Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The mainstay technique for increasing the apneic window is through preoxygenation with spontaneous face-mask ventilation and 100% oxygen.
More recently, there has been increased emphasis on perioxygenation , which also involves providing oxygenation during the apneic period.
Preoxygenation denitrogenates the lungs and creates an alveolar oxygen reservoir. The size of this reservoir can be increased by reducing dependent atelectasis through head-up patient positioning and raising mean airway pressure. Ultimately, however, the size of the oxygen reservoir is fixed at the end of preoxygenation, and, once apnea begins, it does not get replenished.
Preoxygenation techniques include tidal volume breathing of oxygen by face mask for 3 to 5 minutes, deep breathing techniques by face mask up to 2 minutes, continuous positive airway pressure (CPAP), and noninvasive positive-pressure ventilation (NIPPV), or bi-level positive airway pressure (BiPAP).
Apneic oxygenation describes the physiologic phenomenon in which, provided that a patent air passageway exists between the lungs and the exterior (nasopharyngeal and oropharyngeal airspace), the difference between the alveolar rates of oxygen removal from the lungs and carbon dioxide excretion into the lungs generates a negative pressure gradient of up to 20 cm H 2 O that drives oxygen into the lungs from the nasopharyngeal and oropharyngeal reservoirs. Techniques of apneic oxygenation serve to prolong the duration of apnea without desaturation.
Techniques that prolong apnea time include low-flow nasal oxygen at flows less than 15 L/min (NO DESAT), pharyngeal oxygen insufflation, and high-flow humidified nasal oxygen at 30 to 70 L/min (THRIVE).
Head-up positioning is especially useful in that it both improves other efforts at preoxygenation and aids in prolonging the duration of apnea without desaturation.
During induction of anesthesia, maintenance of arterial oxyhemoglobin saturation levels is critical in an apneic patient until airway control has been achieved, with desaturation leading to dysrhythmias, hemodynamic decompensation, hypoxic brain injury, and ultimately death. , These saturation levels are maintained via the process of “perioxygenation” ( Fig. 15.1 ). The term perioxygenation includes both preoxygenation and apneic oxygenation. Preoxygenation ends when apnea starts due to induction of anesthesia and neuromuscular blockade; thereafter, apneic oxygenation (and ventilation) occur. Perioxygenation has become a universally accepted strategy designed to increase oxygen reserves and thereby delay the onset of arterial oxyhemoglobin desaturation during periods of hypoventilation and apnea.
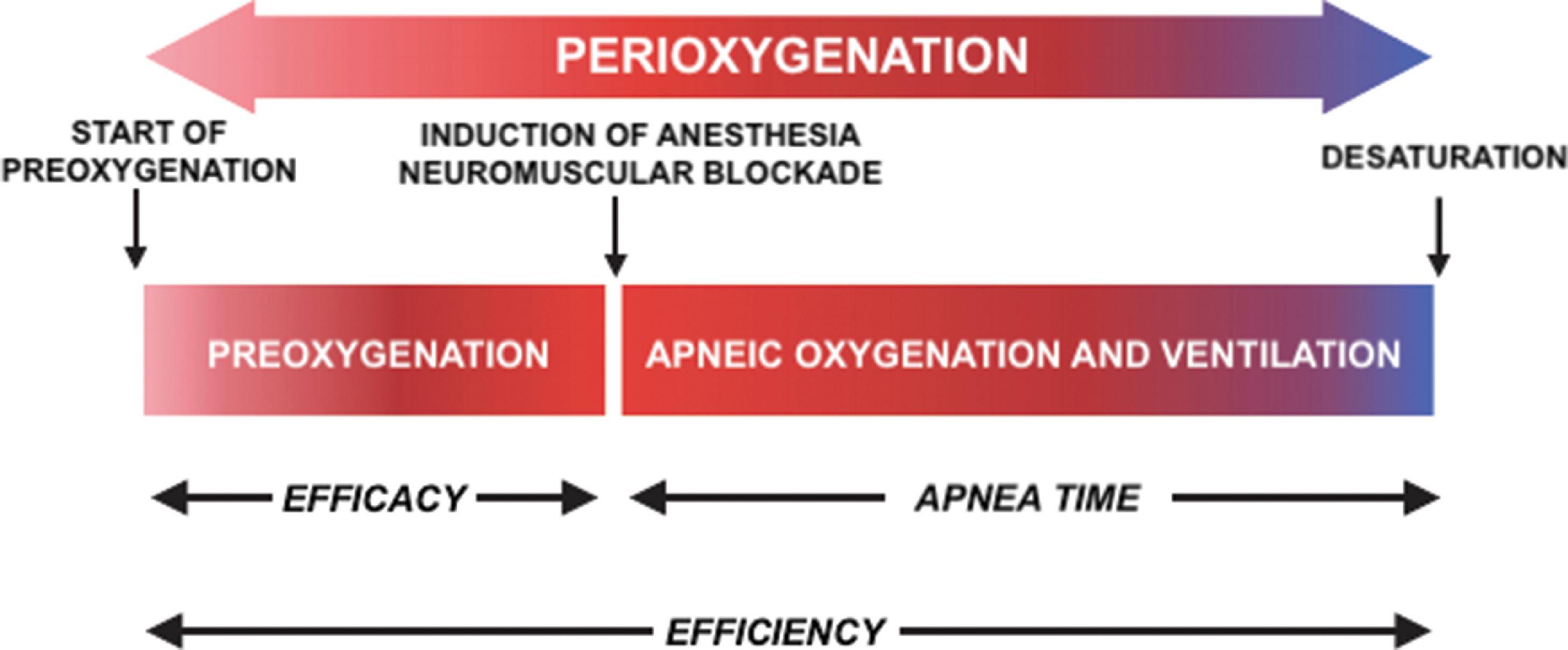
Apnea time is defined as the length of time from cessation of breathing or ventilation until the onset of significant arterial desaturation (typically, an arterial oxyhemoglobin saturation [Sa o 2 ] <90%). The primary method for increasing apnea time during airway management is through preoxygenation with spontaneous face-mask ventilation and 100% oxygen before induction of anesthesia. Preoxygenation denitrogenates the lungs and creates an alveolar oxygen reservoir. The size of this reservoir can be increased by reducing dependent atelectasis through head-up patient positioning and by raising mean airway pressure; ultimately, however, the size of the oxygen reservoir is fixed at the end of preoxygenation, and, once apnea begins, it does not get replenished unless ventilation resumes or other strategies are employed.
