Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Penile cancer occurs in 1 in 100,000 North American and European men (accounting for 0.4%-0.6% of all male cancers). It is 10% of male malignancies in parts of Asia, Africa, and South America.
Human papillomavirus (HPV) types 16 and 18 are detected in 40% to 45% of penile cancers. Moderate or poor differentiation, invasion of the corpora, and the presence of tumor emboli in lymphovascular channels predict regional spread.
Circumcision is recommended for full evaluation of the primary. Magnetic resonance imaging (MRI) is recommended for determination of invasion of the corpora. Computed tomography (CT) for staging lacks sufficient sensitivity for evaluation of lymph nodes. For adverse pathology (high grade, invasion of corpora or lymphovascular spaces) surgical staging of lymph nodes is recommended.
Penile conservation is recommended for Tis, Ta to T1a, and G1 to G2 lesions and can be considered for G3 T1 or T2 lesions (tumor < 4 cm) and for selected T3 tumors. Laser surgery for Tis and T1 tumors yields satisfactory local control and cosmesis. Interstitial brachytherapy for stages T1 to T2 tumors allows penile preservation in 70% to 85%. External beam radiotherapy (EBRT) penile preservation rates are 50% to 65%.
Adjuvant EBRT is recommended after lymph node dissection for patients with multiple positive groin nodes or extracapsular disease.
Locally advanced penile cancer requires a multimodality approach. Treatment may begin with total or partial penectomy and lymph node dissection if surgically resectable. For unresectable disease, combined chemoradiotherapy can be used preoperatively or definitively. Neoadjuvant chemotherapy has a response rate of 50% and may improve resectability.
Unresectable nodes are rarely controlled by radiation alone, but palliation may be possible. A combined approach of concurrent chemoradiotherapy is warranted if the patient's general condition permits.
The penis is divided into three portions: the root, the body (or shaft), and the glans. The root is embedded in the superficial perineum. The shaft consists of the erectile bodies composed of the corpora cavernosa, the corpus spongiosum, and the overlying skin. The glans is the distal part of the corpus spongiosum and is covered by a skinfold known as the prepuce. The coronal sulcus delimits the glans from the shaft.
Early-stage, well-differentiated cancers of the penis can be effectively managed with local therapy, and attention should be paid to preservation of penile function and morphology. Traditional primary surgical management is effective but is associated with considerable psychosexual morbidity. Even partial penectomy can have a profound effect on sexual health and self-image. Suicide or attempted suicide after partial penectomy has been reported. In recent years, emphasis has been increasingly in favor of penile-sparing approaches. Surgical options have been developed in this regard, including procedures such as glans-sparing penectomy. External beam radiotherapy (EBRT) and interstitial radiotherapy are also organ-sparing alternatives that preserve penile morphology and function without compromising disease control or survival in selected patients. Referral to centers specializing in management of this rare cancer is encouraged. Quality of life and sexual health after treatment require more study. Sexuality and expectations should be discussed with the patient and partner when deciding on primary management. Advanced or poorly differentiated tumors require a multimodality approach. A 2013 study of 12 European cancer registries and the Surveillance, Epidemiology, and End Results (SEER) program in the United States showed no improvement in 5-year survival for penile cancer patients since 1990.
Carcinoma of the penis is rare, with an estimated incidence of 1 case per 100,000 men in North America and Europe, where it accounts for 0.4% to 0.6% of cancers. Over the past decades, the incidence has increased in England from 1.1 to 1.3 per 100,000 patient-years, and in Denmark from 1.0 to 1.3 patient-years. This is related to human papillomavirus (HPV) etiology and parallels an increase in HPV-related oropharyngeal squamous cell carcinoma (SCC) seen in the United States over the same time period of 225%. Higher incidences are also seen in parts of Asia, Africa, and South America, where penile cancer represents up to 10% of malignancies in men. The peak incidence is in the sixth decade in developed countries but earlier where the incidence is higher. See the online chapter for additional discussion.
