Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Recent estimates of the magnitude of traumatic brain injury (TBI) suggest that each year nearly 3 million people sustain a head injury in the United States, with the majority of patients having a concussion or a mild TBI. According to data from the Centers for Disease Control and Prevention (CDC), roughly 52,000 people die annually from TBI, while 282,000 people are hospitalized. TBI is a contributing factor in 30% of all injury-related deaths that occur in the United States, illustrating its importance as a public health problem. TBI is the leading cause of death under age 45 in the Western world.
The economic impact of brain trauma in the United States and on the country’s healthcare system is highlighted by estimates of direct medical expenditures and indirect costs (e.g., loss of productivity) attributable to TBI being upward of $76.5 billion in 2010, with much of the cost associated with patients admitted to hospitals. , Between 2007 and 2013, the number of TBI-related emergency department visits and TBI-related admissions increased by 53% and 5%, respectively. Additionally, 5.3 million brain-injured, disabled Americans depend on long-term assistance for help with performing activities of daily living, which further underscores the economic and social significance of TBI in today’s society.
Organized efforts to standardize the assessment and management of patients with TBI have resulted in the recent publication of the 4th edition of the Guidelines for the Management of Severe TBI. A cost-benefit analysis suggested that widespread adherence to and use of these guidelines could result in significant savings: $262 million in annual medical costs, $43 million in annual rehabilitation costs, and $3.84 billion in lifetime societal costs. In addition, the authors posited a potential decrease of 50% in TBI-related deaths. Ultimately, evidence-based management of patients with severe TBI translates to better patient care, improved outcomes, and a decreased economic and societal burden imposed by TBI.
In this chapter, we review key concepts in nonsurgical management of patients with severe TBI. First, we review the assessment of the head-injured patient and the classification of severe TBI. Second, we discuss the key parameters that precipitate secondary brain injury and delve into the principles of TBI management, focusing primarily on methods of avoiding cerebral ischemia and intracranial hypertension. Finally, we summarize important concepts in the medical management of patients with severe TBI and discuss neuroprotective strategies.
The prehospital assessment of a patient’s neurologic condition serves as a guide to the appropriate triage of an individual following a TBI. When transported directly from the scene of injury to a trauma center, patients with severe TBI have lower mortality rates than those who are first taken to nontrauma centers. Furthermore, severely brain-injured patients who are admitted and treated at level I trauma centers have better survival rates and experience superior outcomes compared with matched patients treated at level II facilities.
Over the years, many assessment schemes have been created to determine the neurologic status of head-injured patients, with the Glasgow Coma Scale (GCS) being the most commonly used tool to determine the severity of TBI ( Table 122.1 ). Developed in 1974 by Teasdale and Jennett, the GCS gauges a patient’s level of consciousness through an assessment of eye opening, verbal response, and motor response. For patients who are mechanically ventilated by an endotracheal tube or via a tracheostomy, thus precluding the patient’s ability to communicate verbally, a GCS score of 1 point is given for the verbal response score with a “T” following to indicate the intubated status of the patient. There are instances in which accurately determining a GCS score is difficult or when the applicability of a score is of limited utility, as with the presence of concomitant facial trauma, ingestion of sedative drugs such as alcohol or barbiturates, or iatrogenic paralysis of patients for intubation. Moreover, physiologic derangements such as hypotension and hypoxia at the time of assessment hinder a patient’s neural response to stimuli and thus are important factors that may also lead to inaccurate GCS scores, as well as contributing to secondary brain injury.
| Best Response | Score |
|---|---|
| Eye Opening (E) | |
| Spontaneous | 4 |
| To speech | 3 |
| To pain | 2 |
| Not open | 1 |
| Verbal Response (V) | |
| Conversant | 5 |
| Confused | 4 |
| Nonsense | 3 |
| Sounds | 2 |
| Silence | 1 |
| Intubated | 1T |
| Motor Response (M) | |
| To command | 6 |
| To pain | |
| Localized | 5 |
| Withdrawal | 4 |
| Arm flexion | 3 |
| Arm extension | 2 |
| No response | 1 |
| GCS score = E + V + M (range 3 or 3T to 15) | |
Severe TBI is classified by total GCS scores of 3 to 8 points, whereas scores of 9 to 12 points indicate moderate TBI and those between 13 and 15 points denote mild TBI. GCS scores correlate significantly with outcome, with the motor component of the score being most reproducible and yielding the most prognostic information. Patients whose GCS scores decrease by two points or more between the field and the emergency department are more likely to require surgical intervention. Nearly 80% of patients with an initial hospital GCS score of 3 to 5 points have an eventual outcome of death, severe disability, or vegetative state, with a mortality rate of 65% seen in patients with an initial GCS score of 3. , , Among individuals with a GCS score of 3 on presentation, only 13% have good functional outcomes at the 6-month mark. Age, pupil reactivity, radiologic findings on head computed tomography (CT) scan, and extent of extracranial injuries experienced during the trauma serve as additional independent predictors of patient outcome following severe TBI.