Apneic oxygenation describes the physiologic phenomenon in which, provided that a patent air passageway exists between the lungs and the atmosphere (i.e., the nasopharyngeal and oropharyngeal airspace), the difference between the alveolar rates of oxygen removal from the lungs and carbon dioxide (CO 2 ) excretion into the lungs generates a negative pressure gradient of up to 20 cm H 2 O that drives oxygen into the lungs from the nasopharyngeal and oropharyngeal reservoir. , A continuous flow of nasal oxygen therefore allows maintenance of oxygenation without spontaneous or administered ventilation and increases the apnea time beyond that of standard face-mask preoxygenation. Three techniques use this concept: nasal oxygen during efforts securing an ETT (NO DESAT), pharyngeal oxygen insufflation, and transnasal humidified rapid-insufflation ventilatory exchange (THRIVE).
The first version of the American Society of Anesthesiologists (ASA) Practice Guidelines for Management of the Difficult Airway did not mention preoxygenation. In the updated 2003 report, the topic of “face mask preoxygenation before initiating management of the difficult airway” was added. Routine preoxygenation has become a new minimum standard of care, not only during induction of anesthesia but also during emergence from anesthesia and tracheal extubation.
The principles important to understanding the physiology of perioxygenation include body oxygen stores, the physiology of apnea, and the concept of apneic oxygenation.
Oxygen is carried in the blood in two forms: the greater portion is in reversible chemical combination with hemoglobin (Hb), and the smaller part is dissolved in plasma. The ability to carry large amounts of oxygen in Hb is important, because without it the amount carried in the plasma would be so small that cardiac output would need to be increased 20 times or more to yield adequate oxygen delivery. The amount of chemically bound oxygen is directly related to the concentration of Hb and how saturated the Hb is with oxygen. Arterial oxygen content (Ca o 2 ) can be calculated from the following equation:
where
1.36 = estimated mass volume of oxygen that can be bound by 1 g of normal Hb
Sa o 2 = arterial oxyhemoglobin saturation (when fully saturated, Sa o 2 = 100%)
Pa o 2 = arterial partial pressure of oxygen
0.003 = solubility coefficient of oxygen in human plasma
The Ca o 2 with a Hb concentration of 15 g/dL and 100% Sa o 2 is approximately 20 mL of oxygen per 100 dL of blood. In addition, approximately 0.3 mL of oxygen/100 dL blood is in physical solution at a normal physiologic Pa o 2 ; this amount of dissolved oxygen normally accounts for only 1.5% of the total oxygen content, but its contribution increases when Pa o 2 is increased (dissolved oxygen is linearly related to Pa o 2 ). The venous oxygen content (C V o 2 ) can be calculated with the same formula using mixed venous oxygen tension (P V o 2 ) and mixed venous oxyhemoglobin saturation (S V o 2 ).
The pattern of uptake and release of oxygen by Hb is demonstrated by the oxyhemoglobin dissociation curve, which is a plot of Hb saturation as a function of partial pressure of oxygen (P o 2 ). The sigmoid shape of the curve reflects the fact that the four binding sites on a given Hb molecule interact with each other. When the first site has bound a molecule of oxygen, the binding of the next site is facilitated, and so forth. The result is a curve that is steep up to a P o 2 of 60 mm Hg and shallower thereafter, approaching 100% saturation asymptotically. At a P o 2 of 100 mm Hg (the normal arterial value), 97% of the hemes have bound oxygen; at 40 mm Hg (a typical value for Pv o 2 in a resting person), the saturation declines to about 75%. The shape of the oxyhemoglobin dissociation curve has important physiologic implications. The flatness of the curve above a P o 2 of 80 mm Hg ensures a relatively constant Sa o 2 despite wide variations in alveolar oxygen pressure. The steep portion of the curve between 20 and 60 mm Hg permits unloading of oxygen from Hb at relatively high P o 2 values, which favors the delivery of large amounts of oxygen into the tissues by diffusion.