Important risk factors are phimosis, chronic inflammatory conditions such as lichen sclerosis, treatment with psoralens and ultraviolet A (PUVA) photochemotherapy, tobacco products (dose-dependent association), and a history of genital condylomata (threefold to fivefold increase in risk). Neonatal circumcision is associated with a threefold decrease in risk of penile carcinoma. However, circumcised men with a history of HPV infection remain at increased risk. Oncogenic HPV, especially types 16 and 18, are identified in about 50% of invasive penile cancers. Penile trauma may be another risk factor for penile cancer. There is a threefold increase in development of carcinoma in the scarred penile shaft after penile tears or injury.
Although infant circumcision is highly effective in prevention, it is not recommended on these grounds alone. Instead, the emphasis is on promotion of good hygiene for the normally retractile foreskin, surgical correction of phimosis, and education to increase awareness of the association between HPV infection, venereal warts, and cancer, as well as the premalignant nature of conditions such as lichen sclerosis, which may precede the diagnosis of cancer by many years. In a report from Scandinavia, patients delay an average of 6 months before seeking medical attention.
Genital condyloma acuminata and genital HPV infection are sexually transmitted infections caused by HPV. The number of new infections is estimated at 6.2 million annually in the United States. The prevalence of HPV in western males is more than 20%, but is higher in uncircumcised men. Higher rates of infection are seen with increasing numbers of sexual partners, lack of condom use, and alcohol and tobacco use. More than 100 HPV types have been described as responsible for a variety of human diseases. Approximately 40 different HPV types have specific tropism for the anogenital region. Virus types 6, 11, and 42 to 44 are associated with condylomata acuminata and low-grade dysplasia. The International Agency for Research on Cancer has declared 12 HPV types as group 1 human carcinogens: types 16, 18, 31, 33, 35, and 39 as well as 45, 51, 52, 56, 58, and 59. Immunocompromised men with human immunodeficiency virus (HIV) infection are more susceptible to development of penile lesions and SCC with high-risk HPV types.
Penile intraepithelial neoplasia (PeIN) is a precursor lesion for invasive SCC. Differentiated PeIN is associated with nonviral etiology (inflammation and lichen sclerosis) while undifferentiated PeIN is associated with HPV in 93% of grade 2 PeIN and 100% of grade 3. The quadrivalent HPV vaccine (Gardasil, approved by the Food and Drug Administration, June 2006) protects against HPV types 6, 11, 16, and 18 and was originally recommended for females aged 9 to 26 years. More recently, a nonavalent vaccine was released (Gardasil 9, Merck) which includes additional protection against types 31, 33, 45, 52, and 58. The quadrivalent vaccine is highly immunogenic in men aged 16 to 26 with seroconversion by month 7. It has been shown effective in preventing external genital lesions and possibly confers greater immunity than natural infection. Successful HPV vaccination programs will combat the rising incidence of HPV-related neoplasms for which effective screening programs are lacking, including SCCs of the penis, anus, and oropharynx. Data from 2015 show that in the United States, 28% of male adolescents received HPV vaccination as compared with 42% of females.
The overall incidence of HPV in penile carcinoma is 40% to 45%, as detected by polymerase chain reaction (PCR) amplification of DNA, with HPV 16 and 18 the most frequent. The frequency of HPV detection depends on the histopathological subtype; HPV is seen in 80% to 100% of basaloid and warty penile cancers but in only approximately 35% of verrucous or squamous cell carcinomas. The difference in prevalence of HPV in these two groups reflects different pathogenesis. Penile lesions can be classified into HPV-induced (basaloid, warty, clear cell and lymphoepithelioma) and HPV-independent (SCC usual type, pseudohyperplastic, pseudoglandular, verrucous, papillary, adenosquamous and sarcomatoid-squamous), the latter group often seen on a background of chronic inflammation (lichen sclerosis, bowenoid papulosis). The pathogenesis following HPV infection involves viral transcripts of E6 and E7 oncoproteins. These are found in infected cells and inactivate p53 and retinoblastoma tumor suppression proteins, respectively. Inactivation leads to uncontrolled cell growth without a G1 cell cycle stop. This allows the cell cycle-dependent kinase inhibitor p16 to accumulate in the nucleus. Thus, p16 overexpression is a surrogate for HPV infection and, indeed, the majority of HPV-related cancers can now be detected by hematoxylin and eosin (H & E) staining with an inexpensive stain for p16. Concordance with PCR is 84%.