The pupillary examination is a critical component of a patient’s initial neurologic assessment after TBI. A dilated pupil that does not constrict in response to light indicates ipsilateral uncal herniation, until proved otherwise. Bilaterally dilated pupils can be seen with hypoxia, hypotension, bilateral oculomotor nerve dysfunction, or severe irreversible brain stem injury. A change in the pupillary examination is the most reliable indicator in determining the side with a mass lesion and carries an 80% positive predictive value. Moreover, pupillary size and reactivity are especially important in the assessment of trauma patients with an admission GCS score of 3, because those with bilaterally fixed and dilated pupils have a universally dismal prognosis, with mortality rates of up to 100%.
Many tertiary and quaternary centers have adopted the use of bedside automated infrared pupillometers to provide a quantitative measurement of pupillary response to a calibrated light stimulus of fixed intensity and duration. The integrated infrared camera performs a rapid and precise measurement of the pupil size as well as a number of dynamic pupillary variables. Compared with the standard manual method of subjectively assessing the pupillary light reflex, the use of pupillometry has demonstrated improved reliability and validity. The pupillometer also calculates the Neurological Pupil index (NPi) based on an algorithm that accounts for several measured pupillary variables, including size, percentage constriction, constriction velocity, dilation velocity, and latency. The NPi, a value between 0 and 5, is barely influenced by medications and ambient light, and it accounts for individual baseline pupil size. An NPi of ≥ 3 characterizes normal pupillary light reactivity and an NPi of less than 3 denotes an abnormal pupillary light reactivity; Chen et al. demonstrated an inverse relationship between decreasing pupillary reactivity and increasing intracranial pressure (ICP).
Mass lesions or intracranial hypertension sometimes precipitate a medial displacement of the temporal lobe on the same side. The compression of the midbrain against the tentorium cerebelli and the resultant interruption of corticospinal input from fibers within the ipsilateral cerebral peduncle cause a patient to present with hemiparesis contralateral to the side with the mass lesion. The manifestation of hemiparesis is a key focal neurologic deficit that is useful in establishing the laterality of a mass lesion after TBI. However, the surgeon must be aware that a false localizing sign occurs in cases of the Kernohan notch phenomenon, in which a mass lesion may manifest with ipsilateral hemiparesis due to compromise of the contralateral cerebral peduncle that is pushed against the contralateral tentorial edge.
Primary brain injury refers to damage that occurs as a direct result of the initial traumatic event and may be caused by penetrating, blunt, or blast trauma. Bullets and other projectiles often create a significant amount of destruction to brain tissue along their track, and fragments of the depressed skull can result in injury to brain parenchyma. In addition, laceration of cerebral blood vessels can lead to subarachnoid hemorrhage and/or formation of intracerebral hematomas. Blunt trauma can result in brain injury because of transmission of force directly at the point of impact or at a point distant from impact due to the displaced brain rebounding against the inner surface of the skull, an event referred to as contrecoup injury. The primary injuries of blast TBI are only now becoming understood and represent a regrettable reality of modern warfare and civilian terrorism.
Primary brain injury can be broadly divided into focal and diffuse injuries ( Table 122.2 ). The specific type of injury depends on the nature of the impact during injury (contact or inertial loading), the resultant forces delivered during the impact (rotational, translational, or angular acceleration), and the magnitude and duration of the impact itself.
| Focal | Diffuse |
|---|---|
| Hematoma | Concussion |
| Epidural | Multifocal contusion |
| Subdural | Diffuse axonal injury |
| Intracerebral | |
| Contusion | |
| Concussion | |
| Laceration |
Focal injuries include traumatic intracranial hematomas and contusions. According to data from the Traumatic Coma Data Bank (TCDB), diffuse injuries are more common and constitute upward of 60% of severe TBI, as they span a wide clinical spectrum ranging from concussion to diffuse axonal injury. Higher mortality rates have been seen with focal injuries, highlighting the necessity of expedient cranial imaging in evaluating TBI patients via CT scan, which can confirm the presence of a mass lesion and, in turn, prompt emergent surgical intervention if warranted.