The oxygen-binding properties of Hb are influenced by several factors, including pH, partial pressure of carbon dioxide (P co 2 ), and temperature. These factors cause shifts of the oxyhemoglobin dissociation curve to the right or left without changing the slope of the curve. For example, an increase in temperature or a decrease in pH, such as may occur in active tissues, decreases the affinity of Hb for oxygen and shifts the oxyhemoglobin dissociation curve to the right. As a result, a higher P o 2 is required to achieve a given saturation, facilitating the unloading of oxygen at the tissue. To quantify the extent of a shift of the oxyhemoglobin dissociation curve, the P 50 is used—that is, the P o 2 required for 50% saturation. The P 50 of normal adult Hb at 37°C and normal pH and P co 2 is 26 to 27 mm Hg.
Despite its great importance, oxygen is a very difficult gas to store in a biologic system. In subjects breathing air, oxygen stores are small ( Table 15.1 ). , The relatively steep oxyhemoglobin dissociation curve and the small oxygen stores imply that factors affecting Pa o 2 produce their full effects very quickly. This contrasts with CO 2 , for which the large size of the stores buffers the body against rapid changes. Therefore, in a subject breathing air, a pulse oximeter probably gives an earlier indication of hypoventilation than does CO 2 measurement. In contrast, in a subject breathing a high fraction of inspired oxygen (F io 2 ), CO 2 measurement gives an earlier indication of hypoventilation.
| Storage Site (mL) | Room Air (mL) | 100% O 2 |
|---|---|---|
| In the lungs (FRC) | 450 | 3000 |
| In the blood | 850 | 950 |
| Dissolved in tissue fluids | 50 | 100 |
| In combination with myoglobin | 200 | 200 |
| Total | 1550 | 4250 |
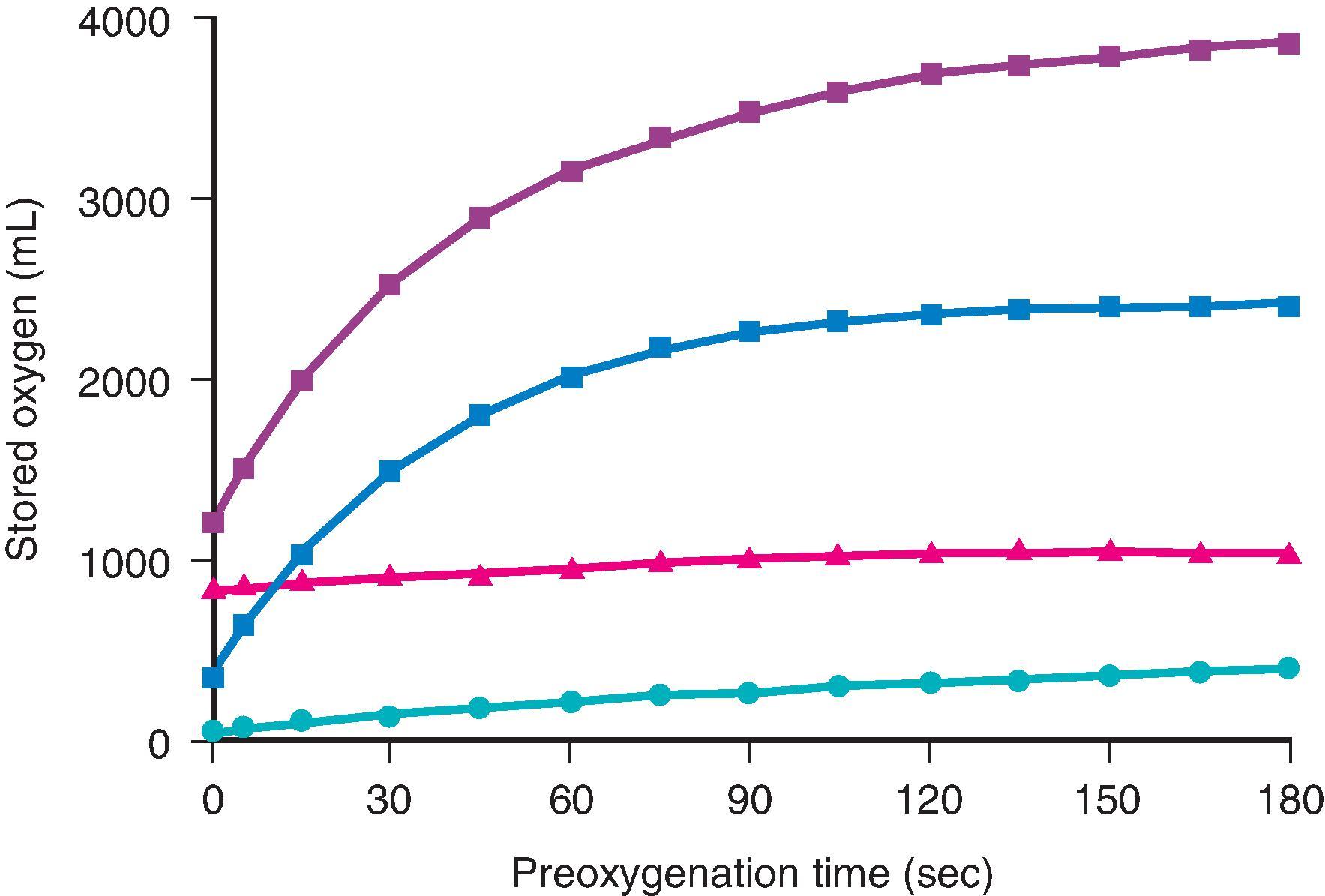
The amounts of body oxygen in the various storage sites of a person breathing air are increased with breathing an F io 2 of 100% ( Fig. 15.2 ; see also Table 15.1 ). , The largest increase in oxygen stores occurs in the functional residual capacity (FRC). Storage of oxygen in the tissue is rather difficult to assess, but assuming that Henry’s law applies and that the partition coefficient for gases approximates the gas-water coefficients, breathing oxygen for 3 minutes significantly increases tissue oxygen stores.
