Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The introduction of liver transplantation over 50 years ago has revolutionized outcomes for patients with both acute and chronic liver failure. Since experimental beginnings, liver transplantation has grown worldwide, with the number of transplants per year ranging from approximately 400 in Australia to over 1000 in the UK and over 8000 in the United States. Concurrently, survival from transplantation continues to improve, with 5-year survival reported between 75% and 85%, depending on reporting jurisdiction. This has been largely the result of improvements in surgical technique, anesthetic management, and postoperative critical care.
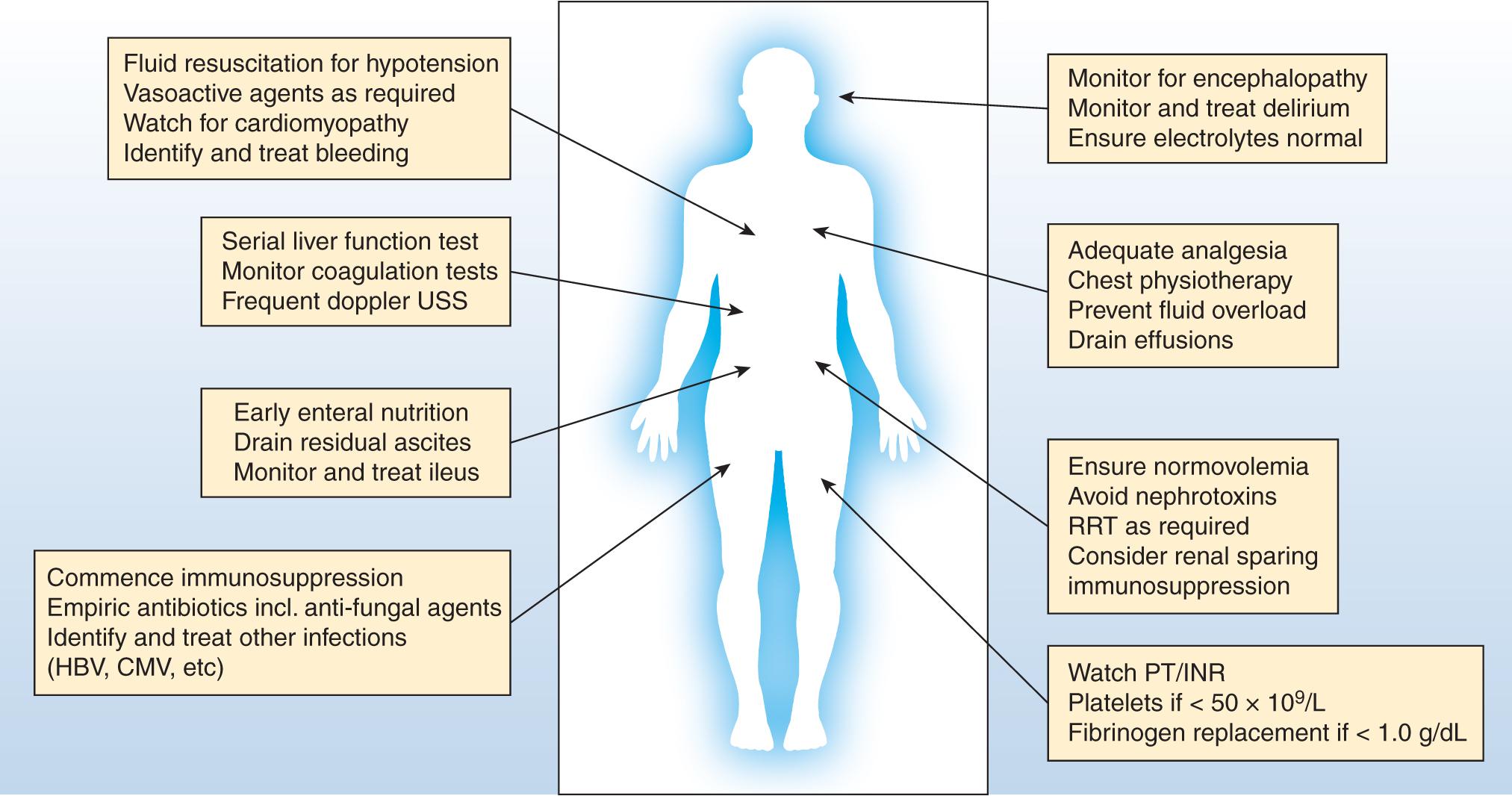
Liver transplantation is a treatment option for patients with both acute and chronic liver failure. Common causes include chronic liver disease as a result of alcohol use disorder; viral hepatitis; metabolic conditions such as Wilson disease and alpha-1-antitrypsin deficiency; and autoimmune conditions such as autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cirrhosis. Selection for liver transplantation in chronic liver disease is largely based on disease severity and the likelihood of death on the transplant waiting list. The Model for End-Stage Liver Disease (MELD) score is the most commonly used criteria for transplantation listing, with the cutoff of 15 used as a trigger for commencement of the transplant assessment process in many jurisdictions. A separate cohort are patients with hepatomas, who are listed on the basis of malignant disease burden. , Eligibility for transplantation has changed such that even critically ill patients are now often listed. These patients often exhibit acute-on-chronic liver failure (AoCLF) and require high-level critical care support for preoperative optimization followed by a frequently slow postoperative recovery. In contrast, patients with acute liver failure (ALF) are almost always managed in the intensive care unit (ICU) at the time of transplant listing. Various predictive criteria have been developed to determine the likelihood of survival without liver transplantation in ALF, although their utility is increasingly questioned as improvements in both understanding of the pathophysiology of ALF and improved critical care management have led to improved outcomes. Regardless of the indication for transplant, an essential requirement for effective care is a systematic and coordinated approach to management ( Table 146.1 and Fig. 146.1 ).
| Body System | Management Challenge |
|---|---|
| Cardiovascular | Vasodilatation, hypotension, cirrhotic cardiomyopathy, redistributed blood volume |
| Respiratory | Fluid overload, hydrothorax, atelectasis, hepatopulmonary syndrome, portopulmonary hypertension |
| Renal | Acute kidney injury, end-stage kidney disease, hepatorenal syndrome |
| Gastrointestinal | Ascites, spontaneous bacterial peritonitis, bacterial translocation, ileus, poor nutrition, hypoalbuminemia |