Secondary brain injury occurs when local phenomena within the skull or systemic factors cause further damage that manifests at the cellular level over the ensuing hours to days after the original insult. The reduction of mortality from severe TBI, even when adjusted for injury severity, age, and other admission prognostic parameters, from 50% to less than 25% over the past 30 years reflects the effect of preventing secondary brain injury specifically through avoiding and treating aggravating factors, such as hypotension, hypoxia, inadequate cerebral perfusion pressure (CPP), and intracranial hypertension. These unwanted physiologic perturbations result in cerebral ischemia, which can significantly compound the effects of the initial injury. The decrement in mortality and improvement in outcomes from TBI that have been achieved are largely attributable to the use of evidence-based protocols, which hold avoidance of cerebral ischemia—through intensive monitoring of neurophysiologic parameters and maintenance of adequate cerebral perfusion—as paramount. , Knowing and understanding the pathophysiologic basis underlying cerebral ischemia and intracranial hypertension, the indications for monitoring, and the available treatments directed toward preventing or mitigating their effects are crucial to any neurosurgeon involved in the care of patients with severe TBI.
At autopsy, 80% of patients who die after TBI have evidence of cerebral ischemia. Cerebral ischemia is defined as cerebral blood flow (CBF) that is inadequate to meet the metabolic demands of the brain. Although the brain constitutes only 2% to 3% of the body’s weight, it consumes 25% of its oxygen and receives about 20% of its cardiac output. The brain is almost completely reliant on adequate blood flow: nearly 95% of the brain’s metabolism is oxidative, without a significant oxygen storage capacity and limited glucose and glycogen reserves. Delivery of oxygen to the brain is contingent on the oxygen content of the blood and CBF; likewise, delivery of glucose and other substrates of metabolism are based on CBF. In turn, a discrete metabolic autoregulatory mechanism exists to couple CBF with the cerebral metabolic rate of oxygen (CMRO 2 ) so that adequate perfusion exists to support local metabolic needs. This principle is illustrated by the Fick equation, CMRO 2 = CBF × AVDO 2 , where AVDO 2 is the arteriovenous oxygen content difference (in milliliters per deciliter) and is measured by subtracting the jugular venous oxygen content from the systemic arterial oxygen content. Under physiologic conditions, changes in CMRO 2 are paralleled by changes in CBF, with AVDO 2 remaining constant. In states in which decreased CBF occurs, as in systemic hypotension or when aberrant cerebral pressure autoregulation exists, the brain increases its extraction of oxygen to avoid ischemia. Current evidence suggests that episodes of inadequate CBF, evidenced by low jugular venous oxygen saturation (S jv O 2 ) values of less than 50%, are associated with increased morbidity and mortality in patients with severe TBI.
Two other important homeostatic mechanisms are involved in prevention of cerebral ischemia. The first mechanism indirectly links CBF to CPP via the partial pressure of carbon dioxide (P a CO 2 ) within the arterial system. In this process, arteriolar dilation and constriction occur secondary to changes in P a CO 2 and concomitant changes in the pH within the perivascular space, with hypercapnia causing vasodilatation and hypocapnia resulting in vasoconstriction. The second mechanism, referred to as pressure autoregulation, similarly links CBF to CPP.
Pressure autoregulation is a fundamental concept in cerebrovascular physiology and is distinct from metabolic autoregulation. Pressure autoregulation allows a constant supply of oxygen and metabolic substrates to be delivered to the brain by maintaining a constant CBF over a range of CPPs. CPP is defined as the mean arterial pressure (MAP) minus the ICP. Under normal circumstances, CBF remains relatively constant over a range of CPPs between 50 and 150 mm Hg, ensuring adequate oxygen delivery through a dynamic system of arteriolar vasoconstriction and dilation. Hence, in situations of low perfusion pressures, arteriolar vasodilation occurs, resulting in decreased cerebral vascular resistance and maintenance of normal CBF. On the other hand, when a state of high perfusion pressure exists, arteriolar constriction is required to maintain normal CBF. At CPPs outside the autoregulatory threshold, however, CBF is unable to be maintained within its normal parameters—an exceedingly low perfusion pressure results in arteriolar collapse and a consequent decrement in CBF; hyperemia occurs at high CPPs outside of the limit of autoregulation because of vessels reaching their capacity for vasoconstriction ( Fig. 122.1 ).