During apnea in the paralyzed patient there is no diaphragmatic movement or lung expansion, and the total body oxygen consumption (V˙o 2 ) remains fairly constant at about 230 mL/min. Consequently, Pa o 2 decreases rapidly because of the depletion of the diminishing oxygen stores in the lungs. If the airway becomes obstructed, oxygen removal will generate a substantial and immediate negative pressure, contributing to a further decrease of Pa o 2 . Although the Pa o 2 falls in direct relation to the alveolar oxygen concentration (P ao 2 ), Sa o 2 remains 90% or more as long as Hb can be reoxygenated in the lungs. , , , Sa o 2 begins to decrease only after lung oxygen stores are depleted and the Pa o 2 is lower than 60 mm Hg. It is for this reason that oximetry is not the best physiologic means, compared with Pa o 2 , for predicting the onset of hypoxemia. However, because it detects decreases in Sa o 2 before other clinical signs, oximetry is an invaluable clinical monitor that adds to the safety of anesthetic management. Critical oxyhemoglobin desaturation may be defined as Sa o 2 80% or less; for patients with Sa o 2 80% or less, the range in the rate of decrease is 20% to 40% per minute during apnea.
The physiologic nomenclature for describing apneic oxygenation has changed several times since the phenomenon was discovered by Volhard in 1908. It has been described as “diffusion respiration” by Draper and Whitehead, as “aventilatory mass flow” by Bartlett and colleagues, and as “apneic oxygenation” by Frumin and colleagues. What all of these studies describe is oxygenation using only the difference in the rates of excretion of CO 2 and absorption of oxygen as the driver of gaseous flow.
Preoxygenation followed by oxygen insufflation during subsequent apnea maintains Sa o 2 by apneic oxygenation. , In the apneic adult, V˙ o 2 averages 230 mL/min, whereas the output of CO 2 to the alveoli is limited to about 20 mL/min, and the remaining CO 2 production (approximately 90%) is buffered within the body tissues. The difference in gas solubility between oxygen and CO 2 and the affinity of oxygen for Hb accounts for the difference in movement of oxygen and CO 2 across the alveolar membrane.
Lung gas volume initially decreases because of the net negative gas exchange rate of 210 mL/min. Therefore, a pressure gradient is created between the upper airway and the alveoli, and if the airway is patent, this results in a mass movement of oxygen down the trachea into the alveoli, prolonging apnea time. Conversely, CO 2 is not exhaled because of this mass movement of oxygen down the trachea, and the alveolar CO 2 concentration (P aco 2 ) shows an initial rise of about 8 to 16 mm Hg during the first minute and a subsequent fairly linear rise of about 3 mm Hg/min.
Fraioli and colleagues emphasized the importance of the ratio of FRC to body weight during apneic oxygenation and demonstrated that patients with a low FRC/body weight ratio could not tolerate apnea for more than 4 minutes, whereas those with a high FRC/body weight ratio (>53.3 ± 7 mL/kg) maintained Pa o 2 at 90% of the control value for 15 minutes or longer. Some studies demonstrated that with a patent airway and an F io 2 of 1.0, Sa o 2 can be maintained at greater than 90% for up to 100 minutes with apneic oxygenation. ,
The success of apneic oxygenation depends on airway patency to allow oxygen to move into the apneic lungs. In the presence of airway obstruction, not only does lung gas volume decrease rapidly, but intrathoracic pressure also decreases at a rate that is dependent on V˙ o 2 and thoracic compliance, subsequently leading to a marked decrease in Pa o 2 . When airway obstruction is relieved, rapid flow of oxygen into the lungs occurs, and with high F io 2 , rapid reoxygenation resumes.
