesthesia, hyperesthesia, paresthesia, or dysesthesia) |
| Weakness |
| Autonomic dysfunction (sweating, discoloration, edema, temperature changes) |
| Incidental finding on imaging studies |
History taking and physical examination are the cornerstones of diagnosis of peripheral nerve tumors. Asymptomatic masses may be present for a long time before a patient presents for evaluation. It is essential to ascertain how long the patient has been aware of the mass and whether it has changed in size or appearance over time. Rapid increases in size or the development of swelling can indicate intrinsic changes in the tumor due to cyst formation, hemorrhage, or malignant transformation. For symptomatic masses, it is important to ascertain the timing of development of symptoms and their progression. Rapid increases in the severity of symptoms (including pain), the size of the tumor, or the development of new symptoms warrant further investigation.
A thorough medical history should be obtained to assess for the presence of other medical conditions that can cause neurologic symptoms (e.g., diabetes mellitus, multiple sclerosis, thyroid disorders). Current and past medications and supplements should be evaluated because some can cause peripheral neuropathy. A full oncologic history should include past cancer diagnoses, tumor resections, chemotherapeutics, and radiation therapy. On occasion, solid tumors metastasize from other organs to peripheral nerves and via perineural spread can reach distant locations from the extremities to the central nervous system (CNS). Blood-based malignancies, such as lymphoma, can infiltrate nerves leading to symptomatic neuropathy and rapid spread. One should inquire about local trauma and secondary injury to any areas of decreased sensation. A family history should be obtained to identify relatives with a history of tumors in brain, spinal cord, or peripheral nervous system or the diagnosis of neurofibromatosis or other syndromes associated with the nervous system tumors (e.g., Weber, Proteus). Occasionally, stigmata of neurofibromatosis type 1 (NF1) are evident with informal observation of relatives accompanying the patient to the clinic. Other times, a more thorough examination of family members is indicated.
A thorough physical examination should be performed on all patients with peripheral nerve tumors. Patients should be examined for diagnostic signs of NF1 ( Table 190.2 ). The neurologic examination includes testing the cranial nerves as well as the motor and sensory function and deep tendon reflexes in all four extremities. A systematic and reproducible examination permits detection of subtle clinical deficits due to the tumor, pathology at higher levels of the neuraxis, or previously undetected tumors elsewhere in the body. Changes in motor function are a late finding; often examination detects only subtle sensory changes or no deficits at all. Discoloration or atrophy of the skin, abnormal sweating, temperature changes, nail bed atrophy, or distal swelling may be findings of autonomic dysfunction.
Two or more of the following:
|
Examination of the mass should include documentation of its size, location, mobility, consistency, relation to adjacent nerves, blood vessels, joints, and muscles. The overlying skin should be evaluated for necrosis, erythema, or changes in pigmentation or hair growth. Palpation of the mass might elicit paresthesias or dysesthesias in the distribution of the affected nerve(s). The affected extremity should be assessed for range of motion, edema, and muscle bulk. Changes in the size, shape, or location of the tumor with changes in joint position should be noted. Benign masses tend to be mobile in a side-to-side direction (but not in an up and down direction); malignant ones tend to immobile.
Historically, the diagnosis of peripheral nerve tumors was based on history, physical examination, and electrophysiologic studies. Although soft tissue masses are not visible on plain film radiographs, these images can show secondary skeletal changes due to tumor mass effect or calcifications intrinsic to the tumor. We believe that all nerve tumors should be imaged. High-resolution ultrasound is being used increasingly. It is relatively inexpensive and is a readily available technology. Computed tomography (CT) also can demonstrate secondary skeletal changes and intrinsic calcifications and offers improved resolution of soft tissues. CT is an imaging modality frequently used for patients with contraindications to magnetic resonance imaging (MRI). Since the 1980s, MRI has emerged as the “gold standard” for imaging peripheral nerve tumors. MRI permits detailed visualization of soft tissue at high resolution and can demonstrate the presence of a mass, the anatomy of involved peripheral nerves, and their relationships to adjacent structures such as major blood vessels and joints. The advent of high-resolution magnetic resonance neurography (MRN) combined with the expansion of the number of neuroradiologists experienced with performing and interpreting these studies has significantly affected the field since the turn of the 21st century. MRN offers improved contrast and resolution that facilitates determining the precise location, size, shape, and resectability of peripheral nerve masses.