| Neurologic | Encephalopathy, delirium, potential increased sensitivity to neuroleptic agents |
| Coagulation | Rebalanced hemostasis, thrombocytopenia, hypofibrinogenemia, potential hyperfibrinolysis |
Patients with end-stage liver disease (ESLD) have a characteristic cardiovascular profile. The classic pattern is a hyperdynamic circulation with low systemic vascular resistance and an increased cardiac output. The mean arterial pressure (MAP) tends to remain low/normal despite these changes. Furthermore, ESLD patients have an abnormal distribution of total body blood volume because of complex alterations in the splanchnic, renal, and systemic circulations. , This involves the redistribution of blood into the splanchnic circulation secondary to the overproduction of nitric oxide (NO) and other proinflammatory mediators. The overproduction of inflammatory mediators is believed to be compounded by gut bacterial translocation in the setting of raised portal pressures. ,
The postoperative hemodynamic course of these patients is influenced considerably by persistence of these preoperative hemodynamic disturbances. The hemodynamic changes of cirrhosis can take days to weeks to fully resolve after transplantation. , Because of the marked hemodynamic instability that can occur during the peritransplant period, invasive monitoring in the ICU is necessary to guide therapy and optimize graft function. Traditionally, the pulmonary artery catheter has been the gold standard for intraoperative and postoperative monitoring; however, its utility has been questioned on the basis of its invasiveness, inability to predict fluid responsiveness, and lack of evidence for improving patient outcomes. , Less invasive techniques such as pulse contour analysis have gained popularity in other critical care populations, but their utility in the complex hemodynamic changes occurring during liver transplantation has recently been questioned. ,
Hypotension is the most common hemodynamic abnormality encountered postoperatively. Causes include postoperative bleeding, persistence of the preexisting vasodilated state, postreperfusion vasoplegia, the effects of narcotics and sedatives, and fluid shifts causing hypovolemia. Refractory vasoplegia postreperfusion can continue into the postoperative period; however, its incidence and impact upon outcomes are not known. Common to other vasoplegic syndromes, hypotension that is refractory to standard vasoconstrictors may be amenable to treatment with methylene blue. ,
Initial treatment aims to restore normovolemia by judicious use of fluid resuscitation. There is no consensus on the preferred fluid in this setting. Experience derived from the care of other critically ill patients suggests that balanced crystalloids may be preferred, as they avoid inducing hyperchloremia. , Albumin-containing solutions are an attractive alternative in patients who are hypoalbuminemic and offer potentially protective effects to the endothelial glycocalyx with improved postoperative outcomes.