Apneic oxygenation can be achieved by preoxygenation followed by insufflation of oxygen through a nasopharyngeal or oropharyngeal cannula or through a needle inserted in the cricothyroid or cricotracheal membrane. This provides at least 10 minutes of adequate oxygenation in healthy apneic patients whose airways are unobstructed and therefore has many practical applications. In patients who are difficult to intubate or ventilate, pharyngeal oxygen insufflation (or tracheal insufflation, in cases of upper airway obstruction) may allow additional time for laryngoscopy and endotracheal intubation. , , This can be advantageous in patients who have decreased oxygen reserves, such as children, pregnant women, obese patients, and patients with acute respiratory distress syndrome (ARDS). The combination of preoxygenation and apneic oxygenation can be used during bronchoscopy and can provide an otolaryngologist adequate time for glottic surgery unimpeded by the presence of an endotracheal tube (ETT) or by a patient’s respiratory movements.
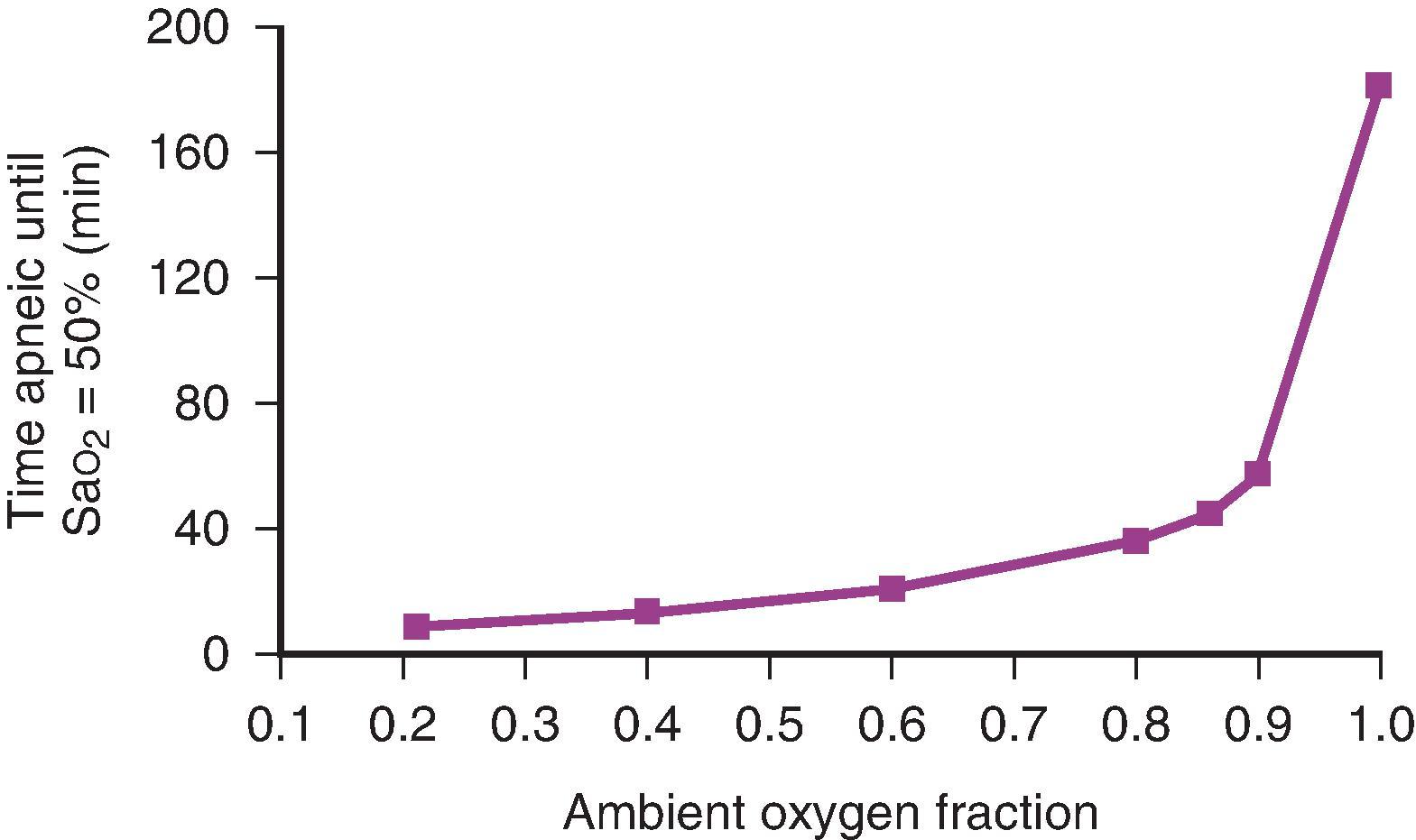
The relationship between the ambient oxygen fraction and the time to development of dangerous hypoxemia (apnea time) has been investigated in a computational modeling analysis by McNamara and Hardman. As the ambient oxygen fraction increases, the onset of hypoxia is delayed irrespective of shunt fraction, and the increase in time to desaturation is very much greater with very high ambient oxygen fractions ( Fig. 15.3 ). Increasing the ambient oxygen fraction from 0.9 to 1.0 more than doubles the time to desaturation compared with increasing the ambient oxygen fraction from 0.21 to 0.9. This effect was maintained over a wide range of shunt fractions (1% to 30% of cardiac output) during apnea. These findings have significant implications for anesthetic practice, where assurance of effective apneic oxygenation involves ensuring the patency of the airway and the provision of 100% oxygen. Failure to provide 100% oxygen to the apneic patient’s open airway greatly hastens the development of hypoxemia.