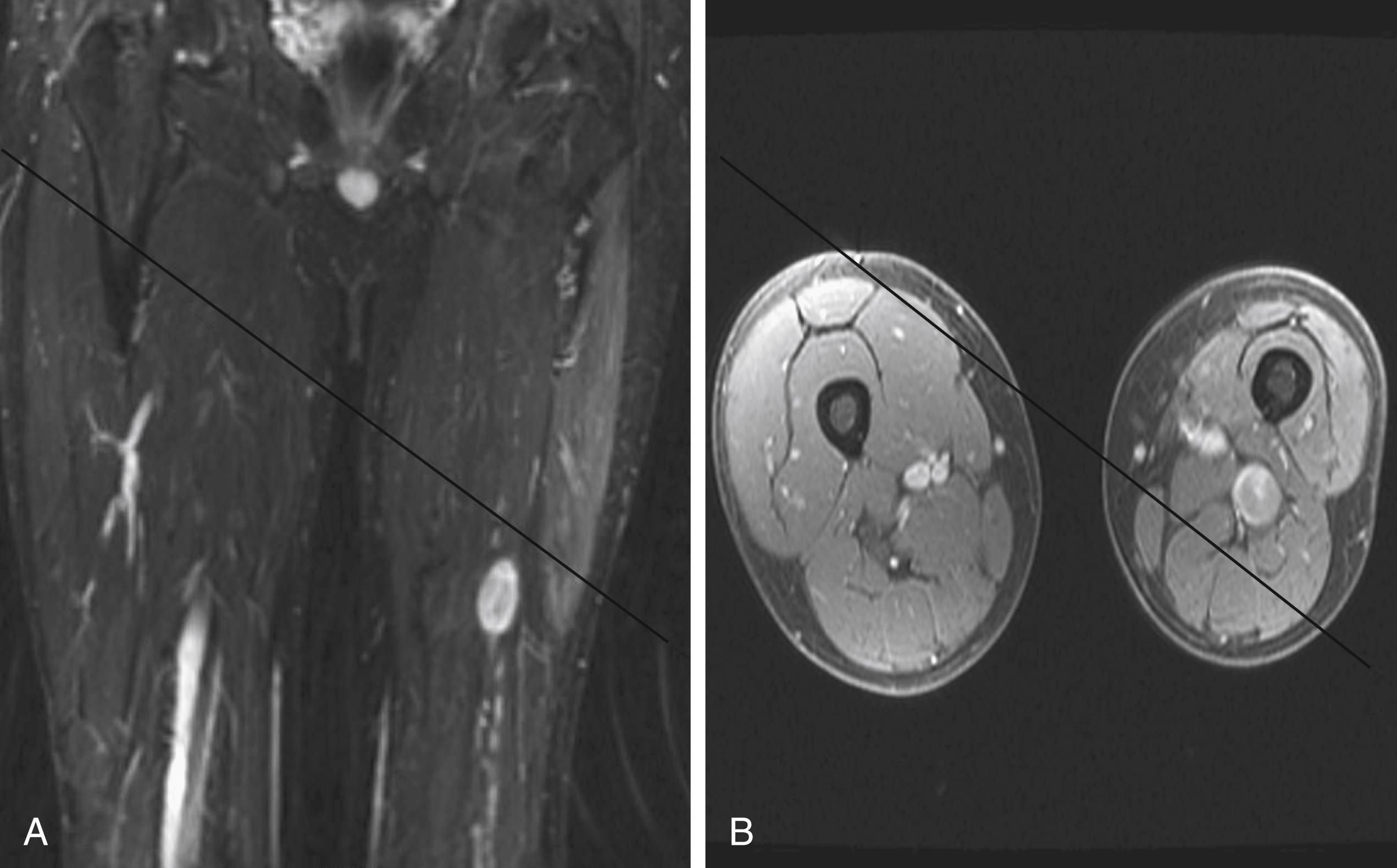
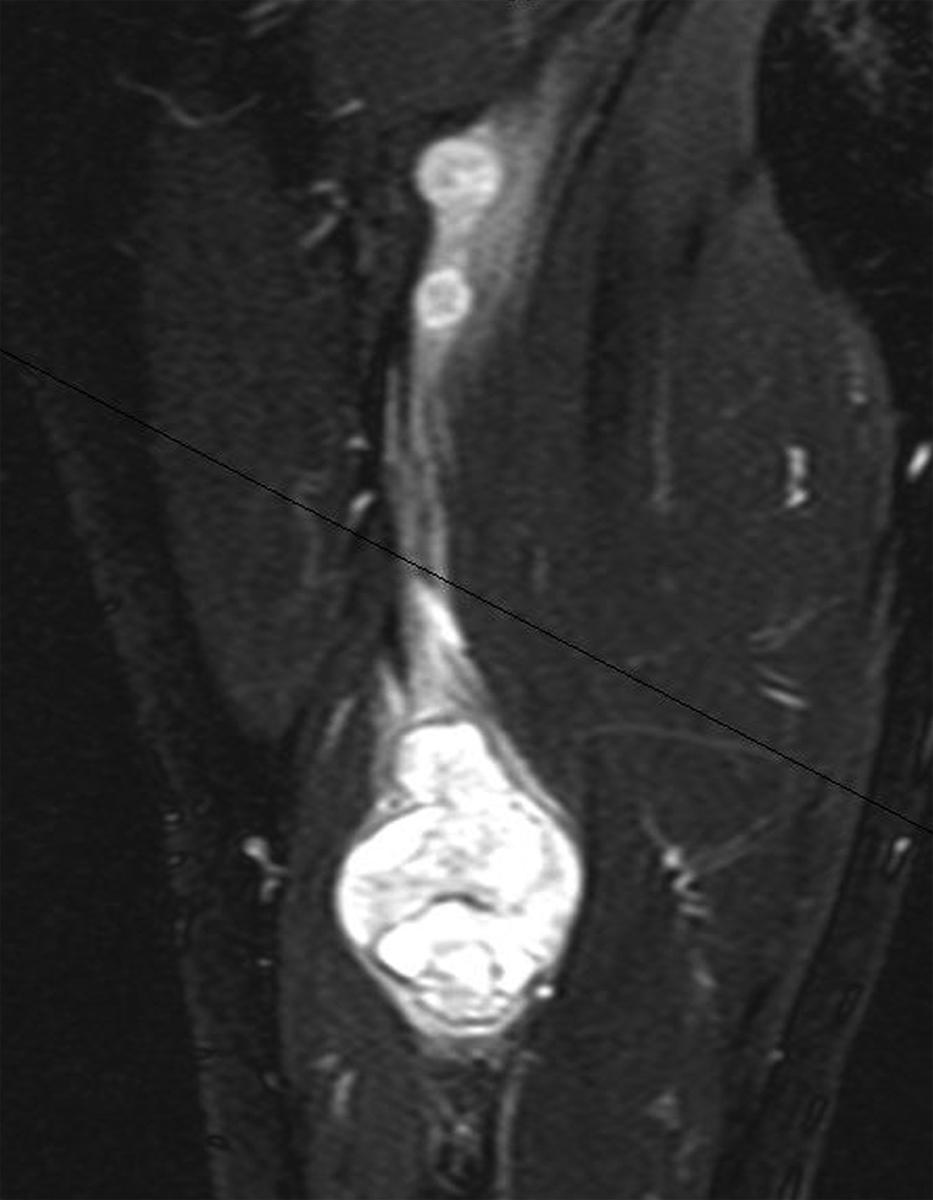
A combination of T1, T2, short tau inversion recovery, and gadolinium-enhanced scans provide clues regarding the nature of the tumor and can help to distinguish benign and malignant lesions. MRI provides crucial information for preoperative planning and aids in determining the specific pathology of the tumor. Further information can be obtained regarding cellular composition and areas of hemorrhage or cysts. For example, benign nerve sheath tumors are often isointense or slightly hyperintense on T1, hyperintense on T2, and heterogeneously enhancing ( Figs. 190.1 and 190.2 ). The majority of neurofibromas, and some schwannomas, display increased central and decreased peripheral T1 signal intensity (the “target sign”) and an opposite pattern of increased peripheral and decreased central T2 signal intensity. Patients with NF1, NF2, or schwannomatosis often have multiple tumors throughout their bodies and can have multiple discrete nodules on an affected nerve or develop large plexiform tumors ( Fig. 190.3 ).