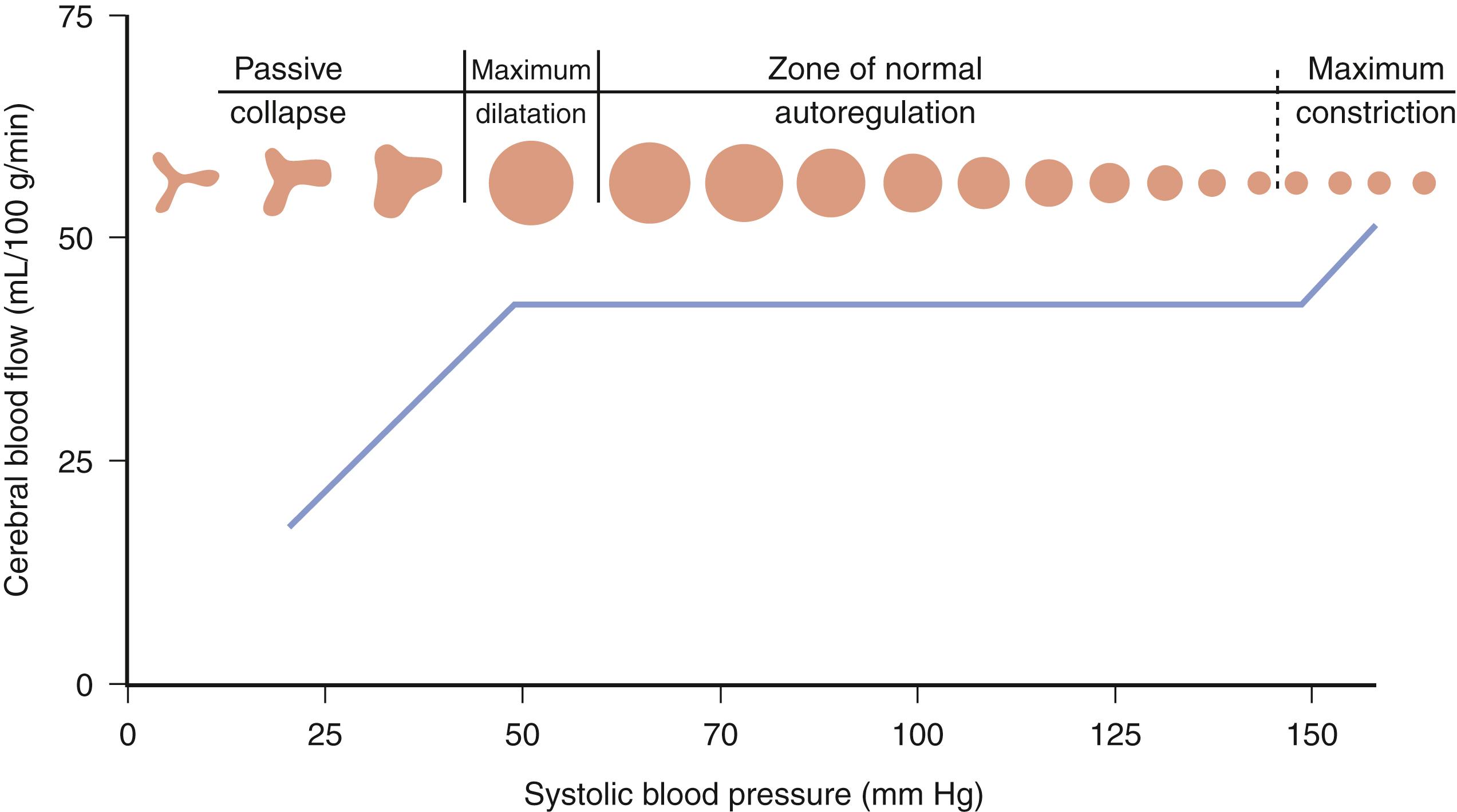
Clinicians must also be able to distinguish CBF from cerebral blood volume (CBV). As already mentioned, CBF is the physiologic parameter that governs cerebral perfusion and oxygen delivery. CBV represents the total volume of blood within the intracranial compartment and is a key component of ICP as described by the Monro-Kellie doctrine. In efforts to control ICP, CBV may be manipulated while maintaining an adequate degree of CBF.