Apneic oxygenation provides very little, if any, CO 2 clearance. Although apneic oxygenation can largely meet physiologic oxygen demands for an increased period, it does not prevent a rapid and eventually fatal rise in CO 2 concentration. In Frumin’s experiments, apneic oxygenation was carried out in 8 human subjects for periods between 15 and 55 minutes; in 2 out of 8 human subjects, the trial was prematurely terminated because of the development of ventricular arrhythmias associated with respiratory acidosis. In an apneic oxygenation study in 12 dogs, 1 died, likely from CO 2 toxicity. There were also suggestions of patient death and altered cerebral function in early studies of apneic oxygenation. , Joels and Samueloff demonstrated that apneic oxygenation caused a progressive respiratory acidosis that rapidly overwhelmed the blood’s buffering mechanisms and progressed into a mixed acidosis that proves fatal. Death is principally caused by the limited tolerance of the myocardial contractile and conductive mechanisms to acidosis. , Joels and Samueloff’s experiments placed the upper limit of the 95% confidence interval for occurrence of death attributed to acidosis at a pH of 6.9.
Studies of preoxygenation have focused on measurements of indices reflecting its efficacy and efficiency. Measurements of alveolar oxygen, alveolar nitrogen, or Pa o 2 reflect the efficacy of preoxygenation, whereas the drop in Sa o 2 during apnea is indicative of its efficiency. , Sa o 2 is misleading as a guide to alveolar denitrogenation. An oxygen saturation as measured by pulse oximetry (Sp o 2 ) of 100% is not a reason to cease preoxygenation and may occur well before the lungs are adequately denitrogenated. Conversely, failure of Sp o 2 to increase substantially during denitrogenation does not necessarily indicate failure of preoxygenation or lack of its value; patients with substantial pulmonary shunting may achieve excellent pulmonary oxygen reservoirs while remaining hypoxemic.
Preoxygenation increases alveolar oxygen concentration and decreases alveolar nitrogen in a parallel fashion ( Fig. 15.4 ); it is the washout of nitrogen from the lungs that is the key to achieving preoxygenation. , Historically, preoxygenation and denitrogenation have been used interchangeably, although a change in focus from preoxygenation to denitrogenation has been suggested. With normal lung function, oxygen wash-in and nitrogen wash-out are exponential functions; therefore the rate of preoxygenation (or denitrogenation) is governed by the time constant (τ) of the exponential curves. This constant is the same for both the wash-in and wash-out curves and is proportional to the ratio of alveolar ventilation (V˙ a ) to FRC. Because the oxygen flow used for V˙ a is delivered via an anesthesia circuit, preoxygenation occurs in two sequential stages according to the time constant, which is the time necessary for a given flow through a container to equal the volume of the container. These are the two stages:
Wash-out of the anesthesia circuit by oxygen flow
Wash-out of the FRC by alveolar ventilation
After 1 τ, the oxygen concentration of the FRC will be increased by approximately 63% of its original value; after 2 τ, to 86%; after 3 τ, to 95%; and after 4 τ, to about 98% of its original value.
To hasten denitrogenation, it is advisable to wash out (flush) the anesthesia circuit with a high oxygen flow before applying the face mask to the patient. During preoxygenation, an oxygen flow rate that eliminates rebreathing should be used.
In summary, three steps should be followed to enhance preoxygenation: (1) the anesthesia circuit is flushed by a high oxygen flow, (2) a nonleaking face mask is used to avoid air entrainment, and (3) an oxygen flow of 5 L/min is used for tidal volume breathing (TVB), and a flow of 10 L/min is used for deep breathing.
The end points of maximal alveolar preoxygenation or denitrogenation have been defined as an end-tidal oxygen concentration (EtO 2 ) of approximately 90% and an end-tidal nitrogen concentration (EtN 2 ) of 5%. , In an adult with a normal FRC and V˙ o 2 , an EtO 2 of 90% or more means that the lungs contain more than 2000 mL of oxygen (8 to 10 times the V˙ o 2 ). Because of the obligatory presence of CO 2 and water vapor in the alveolar gas, an EtO 2 greater than 97% cannot be easily achieved. Factors affecting the efficacy of preoxygenation include F io 2 , duration of breathing, and the V˙ a /FRC ratio ( Box 15.1 ).
The main reasons for failure to achieve an F io 2 close to 1.0 are a leak under the face mask, , rebreathing of exhaled gases, and the use of systems incapable of delivering a high oxygen concentration, such as resuscitation bags. Even the presence of minor leaks may not be fully compensated for by increasing the fresh gas flow (FGF) or by increasing the duration of preoxygenation. Bearded patients, edentulous patients, patients with sunken cheeks, the presence of nasogastric tubes, use of a wrong face mask size, improper use of head straps, and use of systems allowing air entrainment under the face mask are all common factors causing leaks between the mask and the patient’s face that result in a lower F io 2 . Clinical endpoints indicative of a sealed system include movement of the reservoir bag with inhalation and exhalation, the presence of a normal capnogram and end-tidal CO 2 (EtC O 2 ), and measurements of inspired and Et CO 2 values.