The presence of p53 is found in 41% to 75% of invasive penile cancers. Nuclear expression of p53 is seen in tumor cells and basal keratinocytes of p16 (ink4a) negative non–HPV-induced lesions. In multivariate analysis, p53 positivity and lymphatic embolization are predictive of lymph node metastases. Several other biomarkers are under investigation, including PD-L1 (programmed cell death ligand), EGFR (epithelial growth factor receptor) and HER-3 expression.
Premalignant lesions are associated with invasive cancers in up to one-third of cases. Intraepithelial neoplasia such as bowenoid papulosis, Bowen disease, and erythroplasia of Queyrat are precursor lesions of warty and basaloid penile cancers. Lichen sclerosis (balanitis xerotica obliterans) is associated with non-HPV variants of penile carcinoma.
The primary tumor most frequently occurs on the glans (48%) or prepuce (25%), with the glans and prepuce involved in 9%, the coronal sulcus in 6%, and the shaft in only 2%. SCCs represent 95% of invasive cancers of the penis. Other histopathological primary tumor types are malignant melanoma, transitional cell carcinoma, basal cell carcinoma, and sarcoma.
The lymphatics of the prepuce and the skin of the shaft drain into the superficial inguinal nodes located above the fascia lata. The glans and the deep penile structure drain into the superficial or deep inguinal nodes, from which they spread along the femoral vessels to the external iliac, common iliac, and paraaortic regions. The sentinel nodes are located above and medial to the junction of the inferior epigastric and saphenous veins.
Because of the rarity of carcinoma of the penis, reported series often span several decades. Four different staging systems are encountered in a review of the literature of the past 3 decades ( Table 65.1 ).
| Stage | Description |
|---|---|
| Jackson Staging System a | |
| I | Tumor limited to the glans or prepuce |
| II | Tumor extending into the shaft or corpora but without node involvement |
| III | Tumor confined to the shaft but with malignant but operable lymph nodes |
| IV | Invasion beyond the shaft with inoperable lymph nodes or distant metastases |
| TNM (UICC, 1978) b | |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 2 cm |
| T2 | Tumor > 2 cm and ≤ 5 cm |
| T3 | Tumor > 5 cm or deep invasion, including urethra |
| T4 | Tumor invades adjacent structures |
| N1 | Metastases in unilateral inguinal lymph nodes |
| N2 | Metastases in bilateral inguinal lymph nodes |
| N3 | Fixed inguinal lymph nodes |
| TNM (UICC, 1987-2002) c | |
| T1 | Tumor in subepithelial connective tissue |
| T2 | Tumor in corpus spongiosum or cavernosum |
| T3 | Tumor in urethra or prostate |
| T4 | Tumor in other adjacent structures |
| N1 | Tumor in one superficial inguinal lymph node |
| N2 | Tumor in multiple or bilateral superficial inguinal lymph nodes |
| N3 | Tumor in deep inguinal or pelvic lymph nodes |
| TNM (UICC, 7th Edition 2009) d | |
| Tis | Carcinoma in situ |
| Ta | Noninvasive verrucous carcinoma |
| T1 | Tumor invades subepithelial connective tissue T1a: Without lymphovascular invasion; not poorly differentiated or undifferentiated T1b: with lymphovascular invasion or poor differentiation |
| T2 | Tumor invades corpus spongiosum or cavernosum |
| T3 | Tumor invades urethra |
| T4 | Tumor invades other adjacent structures |
| N1 | Palpable mobile unilateral inguinal lymph node |
| N2 | Palpable mobile multiple or bilateral inguinal lymph nodes |
| N3 | Fixed inguinal nodal mass or pelvic lymphadenopathy unilateral or bilateral |
a From Jackson S. The treatment of carcinoma of the penis. Br J Surg. 1966;53:33-35.
b From Harmer M. Penis (ICD-0187). In: TNM Classification of Malignant Tumours . 3rd ed. Berlin: Springer-Verlag; 1978:126-128.
c From Hermanek PS, Sobin LH. Penis (ICD-0187). In: Hermanek PS, Sobin LH, eds. TNM Classification of Malignant Tumours . 4th ed. Berlin: Springer-Verlag; 1987:130-132.
d From Sobin L, Gospodarowicz M, Wittekind C. Penis (ICD-O C60). In Sobin L, Gospodarowicz M, Wittekind C, eds. TNM Classification of Malignant Tumours . 7th ed. Oxford: Blackwell Publishing; 2010:239-242.