The optimal target for arterial blood pressure has not been established; however, a MAP of 65 mm Hg seems appropriate in these patients to ensure adequate end-organ perfusion, especially for the engrafted liver. A higher MAP may be required in the presence of poor hepatic arterial flow because of high resistive indices. A central venous pressure of 8–12 mm Hg appears to be safe; however, further resuscitation and increases in volume state may have deleterious effects on hepatic outflow, leading to graft congestion and poor function. , In the presence of hypotension not related to bleeding or resolving with judicious fluid resuscitation, the application of vasoconstrictor agents is required. There is little evidence on which to prefer one agent over another, although norepinephrine is often used. Terlipressin is commonly used in the preoperative setting for decompensated liver failure, and in the postoperative setting some evidence exists suggesting use may decrease ascitic drain output and the incidence of acute kidney injury; however, its routine use is not recommended.
Although cardiac performance is generally preserved in patients undergoing liver transplantation, there are occasional instances where this may not hold true. Cirrhotic cardiomyopathy occurs to some extent in up to half of cirrhotic patients. , In contrast to traditional cardiomyopathies, patients with cirrhotic cardiomyopathy have normal cardiac function in terms of both output and contractility, but have evidence of blunted systolic contractile response to stressors and the presence of diastolic dysfunction at rest. As liver transplantation is a major physiologic stressor, overt cardiac dysfunction may only become apparent during the perioperative period. The major implications for management in the postoperative period are to ensure normovolemia and prevent significant fluid shifts. Additionally, prevention of electrolyte abnormalities and avoidance of other factors that may further depress cardiac function (e.g., hypothermia, severe acidosis) is required. In the presence of an established low cardiac output state with a corresponding decrease in end-organ perfusion, the use of inotropic agents such as milrinone or dobutamine may be necessary.
As patients waitlisted for liver transplant are increasingly older and more comorbid, underlying cardiovascular disease has become more common. , Larger numbers of patients with nonalcoholic steatohepatitis cirrhosis and metabolic syndrome also make this scenario more common. , It is recognized that patients with established coronary artery disease undergoing liver transplant have poorer outcomes posttransplantation. , Correspondingly, an appropriate risk assessment and consideration for revascularization is required for those being evaluated for transplantation. Recent studies have shown a higher rate of arrhythmic complications but a low rate of ischemic events. , The timing of recommencing antiplatelet agents is challenging and requires multidisciplinary review to ensure the maximal protection of existing stents is balanced against the risk of bleeding.
Patients undergoing transplantation for ALF often have additional cardiovascular challenges, with vasoplegic shock similar to that seen in severe sepsis being typical, especially for hyperacute presentations. Massive hepatic necrosis releases inflammatory mediators, which persist in the circulation because of a lack of hepatic clearance. This pattern may take days to resolve after successful liver transplant.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here