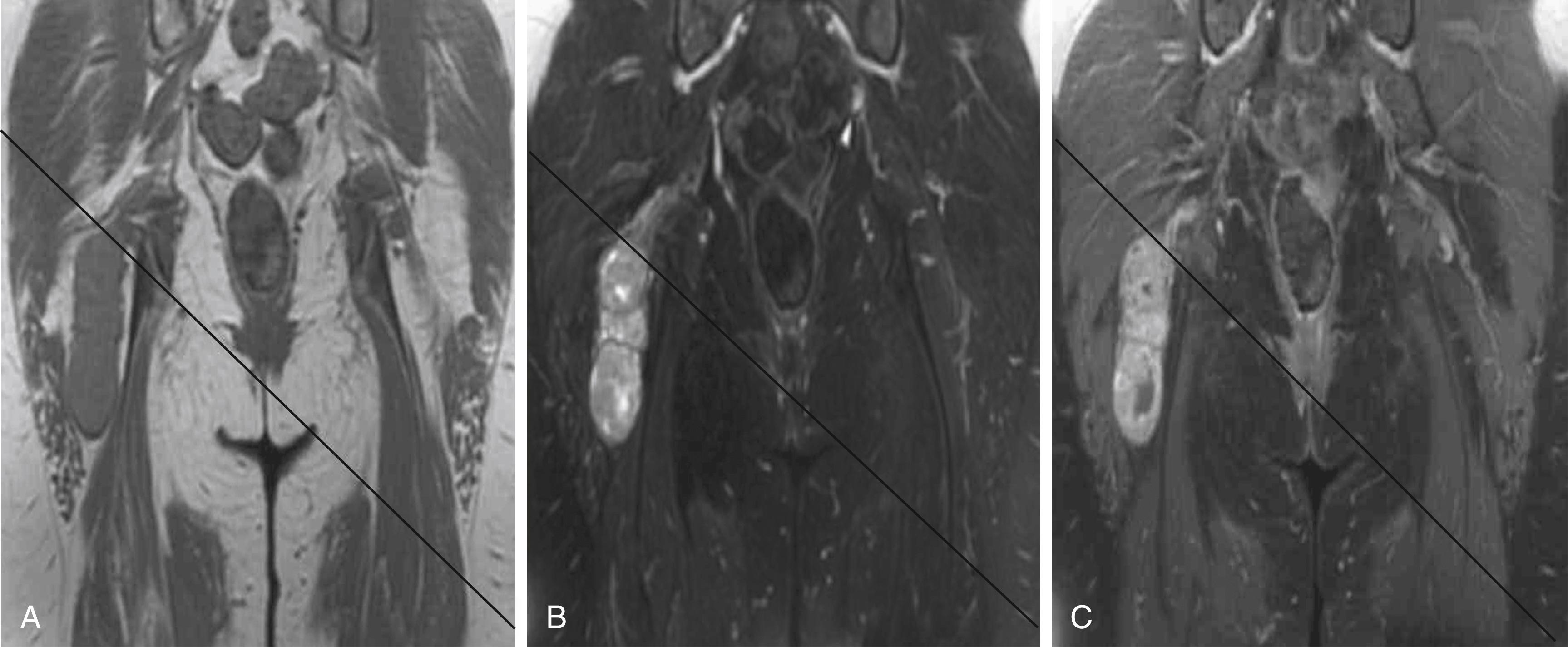
The capacity to accurately assess tumor grade or malignancy based on MRI remains limited. In general, malignant tumors heterogeneously enhance, are less discrete, may demonstrate edema in surrounding tissues, and are more likely to have areas of necrosis or hemorrhage ( Fig. 190.4 ). However, benign tumors can also have all of these characteristics. A rapid increase in the size of a tumor on serial MRIs can suggest malignancy, but small increases in tumor volume are difficult to detect.