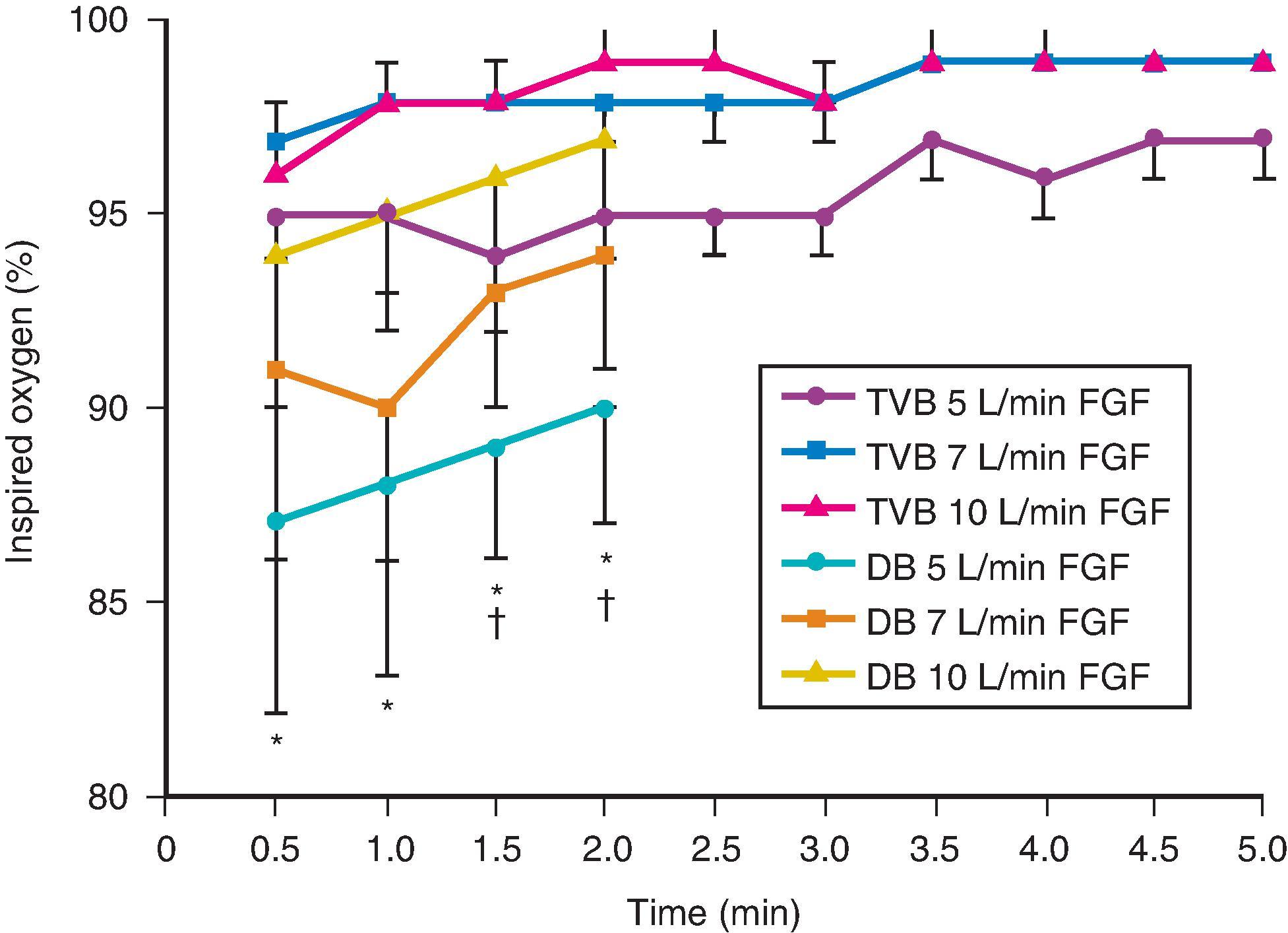
Although anesthetic circuits can deliver 100% oxygen, the F io 2 is influenced by the type of breathing, the level of FGF, and the duration of breathing. In a study that compared preoxygenation techniques using a semiclosed circle absorber with varying FGFs in the same subjects, it was found that with TVB, inspired oxygen concentration was 95% with FGF of 5 L/min, increasing to 98% with FGFs of 7 and 10 L/min. However, with deep breathing, the inspired oxygen concentration was only 88% at 5 L/min, 91% at 7 L/min, and 95% at 10 L/min FGF (see Fig. 15.4 ). These findings imply that increasing the FGF from 5 to 10 L/min has little impact on increasing F io 2 during TVB but has a noticeable effect during deep breathing. Because of the breathing characteristics of the circle system, the minute ventilation during deep breathing may exceed the FGF, resulting in rebreathing of exhaled gases (N 2 ) and consequently decreasing the F io 2 ; in contrast, during TVB, rebreathing of exhaled gases is negligible, and increasing the FGF from 5 to 10 L/min has only a slight effect on F io 2 . ,
Sufficient time is needed to accomplish maximal preoxygenation. With an F io 2 close to 1.0, most healthy adult patients can reach the target level of Et O 2 90% or more (or EtN 2 ≤5%) within 3 to 5 minutes of TVB. The half-time for exponential change in fraction of alveolar oxygen concentration (F ao 2 ) with an immediate change in F io 2 for a nonrebreathing system is described by the equation:
where
V frc = volume of functional residual capacity.