Low CPP has been shown to be associated with higher mortality rates and greater morbidity among survivors following TBI, especially when systemic hypotension occurs concomitantly. Moreover, the normal response of cerebral arterioles to changes in CPP is frequently absent or impaired in 49% to 87% of patients after severe TBI. Aberrant pressure autoregulation at a range outside normal physiologic parameters may occur after TBI ( Fig. 122.2 ), , with significant consequences. Shifting the lower limit of autoregulation from 50 mm Hg to a supranormal range of 70 to 90 mm Hg could precipitate cerebral ischemia, , , which is especially noteworthy when considering that up to 60% of patients with severe brain injury experience a reduction in CBF during the first few hours after their trauma. Prolonged systemic hypotension or aggressive hyperventilation therapy would further exacerbate ischemia in vulnerable areas and could lead to subsequent cerebral edema. Conversely, autoregulation occurring at a lower-than-typical range of CPPs could result in malignant hyperemia or significant damage to the microcirculation, leading to secondary hemorrhages. Thus, absent or impaired cerebral pressure autoregulation represents a particularly significant risk factor for the development of secondary brain injury, especially early in the postinjury period.
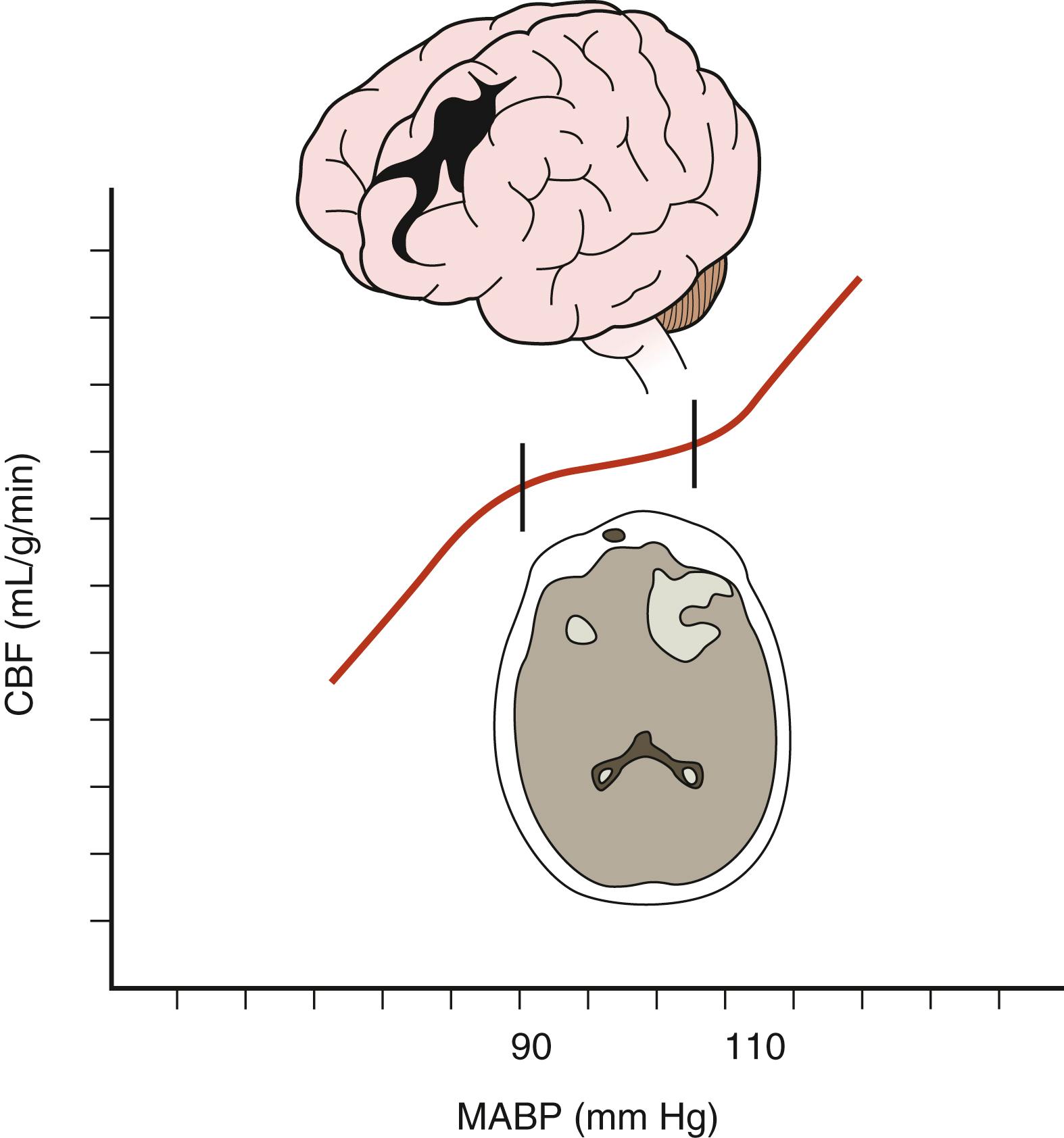
Based on the preceding information, CBF is assumed to have a linear relationship with CPP in managing patients with severe TBI. Other key factors that affect CBF include local factors such as physical compression of vessels by mass lesions or edematous brain, reduced cerebral metabolism of oxygen, and post-traumatic vasospasm. , Nearly 33% of severely head-injured patients have CBF values near the ischemic threshold (<18 mL/dL) within the first 24 hours after primary injury, , which is seen most often in individuals with diffuse cerebral edema or acute subdural hematomas. Low CBF values during the first 7 days after injury are associated with significantly higher mortality rates and increased morbidity. , ,
CPP thus serves as an important gauge of the adequacy of CBF and, in turn, must be monitored and maintained in patients with severe TBI. Based on studies of brain tissue oxygen tension (P bt O 2 ) and S jv O 2 that correlate with unfavorable outcomes, some investigators have suggested a treatment and maintenance threshold CPP of 60 mm Hg, , while other groups recommend a higher value of 70 mm Hg , to avoid cerebral oxygen desaturation. Based on the results of cerebral microdialysis