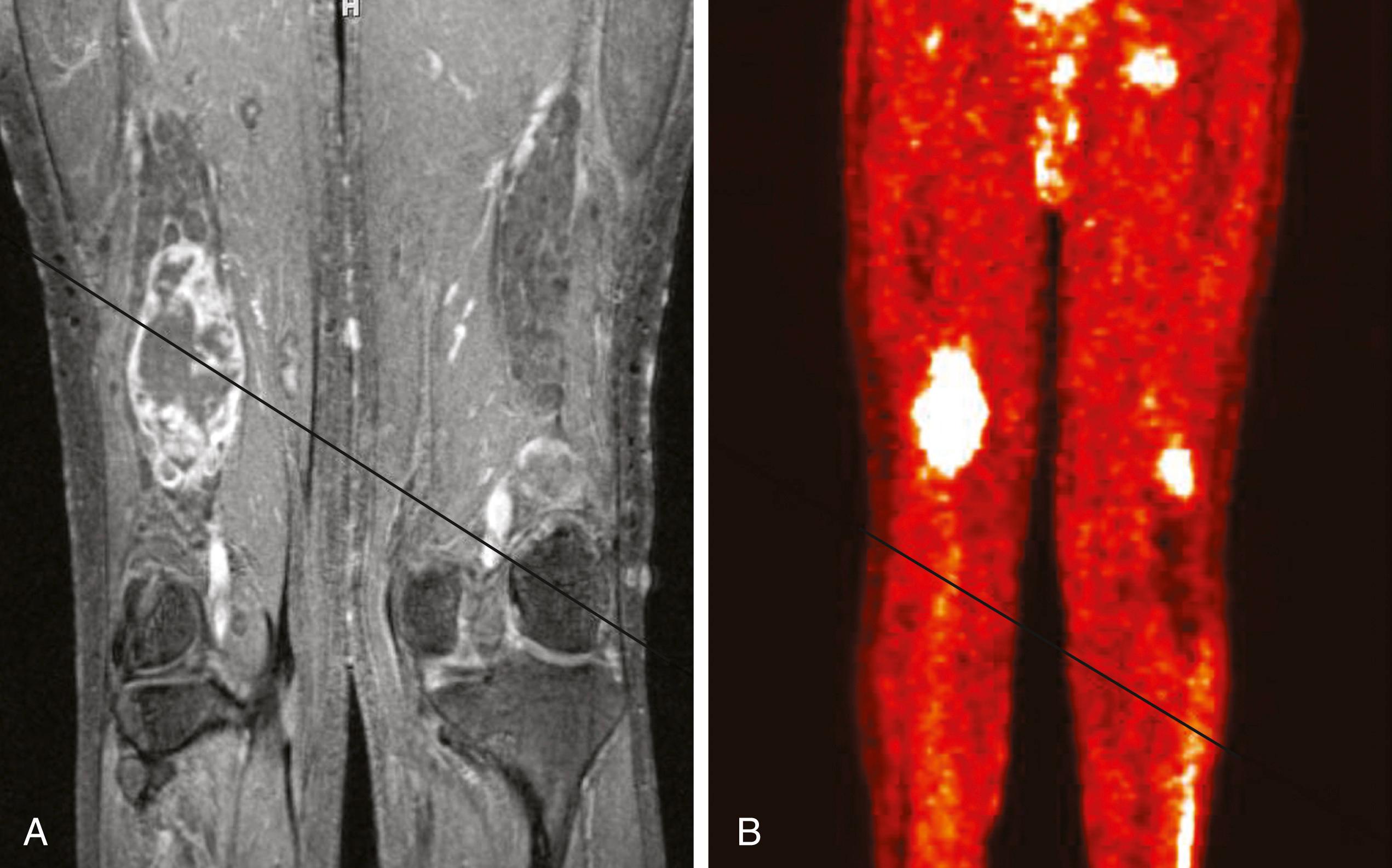
Metabolic imaging using with 18 fluorodeoxyglucose ( 18 FDG) positron emission tomography (PET) often adds valuable information regarding the grade of a tumor (see Fig. 190.4). Ferner and colleagues introduced the use of 18 FDG-PET to detect malignant transformation of benign plexiform neurofibromas to MPNSTs. Several small series have reported sensitivity ranging from 89% to 100% and specificity ranging from 83% to 95% depending on the time of scanning after injection and the cutoffs used for standard uptake values. The addition of CT anatomic imaging to 18 FDG-PET can facilitate targeting biopsies to metabolic hot spots, to further augment diagnostic sensitivity.