With a V frc of 2.5 L, the half-times are 26 seconds when V˙ a = 4 L/min and 13 seconds when V˙ a = 8 L/min. Therefore most of the oxygen that can be stored in the lungs may be brought in by hyperventilation with an F io 2 of 1.0 for a shorter period of time than that needed with TVB. This is the basis for the deep breathing techniques, which have been introduced as an alternative to TVB. ,
Changes in V˙ a and FRC can have a marked effect on the rate of rise in EtO 2 (and decrease in EtN 2 ) during preoxygenation. In pregnant women, because of an increased V˙ a and decreased FRC, EtO 2 rises faster than in nonpregnant women. , , Similarly, preoxygenation can be accomplished faster in infants and children than in adults.
Preoxygenation can markedly delay arterial oxyhemoglobin desaturation during apnea. In healthy individuals breathing room air, desaturation to 70% can occur within 1 minute, whereas with adequate preoxygenation, desaturation occurs after 5 minutes. The delay in desaturation during apnea depends on the efficacy of preoxygenation, the capacity for oxygen loading, and V˙ o 2 (see Box 15.1 ). Patients with a decreased capacity for oxygen loading (decreased FRC, Pa o 2 , Ca o 2 , or cardiac output) or with increased V˙ o 2 , or both, desaturate much faster during apnea than healthy patients do. The main difference in the rate of apnea-induced oxyhemoglobin desaturation after different preoxygenation techniques is observed between Sa o 2 levels of 100% and 99%. , , , This range represents the flat portion of the oxyhemoglobin dissociation curve. When oxygen reserves are depleted, rapid desaturation occurs regardless of the technique of preoxygenation and is like that observed in patients breathing air.
Farmery and Roe developed a computer model describing the rate of oxyhemoglobin desaturation during apnea. This model was found to agree reasonably well with actual data from patients whose weight and degree of preoxygenation were reliably known ( Fig. 15.5 ). , Because it would be dangerous to obtain data on time to marked oxyhemoglobin desaturation in humans, this model is uniquely useful for analysis of oxyhemoglobin desaturation below 90%. , As the pre-apnea F ao 2 is progressively decreased from 0.87 to 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, and 0.13 (F ao 2 at room air) in a healthy 70-kg patient, the apnea time to 60% Sa o 2 is progressively decreased from 9.9 to 9.31, 8.38, 7.30, 6.37, 5.40, 4.40, 3.55, and 2.8 minutes, respectively. , Fig. 15.5 shows that for a healthy 70-kg adult, a moderately ill 70-kg adult, a healthy 10-kg child, and an obese 127-kg adult, 80% Sa o 2 is reached after 8.7, 5.5, 3.7, and 3.1 minutes, respectively, whereas 60% Sa o 2 is reached at 9.9, 6.2, 4.23, and 3.8 minutes, respectively. ,
![Fig. 15.5, Arterial oxyhemoglobin saturation (Sa o 2 ) versus time of apnea in an obese adult, a 10-kg child (low functional residual capacity [FRC] and high oxygen consumption [V˙o 2 ]), and a moderately ill adult, compared with a healthy adult. Fao 2 , Fractional alveolar oxygen concentration; Ve, expired volume. Fig. 15.5, Arterial oxyhemoglobin saturation (Sa o 2 ) versus time of apnea in an obese adult, a 10-kg child (low functional residual capacity [FRC] and high oxygen consumption [V˙o 2 ]), and a moderately ill adult, compared with a healthy adult. Fao 2 , Fractional alveolar oxygen concentration; Ve, expired volume.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Perioxygenation/4_3s20B9780323795388000151.jpg)
Various techniques and regimens have been described to ensure (1) adequate preoxygenation to optimize the oxygen reservoir before induction of anesthesia and (2) techniques to prolong the duration of apnea after the induction of anesthesia and administration of muscle relaxants. Regardless of the technique used, preoxygenation and prolongation of the apnea time after induction of anesthesia have become an integral component of the rapid sequence induction and intubation (RSI) technique and are particularly important if manual ventilation is not desirable following the induction of anesthesia, if difficulty with ventilation or endotracheal intubation is anticipated, and in patients with oxygen transport limitations. Because the “cannot intubate, cannot oxygenate” (CICO) situation is largely unpredictable, the desirability of maximal preoxygenation is theoretically present for all patients.
Several methods of preoxygenation are described in the scientific literature. Traditional techniques include TVB, deep breathing, and positive airway pressure ( Box 15.2 ). THRIVE can also be used for preoxygenation (see later discussion).
Traditional tidal volume breathing (3 to 5 minutes)
One vital capacity breath followed by tidal volume breathing
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here