monitoring, others have discussed a lower CPP threshold of 50 mm Hg. In a prospective study in which a CPP-focused management strategy was used, Rosner et al. demonstrated a mean mortality rate of 21% in patients with severe TBI when CPP is maintained above 70 mm Hg, as compared with the 40% mortality rate in the TCDB series. Current practice guidelines dictate that CPP should be maintained between 60 and 70 mm Hg and that taking into account the integrity of the patient’s autoregulatory status is important when considering minimum CPP threshold. Consequently, among patients with aberrant pressure autoregulation, further adjustments to CPP can be made based on ancillary neurophysiologic monitoring of CBF, oxygenation, metabolism, and the individual patient’s pressure autoregulatory status.
Systemic hypotension and hypoxia have deleterious consequences on patient outcomes after TBI because they contribute to secondary brain injury. Episodes of hypotension, the number of hypotensive events, inclusive of prehospital hypotensive events, , and the increased total duration of such episodes increase patients’ mortality and morbidity, with one study showing a 150% increased risk of mortality in patients with severe TBI who experienced at least one episode of hypotension. The latest iteration of the Brain Trauma Foundation’s guidelines suggests maintenance of systolic blood pressure above 100 mm Hg in patients between 50 and 69 years old and above 110 mm Hg in patients between 15 and 49 years of age and those who are over 70 years old.
Similarly, hypoxia portends poor outcomes after TBI. Stocchetti et al. noted that among severely brain-injured patients, those who had documented oxygen saturations of less than 60% had a mortality rate of 50%, with all survivors being severely disabled, in contrast to a mortality rate of 14.3% and a 4.8% rate of severe disability among nonhypoxic patients. As with hypotension, the number of hypoxic events, along with the duration of hypoxia, is predictive of worse outcomes. Establishing an airway early in the postinjury period is one important mechanism to prevent hypoxia; early intubation improves outcomes in patients with severe head injuries.
Therapies directed toward avoidance of cerebral ischemia are founded on CPP calculation. Thus, systemic blood pressure and ICP monitoring, with direct transduction of blood pressure by an indwelling arterial catheter and an ICP monitor, are warranted. Systemic arterial oxygen saturation and blood hemoglobin/hematocrit are also monitored as standard practice among all trauma patients.