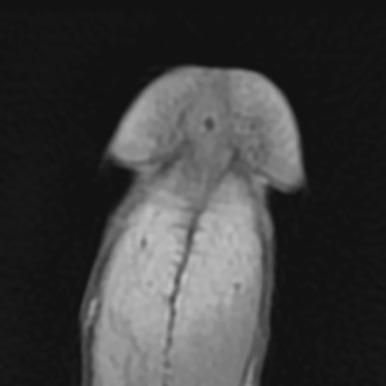
As clinical staging of carcinoma of the penis is subjective, it may be difficult to distinguish T1 (i.e., invasion of subepithelial connective tissue) from T2 (i.e., invasion of corpus spongiosum or cavernosum). For this reason, techniques that treat less than the full thickness of the penis must be restricted to carefully selected cases. High-resolution magnetic resonance imaging (MRI) is the gold standard for evaluating the primary tumor and local extension. Although small-volume lesions on the glans can be adequately staged by palpation in most cases, MRI—especially with artificial erection by intracorporal injection of prostaglandin E1—improves staging of the primary tumor where invasion of the corpus cavernosum is suspected ( Fig. 65.1 ). Any patient who presents with phimosis and chronic discharge, bleeding, balanitis, or swelling in the region of the coronal sulcus or glans under an unretractable foreskin should have a dorsal slit of the foreskin to allow inspection of the glans, preferably followed by a full circumcision. Any suspicious lesions should be sampled. Fig. 65.2 shows the diagnostic algorithm for a patient with clinically node-negative disease.
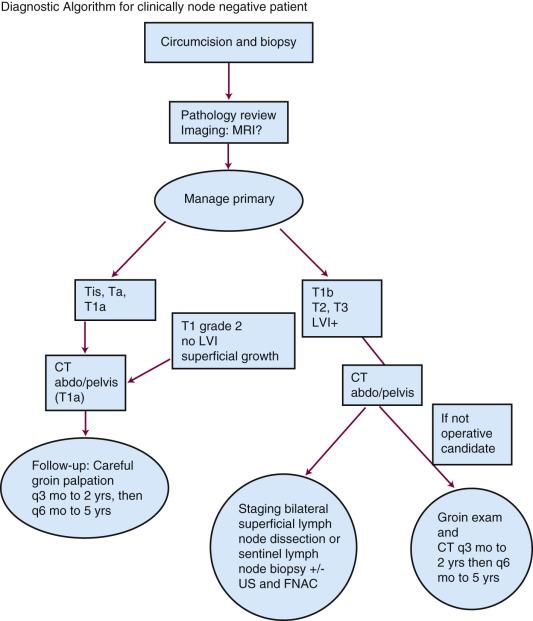
Management of the patient with clinically negative groins has been considerably clarified in the past decade. Although many radiotherapy series have advocated a “wait-and-see” policy, with no systematic staging investigations, such as computed tomography (CT) or fine-needle aspiration cytology (FNAC), the predictive factors for lymph node involvement have been established and allow selective surgical staging. Overall, only about 20% of clinically negative nodes have micrometastases; thus, staging lymph node dissection is not warranted for all patients. Traditional inguinal node dissection may be complicated in one-third of cases by infection, skin flap necrosis, deep vein thrombosis, or severe leg edema. Nodal status is, however, the strongest predictive factor for overall survival (OS) and lymph node dissection may be curative for men with microscopic regional spread. Several surgical series have identified that therapeutic node dissection confers an inferior survival compared with prophylactic node dissection. McDougal found a 5-year OS after inguinal node dissection of 92% for patients with clinically N0 disease, compared with 33% for those with clinically involved nodes. Modified inguinal lymphadenectomy—sparing the saphenous vein and limiting the dissection laterally, distally, and proximally—may reduce morbidity.