Electromyography (EMG) and nerve conduction studies (NCSs) are often obtained in patients with a tumor involving a peripheral nerve. EMG studies and NCSs assist in localizing the tumor, provide documentation of baseline function for later comparison, and can detect subclinical deficits. Electrophysiologic studies are especially useful for tumors arising proximally at the plexus level that involve multiple nerves of the extremity. Care should be taken to focus the studies on the specific muscles or muscle groups of the affected nerve(s).
The majority of peripheral nerve tumors are benign. Intraneural tumors can arise from the nerve sheath (schwannoma, neurofibroma, intraneural perineuroma) or other intrinsic structures of the nerve (intraneural ganglia, adipocytic [lipomatous] tumors, ganglioneuroma, and others). Extraneural tumors represent a diverse population of entities that can arise from or metastasize to adjacent soft tissue, bones, or joints and compress or invade peripheral nerves. This section focuses on schwannomas and neurofibromas.
Schwannomas (neurilemmomas) are benign tumors that arise from the nerve sheath. They are typically solitary and slow growing. A schwannoma can arise from any peripheral nerve with a Schwann cell, including distal portions of cranial nerves. Sporadic schwannomas can occur at any age but are most common in the third to sixth decades of life.
Bilateral acoustic schwannomas are pathognomonic for the diagnosis of NF2, but there is no association between peripheral schwannoma and NF1. Since the turn of the 21st century the diagnosis of schwannomatosis has gained acceptance for patients with multiple peripheral nerve schwannomas in the absence of acoustic schwannomas. The specific diagnostic criteria for schwannomatosis are listed in Table 190.3 . The annual incidence of new diagnoses has been estimated to be 1 in 1,808,300 in a Finnish population-based study, which would correlate to a birthrate of approximately 1 in 80,000. Schwannomatosis usually occurs sporadically, but cases of autosomal dominant transmission have been reported. The tumors that develop may be conventional globular schwannomas or plexiform and arise throughout the body or may be limited to a single segment or nerve. Based