Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Endovascular therapy has evolved into an attractive first-line therapy for patients with peripheral arterial occlusive disease. Plaque excision techniques, such as atherectomy and laser ablation, attempt to improve primary patency as a standalone procedure or in conjunction with balloon angioplasty. Ideally, plaque debulking would minimize arterial wall trauma and recoil after angioplasty, thereby reducing the need for stent deployment.
Cryoplasty attempts to achieve these results by using cold thermal energy. Laser angioplasty is a means of atheroma ablation because laser light delivers precisely focused, intense energy. This energy can be generated outside the body and transmitted through optical fibers virtually without loss to target tissue. Ideally, laser energy would vaporize atheroma without damage to surrounding arterial tissue, with a resultant favorable healing response. Both techniques may be especially important in the below-knee revascularization often required for limb salvage and wound healing.
Laser is an acronym for the l ight a mplification by s timulated e mission of r adiation. T. H. Mailman first used a ruby crystal to create a laser beam in 1960. Since then, a variety of media have been used to produce laser light of varying wavelengths: 10 to 400 nm in the ultraviolet range, 400 to 700 nm in the visible range, and greater than 700 nm in the infrared range. Lasing media can include solid-state crystals (yttrium–aluminum–garnet [YAG]), gas (helium–neon, argon, CO 2 ) or liquid (organic dyes, semiconductors such as gallium arsenide).
Laser energy can be delivered as a continuous or pulsed wave. The continuous-wave mode delivers a steady flow of energy resulting in tissue ablation but can cause thermal damage to surrounding tissue. Pulsed laser energy reduces surrounding damage by allowing tissue to cool between individual laser pulses. However, not all wavelengths are amenable to pulsed delivery because a high repetition rate decreases the tissue relaxation time, resulting in similar thermal damage as occurs with continuous-wave energy.
A major advance in laser angioplasty was the development of optical fibers that carry laser energy without breakage. Optical fibers contain a transparent core, an outer cladding, and a protective outer coating or sheath. The core has a higher refractive index than the surrounding cladding so that laser light is internally reflected. To maximize the transmission of energy to the distal tip of an optical fiber, laser wavelengths are chosen to generate high-quality beams with low divergence that can be focused onto a single optical fiber. This becomes especially important with newer lasers that use short high-power pulses such as the excimer laser.
Laser light causes a variety of effects on tissue depending on the concentration of energy delivered and the interval of energy exposure ( Figure 1 ). The dominant effect between laser energy and biologic tissue is a photothermal reaction as tissue chromophores absorb the laser light with conversion to thermal energy. A high absorption coefficient favors efficient thermal ablation at lower energy densities, with decreased surrounding zones of thermal injury. Early thermal lasers heated the atheroma and arterial wall in order to coagulate or weld the artery in an open, dilated position. The destruction of endothelium and vascular smooth muscle was initially deemed beneficial for the inhibition of thrombosis and restenosis. Subsequent laser angioplasty systems have emphasized the use of laser energy for the direct ablation of atheroma, with minimal thermal damage to surrounding tissue.
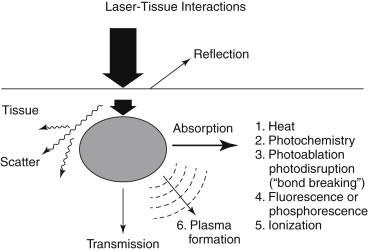
The versatility of laser light has led to a variety of laser angioplasty systems with different theoretical advantages. The goal in each system is to precisely vaporize atheroma of variable composition while minimizing injury to the adjacent arterial wall. It is important to understand the mechanism of action for each system in order to determine the advantages and disadvantages as compared to other interventional devices and to improve the methodology for future systems.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here