The reliability of physical examination is diminished markedly in the obese patient; thus, imaging is paramount. Both CT and MRI will demonstrate gross lymph node enlargement but will not detect a small metastasis in a normal-sized node. Nonetheless, CT is the imaging modality recommended to examine the inguinal regions and pelvis as well as to rule out more distant metastases, with a sensitivity and specificity of 36% and 100%, respectively. Although sensitivity as high as 80% has been reported with positron emission tomography (PET)/CT using the radiopharmaceutical fluorodeoxyglucose (F-FDG), a meta-analysis of 7 studies showed a pooled sensitivity per groin of only 57%. Since positive predictive values were only 25% to 37%, surgical staging remains necessary in order to detect small inguinal metastases. PET/CT performs better in staging pelvic nodes in patients with known inguinal metastases, with sensitivity over 90%. The combination of ultrasound and FNAC has been shown to detect 80% of metastatic disease in nonpalpable nodes (12 out of 15) with an overall sensitivity and specificity of 87% (48 out of 55) and 99%, respectively.
Stage and histopathological factors should be used to identify patients at high risk for microscopic regional spread to selectively offer surgical staging of lymph nodes. Chaux and Cubilla have published a stratification system to estimate the likelihood of lymph node metastases based on grade, extent and depth of invasion, and the presence of perineural invasion. T category and lymphovascular invasion are also reported to be predictive for nodal relapse. The risk of nodal involvement in patients at high risk is 64% to 83%, 20% to 33% in intermediate risk, and 0% to 8% in low risk.
The difficulty with applying histopathological information to decision-making in radiotherapy is that the primary tumor is not available for complete histopathological examination. Invasion of the corpora may be underappreciated clinically. Diagnostic biopsies are often superficial and unreliable in determining the depth of invasion, the presence of lymphovascular invasion, or the ultimate tumor grade. However, if the biopsy shows high-risk features, surgical assessment of regional lymph nodes is recommended. Ultrasound-guided FNAC is also a valuable tool to evaluate suspicious inguinal nodes, with reported sensitivity and specificity more than 90%. A 4- to 6-week trial of antibiotics is no longer recommended.
The European Association of Urology (EAU) guideline recommends observation of lymph nodes for carcinoma in situ (Tis), verrucous (Ta), and T1 grade 1 tumors because these are associated with less than a 10% incidence of lymph node positivity. T1 grade 2 tumors are classified as intermediate risk and observation is recommended only for those with a superficial growth pattern and no vascular invasion. Category T2 or higher, or grade 3 tumors, and those with vascular invasion have a higher than 50% risk of lymph node involvement and should have surgical staging. Patients with T1 grade 2 tumors with high-risk features such as tumor thickness or vertical growth should also be considered for surgical staging. Close follow-up of patients at high risk is important. See the online chapter for additional discussion.
Dynamic sentinel lymph node mapping uses a gamma probe after intradermal peritumoral injection of technetium-99m and is becoming more widely available for detection of early lymph node involvement ( eFig. 65.1 ). False-negative rates, although initially in the 18% to 25% range, have fallen to 5% to 7% in the recent literature from high-volume centers with experienced surgeons. Complication rates of 5% are reported. Lam et al. described long-term follow-up of 500 inguinal basins in 264 patients with T1G2 or higher disease and nonpalpable nodes managed with ultrasound or FNAC and DSNB. Sensitivity of dynamic sentinel node biopsy (DSNB) alone per inguinal basin was 92% and with ultrasound, with or without FNAC, 95%. Routine serial sectioning along with cytokeratin immunohistochemistry, exploration of groins with low or no signal, and ultrasound with FNAC to detect positive lymph nodes that may have altered lymphatic flow have helped to decrease false negatives. The use of PET/CT may further decrease the risk of false negatives caused by tumor blockage and rerouting of lymphatics. Superficial inguinal lymph node dissection is still considered the gold standard in centers lacking the expertise for DSLB because the consequences of a false negative may be an increased risk of death from disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here