Brain oxygen monitoring is a means of directly assessing the adequacy of oxygen delivery to brain tissue. Commonly employed modalities toward this goal include S jv O 2 sampling via an internal jugular vein catheter directed toward the jugular bulb and direct brain tissue oxygen monitoring by a fiber optic catheter. Episodes of jugular venous oxygen desaturation (S jv O 2 <50%) are known to be associated with higher mortality and worse outcomes in patients after severe TBI. , Low values of P bt O 2 (<10 to 15 mm Hg) and the extent of their duration (>30 minutes) are also associated with high rates of mortality. The current guidelines for severe TBI management advocate jugular bulb monitoring of arteriovenous oxygen content difference with a treatment threshold for S jv O 2 at 50%.
Modalities aimed at directly measuring CBF include placement of thermal diffusion probes and noninvasive techniques such as transcranial Doppler sonography, xenon CT, nitrous oxide uptake, and positron emission tomography. Assessing the metabolic state of the brain via cerebral microdialysis may gauge the adequacy of CBF. Finally, laser Doppler flowmetry and laser flow flowmetry, performed during surgery with the brain exposed, represent other technologies that measure CBF. ,
Avoidance of secondary brain injury due to cerebral ischemia is a fundamental principle guiding care after severe head injury. The surgical team must ensure adequate CBF by keeping CPPs between 60 and 70 mm Hg, as well as avoiding systemic hypotension, hypoxia, and anemia. Maintenance of CPPs above 70 mm Hg via use of vasopressors and volume expansion has been shown to significantly increase the risk of developing acute respiratory distress syndrome, thus obviating the need to aggressively treat patients in such a manner. ,
In patients with inadequate CPP, systemic causes of hypotension such as cardiac or spinal cord injury, tension pneumothorax, and bleeding must be ruled out. Intravascular volume may be assessed via central venous pressure monitoring. Administration of intravenous isotonic fluids is a mainstay of management, but vasopressors are often necessary to maintain adequate CPP. The profiles of commonly used vasopressors are listed in Table 122.3 . All listed agents are sympathomimetics, with phenylephrine being the most commonly used. Although these agents are of significant importance in the management of patients after severe head injury, their use may be associated with complications such as pulmonary edema and/or expansion of preexisting hematomas or contusions, and so they must be used with caution.
| Agent | MAP | CBF | ICP |
|---|---|---|---|
| Dopamine | |||
| <2 μg/kg/min | ↔ | ↔ | ↔/↓ |
| 2–6 μg/kg/min | ↑ | ↔/↑ | ↑ |
| 7–20 μg/kg/min | ↑ | ↔/↑ | ↓/ |
| Phenylephrine | ↑ | ↔ | ↔ |
| Norepinephrine | ↑ | ↔ | ↔ |
| Epinephrine | ↑ | ↔ | ↔ |
| Dobutamine | ↔/ | ↑ | ↔/↑ |
Delivery of oxygen to brain tissue is predicated on not only sufficient CBF but also oxygen content of the blood. As such, anemia can lead to cerebral ischemia. Despite evidence indicating that low hematocrit is associated with higher mortality and morbidity in patients with severe TBI, no consensus exists as to a particular threshold for transfusion of blood. Although anemia is associated with worse outcomes, there is little support for the aggressive use of packed red blood cells to correct for anemia based on extant data: some studies have noted increasingly negative neurologic outcomes in patients with severe TBI who underwent transfusion, while other authors have shown no difference in mortality rates among patients treated under an aggressive transfusion protocol as opposed to those treated along a more restrictive one. Interestingly, studies have demonstrated that between 26% and 43% of patients with severe head injuries experience a paradoxical decrement in P bt O 2 values in the setting of blood transfusion, , with no effect seen on cerebral metabolism.
Animal experiments, however, have shown that a hematocrit in the range of 30% to 33% provides an optimal balance between blood viscosity and oxygen-carrying capacity. Kee and Wood noted that brain tissue oxygen delivery begins to decrease at a hematocrit less than 33%, lending credence to the belief that maintenance of a hematocrit of approximately 30% to 33% is optimal. As with all trauma patients, a hematocrit refractory to blood transfusion should prompt a search for extracranial bleeding sources.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here