on current evidence, it does not appear that a diagnosis of schwannomatosis increases the risk of malignant transformation of any given schwannoma. Prophylactic screening imaging is somewhat controversial. Many do not recommend it for patients with schwannomatosis. Imaging should be performed in these patients if new symptoms develop in a nerve distribution or if a new palpable or symptomatic mass is discovered.
| Definite Schwannomatosis |
| Two or more pathologically proven schwannomas and Lack of radiographic evidence of vestibular nerve tumor at greater than 18 years of age |
| Presumptive or Probable Schwannomatosis |
| Two or more pathologically proven schwannomas, without symptoms of cranial nerve VIII dysfunction at >30 years of age. Two or more pathologically proven schwannomas in an anatomically limited distribution (single limb or segment of the spine) without symptoms of cranial nerve VIII dysfunction at any age |
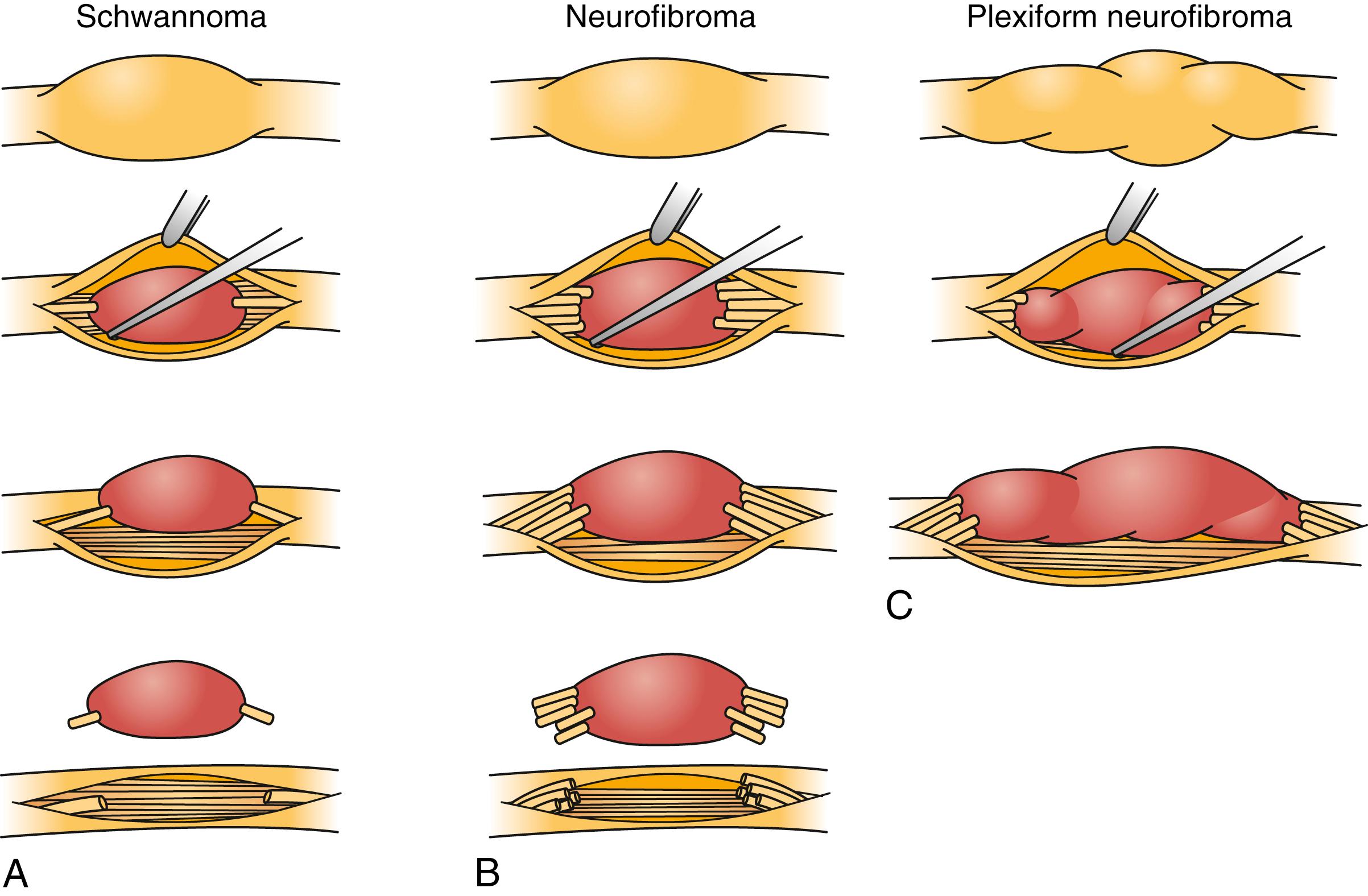
Schwannomas can be broadly classified into four subcategories: conventional schwannoma (the focus of this section); cellular schwannoma, a benign tumor that histologically resembles MPNST; plexiform schwannoma, which simulates plexiform neurofibroma or in its cellular form, MPNST; and melanocytic schwannoma, which is often mistaken for a melanoma. Conventional schwannomas are usually globoid and homogeneously firm, but they can have ballotable cystic areas. On gross examination the tumor has a well-defined capsule, appears shiny, and is tan-yellow. Histologically, they are distinguished from other nerve sheath tumors by being composed exclusively of Schwann cells; perineural cells and fibroblasts are not present. Diagnostic features include a fibrous capsule, hyaline vessels, cellular (Antoni A) and loose-textured (Antoni B) areas, and Verocay bodies (opposing rows of spindle nuclei separated by anucleate rows of eosinophilic processes). Classically, schwannomas arise eccentrically within the nerve sheath and displace the fascicles whereas neurofibromas may be more centrally located ( Fig. 190.5 ). However, often a small fascicle entering or exiting the proximal and distal poles of the tumor can be identified.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here